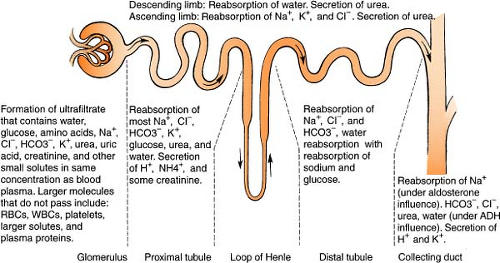
Renal System
Acute Renal Failure
Acute renal failure is the rapid deterioration of renal function with a high blood concentration of nitrogen waste products. There are three major categories:
Prerenal (Hypoperfused Kidney)
Prerenal events occur before blood reaches the kidney, leading to renal hypoperfusion and resulting in decrease of glomerular perfusion, CHF, and low blood pressure (BP). This is the most common cause of acute renal failure.
Intrarenal (Diseased Kidney)
Intrarenal failure is due to intrinsic damage to the kidney. There are two types:
Glomerulonephritis has an immunologic basis and causes change in the glomerular membrane and/or cellular structure. The glomerulus loses its ability to be semipermeable, and protein and red blood cells (RBCs) pass through into the urine, resulting in hematuria and proteinuria.
Acute tubular necrosis has two origins:
Ischemic (oliguric) necrosis is related to catastrophic hypotension from many sources, for example, surgery, cardiac or septic shock, trauma, hemorrhage. It is more serious than nephrotoxic necrosis because cells are destroyed in a layer that cannot regenerate (the “basement membrane”).
Nephrotoxic (nonoliguric) necrosis is related to environmental or occupational insults to the kidney, for example, large doses of iatrogenic agents such as gentamicin, amikacin, or carbenicillin. It has a good prognosis for recovery because the damage involves only the tubuleís layer of epithelial cells, which can regenerate.
Use of Laboratory Values in Differentiating Acute Tubular Necrosis from Decreased Renal Perfusion
| Acute Tubular Necrosis | Reduced Renal Blood Flow | |
|---|---|---|
| Urine Volume | <400 mL/24 hr | <400 mL/24 hr |
| Sodium | >40 mEq/L | <20 mEq/L |
| Specific gravity | 1.01 | Usually>1.020 |
| Osmolality | 250–350 mOsm/L | Usually>400 mOsm/L |
| Urea | 200–300 mg/100 mL | Usually>600 mg/100 mL |
| Creatinine clearance | <60 mg/100 mL | Usually>150 mg/100 mL |
| FeNa | >3.0% | <1.0% |
| Blood BUN/Cr ratio | 10:1 | Usually >20:1 |
| Responses to Mannitol | None | None or flow increases to >40 mL/hr |
| Furosemide | None | Flow increases to >40 mL/hr |
Postrenal (Obstructed Kidney)
Postrenal failure is caused by blockage of flow in the urinary tract. Treatment of acute renal failure in the first 24 to 48 hours includes correcting hypotension or fluid deficits, administering furosemide (Lasix) (80–320 mg initial dose) or mannitol (12.5–25 g), and/or giving a trial of low-dose dopamine (1–3 μg/kg/min) plus Lasix.
Acute Tubular Necrosis
(see Acute Renal Failure, Intrarenal, p. 247)
Adh
(see also Aldosterone, p. 249; Renin-Angiotensin System, p. 261)
Antidiuretic hormone (ADH) is controlled by osmoreceptors (sensitive to osmolar change) and baroreceptors (sensitive to pressure change). Normally, osmoreceptors in the hypothalamus stimulate the posterior pituitary to release ADH in response to a rise in serum osmolality (i.e., when water is lost). ADH then acts on the renal tubule to increase water permeability, and water is reabsorbed. The reverse is true: When serum osmolality falls (water is retained), excess water is excreted by the kidney.
Baroreceptors influence release of ADH in pathologic states. When blood volume is down about 10% or blood pressure falls, these receptors in the heart and various blood vessels contract and initiate a stimulus for ADH release. Water is then retained by the kidney. The reverse is true when baroreceptors are stretched more than normal (i.e., when blood volume rises).
Baroreceptors override osmoreceptors. For example, if blood volume is low, water is retained even though osmolality drops. This is a protective mechanism whereby the body attempts to maintain blood volume even though hemodilution results.
Aldosterone
(see also ADH, p. 249; Renin-Angiotensin System, p. 261)
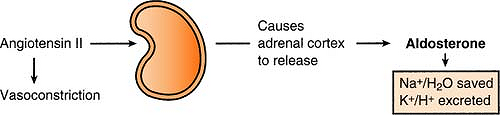
Aldosterone is released by the adrenal cortex in response to angiotensin II, causing sodium and water to be reabsorbed and potassium and hydrogen to be excreted.
Remember:
Because aldosterone causes sodium and water to be saved, the actual concentration of sodium does not change, only the extracellular volume.
Aldosterone is released for:
Increased potassium (to decrease the K+ level)
Decreased sodium (to save Na+)
Hypovolemia (to save H2O)
No aldosterone is released in cases of:
Increased sodium (to decrease the Na+ level)
Decreased potassium (to save K+)
Anion Gap
The anion gap is a formula used to signal the presence of a metabolic acidosis, differentiate the cause, and confirm other findings. In maintaining chemical neutrality, the total concentrations of cations and anions in the blood (and other body fluids) are equivalent. Sodium accounts for 95% of all cations, and chloride and bicarbonate account for 85% of all anions. The concept of an anion “gap” developed when blood chemistries began to be reported as a “panel.” Since only the major anions and cations are reported on these panels, it is normal for a “gap” of 8 to 16 mEq/L to exist (reflecting the absence of the anions/cations not accounted for). The formula to calculate the anion gap is:
An anion gap of >16 mEq/L indicates an increase in the number of unmeasured anions. Because most of these unmeasured anions are acids, the increase always reflects a metabolic acidosis. Differential diagnosis includes diabetic ketoacidosis; uremia (renal failure); lactic acidosis from sepsis; shock; ischemic bowel; or intoxication with aspirin, ethanol, antifreeze, or INH.
While a decrease in the anion gap is rare, the clinical significance is equally important, indicating hyponatremia, hypermagnesemia, multiple myeloma, or hypoalbuminemia (a 1-g drop in albumin will decrease the anion gap by 2.5–3 mEq).
Remember:
An actual high anion gap (acidotic) in a patient with a low albumin level may appear as a false normal.
Cavh, Cavhd
(see Continuous Renal Replacement Therapy, p. 253)
Continuous Renal Replacement Therapy
(see also Hemodialysis, p. 256; Peritoneal Dialysis, p. 259)
Continuous renal replacement therapy (CRRT) encompasses “out of body” blood purification methods intended to replace kidney function for extended periods of time, done continuously (24 hours/day). The general principles include blood flowing from a vascular access point, being purified in some manner outside the body by use of filters, creation of an “effluent” (urine), and returning the purified blood to the body via another vascular access. Depending on the mode used, access can be either venovenous or arteriovenous.
Treatment goals include promoting hemodynamic stability, allowing for nutritional support, maintaining fluid and electrolyte balance, maintaining pH balance, and promoting healing and renal recovery.
Modes: SCUF, CVVH, CVVHD, CVVHDF, CAVH, CAVHD
Indications for Crrt
Oliguria (output <200 mL/12 hr)
Hyperkalemia (K+ >6.5 and rising)
Acidemia (pH <7.1)
Pulmonary edema
Azotemia
Uremic encephalopathy
Uremic pericarditis
Severe sodium alteration (<115 or >160 mEq/L)
Hyperthermia
Drug overdose (lithium, vancomycin, procainamide)
Anasarca (accumulation of serum in subcutaneous connective tissue and serous cavities of body)
Diuretic-resistant cardiac failure
In Summary, All of the Above Fall Into Four Categories:
Volume overload
Acid-base imbalance
Uremic syndromes (and their sequelae)
Drug/toxin overdose
The contraindications for CRRT are all relative. In general, they include severe vascular disease and bleeding/clotting disorders.
Crrt Terminology
Ultrafiltration: The process of removing excess H2O by creating a pressure differential between the blood and fluid compartments. The addition of positive pressure in the blood path and negative pressure in the dialysate path causes excess H2O to move from the patient (high pressure) to an area of lower pressure (the dialysate). The negative pressure is an actual suctioning force applied to the membrane.
Convection: Movement of solutes (solids) through a membrane via a “sweeping” action called a solvent drag
Diffusion: Spontaneous movement of solutes from an area of higher concentration to one of lower concentration
Osmosis: Spontaneous movement of water from an area of lower concentration to one of higher concentration
Hemofiltration: Movement of large volumes of fluid via ultrafiltration, and some movement of solutes via convection
Replacement fluid: Approximates normal plasma, flexible to meet patient needs (Ringer’s lactate, normal saline, albumin, bicarbonate, electrolytes)
Dialysate: Solution that surrounds the patientís blood, separated by a filter, and allows solutes to cross from one side to the other
CRRT methods can be either arteriovenous (AV) or venovenous (VV). The differences are listed in the table.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree