Chapter 50
Chest Pain and Myocardial Ischemia
Pathophysiology of Myocardial Ischemia and Acute Coronary Syndromes
In the postoperative patient, this oxygen supply may be also diminished as a result of intraoperative blood loss or iatrogenic hypotension. Like critical illness, recovery from major trauma or surgical interventions places extra demands on the myocardium by increasing total-body minute oxygen consumption (see Chapter 8). Finally, oxygen supply-demand balance may also be offset by pharmacologic interventions. For example, vasopressors and inotropic agents may cause tachycardia and augment myocardial contractility, thus increasing myocardial oxygen demand.
The triggers of acute plaque rupture are not fully defined. Most likely, acute hemodynamic stress is the trigger, but acute and chronic endothelial inflammation may also play a role. There is a circadian variation in the occurrence of all acute cardiovascular events, including myocardial infarction (MI), sudden death, and stroke, with their peak frequency in the first 2 hours after awakening. On arising and assuming an upright posture there are a variety of acute circulatory adjustments, including the release of catecholamines, which results in increased cardiovascular tone and hemodynamic stress. In addition, in these hours there is an increase in blood viscosity and platelet aggregation. Beta-blocker therapy eliminates the higher morning incidence of acute cardiovascular events, which suggests that catecholamine release and hemodynamic stress are important causative events.
Causes of Chest Pain
The differential diagnosis of chest pain in the ICU patient is extensive and includes a number of cardiovascular and noncardiovascular causes (Table 50.1). Esophageal pain shares some features with classic angina; it is typically retrosternal and frequently relieved with nitroglycerin. Reflux pain is often burning in nature, aggravated by lying down, and related to eating. Pain aggravated by movement or by palpation of the affected area is likely to be due to musculoskeletal causes. The description of chest pain may be most helpful in differentiating other cardiopulmonary causes. For instance, the chest pain of pericarditis is typically sharp and pleuritic, relieved by leaning forward. The chest pain of aortic dissection is typically “tearing” in quality, reaches maximal intensity instantaneously, and radiates to the back or flanks. The chest pain of pulmonary embolism, when present, is more often sharp and associated with dyspnea, hypoxemia, or hemoptysis in the absence of other signs of left-sided heart failure. Pneumothorax pain is usually sharp, sudden, and accompanied by dyspnea. Frequently, many patients are admitted to the hospital with a diagnosis of “rule out MI” and are discharged after a cardiac enzyme evaluation is negative and a stress test is normal. These patients have a great deal of uncertainty regarding the actual cause of their chest discomfort, and further evaluation and intervention are essential in their postdischarge care.
TABLE 50.1
Causes of Chest Pain in the Intensive Care Unit
| Cardiovascular | Angina Acute coronary syndrome/myocardial infarction Aortic dissection Congestive heart failure Pericarditis |
| Pulmonary | Pulmonary embolism with/without pulmonary Infarction Pneumothorax Pneumonia Pulmonary hypertension Pleurisy/pleuritis Tracheobronchitis Chest tubes |
| Gastrointestinal | Esophageal reflux Esophageal spasm Peptic ulcer disease Gallbladder disease Pancreatitis Hepatic capsular distention Subdiaphragmatic abscess |
| Musculoskeletal | Costochondritis Arthritis Postcardiopulmonary resuscitation (CPR) trauma Postoperative incisional or other related pain Cervical spine disease |
| Infectious | Herpes zoster |
| Psychological | Anxiety Panic disorder |
Clinical Presentation of Angina and Chest Pain
Symptoms
Recognition and treatment of coronary ischemia in the ICU are important. Ischemia may occur spontaneously or, more often, may occur in the setting of precipitants such as anemia, fever, or postoperative stress. Typical angina may range from a minor discomfort to severe pain. The discomfort is often described as pressure, heaviness, or indigestion. If “pain” is present, it is frequently described as crushing, squeezing, or burning. Classically, angina is located substernally and radiates to the left arm, neck, or jaw. Superficial discomfort is less likely to be caused by myocardial ischemia. Moreover, the chest discomfort of myocardial ischemia does not begin suddenly at maximal intensity but rather crescendos in intensity.
Symptoms of ischemia or myocardial infarction may be difficult to discern in the critically ill patient because of comorbid illnesses and sedating medications. Even when patients have the capacity to communicate, nearly 20% of those with acute myocardial ischemia or infarction will not develop chest discomfort. In this case, ischemia is “silent”—that is, it manifests only as objective signs in the absence of patient awareness. Recognition of ischemia in the ICU therefore requires careful scrutiny of hemodynamics, targeted physical examinations, and careful review of electrocardiographic (ECG) changes from baseline.
Diagnostic Evaluation
Electrocardiogram (ECG)
Repolarization Abnormalities
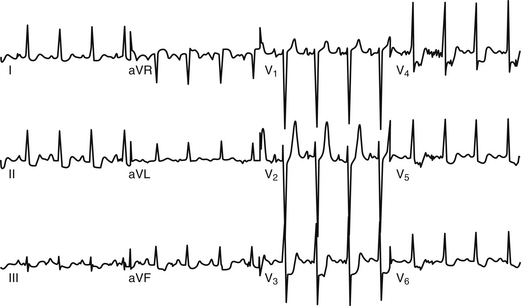
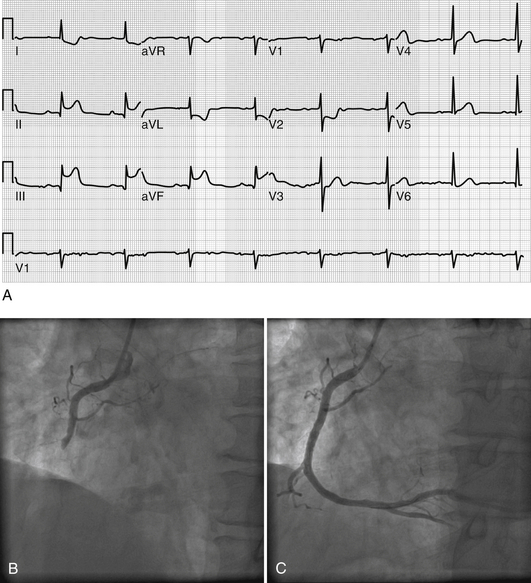
The classic ECG manifestation of ischemia is downward displacement of the ST segment (Figure 50.1). Horizontal and downward-sloping ST segments are generally more specific than upsloping ST segments. Although less specific, flattening of the ST segment with increased angulation at the ST-segment/T-wave junction also suggests ischemia. With resolution of ischemia, ST segments typically return to baseline. When ST segments remain depressed, subendocardial myocardial infarction should be suspected. ST-segment elevation is consistent with acute transmural myocardial infarction and is often followed by T-wave inversion and the appearance of Q waves (Figure 50.2A). A new left bundle branch block (LBBB) is felt to be equivalent to an ST-segment elevation infarction as it may represent complete occlusion of the LAD prior to septal branches that perfuse the left bundle branch. Less often, ST-segment elevation reflects transient myocardial ischemia secondary to vasospasm (Prinzmetal’s angina). Diffuse, widespread ST elevation may be secondary to pericarditis.
Arrhythmias (see Chapters 33 and 34 for more details)
Arrhythmias may be additional ECG manifestations of myocardial ischemia or infarction, although they may also occur as a manifestation of structural heart disease (hypertension, left atrial abnormalities, etc.). These can include atrial arrhythmias (atrial tachycardia, atrial fibrillation, and atrial flutter), sinus tachycardia, and ventricular tachycardia/fibrillation. Conduction blocks ranging from first-degree atrioventricular (AV) block to complete heart block can also be seen.
Chest Radiography
In the setting of myocardial ischemia, chest radiographs frequently demonstrate evidence of interstitial or alveolar edema. When it appears in an interstitial pattern, edema manifests as haziness among pulmonary vascular markings, owing to pulmonary venous engorgement as well as peribronchial cuffing. This is accompanied by thickening of interlobular septa with engorged lymphatic vessels, known as Kerley B lines, which appear as short, horizontal, linear densities at the lung periphery. If interstitial fluid accumulates rapidly, it may flood alveoli, resulting in confluent opacification, usually in a perihilar or “batwing” distribution. Unilateral or bilateral pleural effusions may also accompany acute ischemic cardiac failure.
Cardiac Enzymes
Myocardial injury results in the release of a variety of intracellular biochemical markers. The most commonly measured of these include creatine kinase (CK), its dimeric isoform CK-MB (isoenzyme of creatine kinase with muscle and brain subunits), and troponins I and T (Table 50.2). Typically, postinfarction CK and CK-MB appear within 4 to 6 hours and peak within 18 to 24 hours. Both remain elevated for 48 to 72 hours following infarction, but, as a result of faster clearance, CK-MB usually returns to baseline before total CK. Measurement of CK and its isoenzymes is very sensitive, but trace amounts of CK-MB are also found in a number of other tissues, so care must be taken in interpreting rises in CK-MB after surgery or trauma.
TABLE 50.2
Assessment and Management of Chest Pain in the Intensive Care Unit
| History | Character, timing, radiation of pain Associated symptoms Cardiac risk factors |
| Physical Exam | Vital signs Focused cardiac and pulmonary examination Signs of congestive heart failure |
| Diagnostic Evaluation | ECG (ST-T new wave changes consistent with ischemia/infarction; Q waves; arrhythmias) Cardiac biomarkers (troponin, CK, CK-MB) |
| Risk Stratification | TIMI risk score Echocardiography |
| Contributing Causes | Consider precipitants for ischemia other than acute coronary syndrome —Sepsis —Hypovolemia —Trauma —Anemia —Stress from postoperative state |
| Medical Therapy | Anti-ischemic therapy —Oxygen —Analgesia —Beta-blockers —Nitrates Antiplatelet therapy —Aspirin —Thienopyridines (i.e., clopidogrel) —Glycoprotein IIb/IIIa inhibitors Anticoagulant therapy —Heparin, low-molecular-weight heparin Statins ACE inhibitors |
| Invasive Therapy | STEMI —Prompt angioplasty/PCI —Thrombolytics if PCI not available NSTEMI/UA —Consider invasive approach within 24–48 hours |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree