Hematologic and Immune Systems
Aids
AIDS is an infectious disease caused by human T-cell lymphotrophic Type III virus (HTLV-III). It is an acquired deficiency of the body’S immune system. The immune system continually produces cells and antibodies that attack and destroy germs that otherwise would render a patient ill. The AIDS virus zeros in on certain T lymphocytes and breaks down the immunity, thus removing any natural resistance and leaving the patient vulnerable to a variety of viruses, bacteria, yeasts, fungi, and parasites. The virus is carried in the blood and transmitted through the blood.
A phase of infection with HIV, described clinically but no longer commonly diagnosed in practice, is AIDS-related complex (ARC). Patients with ARC usually experience fatigue, weight loss, and night sweats, along with superficial fungal infections of the mouth (oral thrush) and fingernails and toenails. They do, however, lack the opportunistic infections and neoplasms that define AIDS. It is uncommon for HIV-infected persons to die at the stage of ARC.
Pneumonia is the major cause of death, usually caused by the protozoa Pneumocystis carinii or by the cytomegalovirus (CMV). The most characteristic cancer that develops is Kaposi’S sarcoma. It spreads rapidly and is usually fatal. It initially may be limited to skin (red-brown lesions) but quickly spreads internally. Another cancer that often develops is lymphoma, a cancer of the lymphatic system, which eventually causes death.
The screening tests for AIDS are (1) the enzyme-linked immunosorbent assay (ELISA) and (2) the Western blot test. Both identify antibodies produced by the immune system after infection by the virus and are therefore not accurate until a person has been infected for 4 to 12 weeks.
Aids Profile
Lymphocytopenia, thrombocytopenia
Low T4:T8 ratio
Brain dementia (30%–40%)
Unexplained weight loss
Night sweats
Red-brown spots on skin or mucous membranes
Swollen lymph nodes lasting more than 1 month
Persistent white spots or unusual blemishes in the mouth
Fever >99°F for more than 10 days
Persistent cough and shortness of breath
Persistent diarrhea
Treatment
The recommended therapy for HIV infection is the HAART regime (highly active antiretroviral therapy). It combines three or more anti-HIV medications in a daily dosing program. While the therapy does not cure the HIV infection (and the individual can still transmit HIV to others), the goal is to control the reproduction of the virus and slow the progression of the disease.
There are multiple categories of anti-HIV medications currently approved by the FDA with countless clinical trials underway investigating new agents. Classes of anti-HIV medications known thus far include:
Nucleotide reverse transcriptase inhibitors (NRTIs): These drugs are used to prevent healthy T cells in the body from becoming infected with HIV and are sometimes referred to as “nukes.” They are, in effect, “faulty” versions of building blocks that HIV needs to make more copies of itself. When HIV uses an NRTI instead of a normal building block, reproduction of the virus is stalled.
Nonnucleoside reverse transcriptase inhibitors (NNRTIs): Sometimes called “non-nukes,” these drugs attach themselves to reverse transcriptase and prevent the enzyme from converting RNA to DNA. In turn, the genetic material of HIV cannot be incorporated into the healthy genetic material of the cell, and this prevents the cell from producing new virus. A new NNRTI, the first in over a decade, has recently been approved, Intelence (etravirine).
Protease inhibitors (PIs): These inhibitors are designed to attack HIV by blocking the protease enzyme. When new viral particles break off from an infected cell, protease cuts long protein strands into the sections needed to assemble a mature virus. When the protease enzyme is blocked, the new viral particles cannot mature. PIs, when used in conjunction with other AIDS drugs, have been noted to cause the HIV levels in the blood to decrease dramatically and the numbers of WBCs to rise. Rapid resistance to this class of drug has been noted. As a consequence, several firms are researching the development of a new PI that will not be cross-resistant with existing drugs. A new PI drug, Prezista (darunavir) is now available.
Vaginal HIV Microbicides: Clinical trials are now underway to evaluate the safety and efficacy of this agent. Research has demonstrated that although called “microbicides,” most do not actually kill the virus, but instead impede its ability to infect the host cells.
Entry Inhibitors: This is a new generation of antiviral compounds, presently undergoing active preclinical and clinical development as potential therapies for HIV-1 infection.
Attachment and Fusion Inhibitors: This is a new class of anti-HIV drug that is intended to protect host cells from HIV infection by preventing attachment of the virus to a new cell and breaking through the cell membrane. Preventing attachment blocks HIV entry into the cell. Digestive acids break this drug down, so most are given by IV infusion.
CCR5 Antagonists: A new class of antiretroviral drugs that could provide an alternative to HIV positive patients with resistance to multiple drugs. An example of a drug in this class, Selzentry (maraviroc), works by blocking a protein, CCR5, on the immune cells that HIV uses as a portal to enter and thereby infect the cell. Maraviroc carries a warning as it poses an increased risk of heart attack.
Other categories: Gene Therapy, Integrase Inhibitors, Maturation Inhibitors, Zinc Finger Inhibitors, Viral Decay Accelerator.
There are currently no HIV/AIDS vaccines approved for use, although many are in current clinical studies.
Albumin
Albumin is used as a blood volume expander in the treatment of hypovolemic shock, hemorrhage, trauma, acute hemodilution, or acute vasodilation. It improves “third space” fluid loss, including that occurring in acute peritonitis, mediastinitis, burns, and after radical surgery. It is used to reduce cerebral edema caused by neurosurgery or anoxia. It is also used in the treatment of hypoproteinemia, hepatic cirrhosis, and nephrosis.
Volume
5% (isotonic and isosmotic; 95% NS + 5% albumin) 250 to 500 mL bottles
25% (salt poor; 75% NS + 25% albumin) 50 mL (12.5 g) or 100 mL (25 g)
Infusion Rate
Tubing accompanies albumin. No filter is necessary. No saline needed. May piggyback into mainline IV.
5% albumin:
For shock: Initial dose of 500 mL given as rapidly as possible. Additional 500 mL may be given in 30 minutes.
For patients with low blood volume: Administer at 2–4 mL/min.
25% albumin:
Therapy based on clinical response. Administer as rapidly as tolerated.
For patients with low blood volume: 1 mL/min.
Special Considerations
No ABO blood group antibodies are present; therefore, compatibility is not a factor.
Anaphylactic Shock
(see Shock, Anaphylactic, p. 293)
Anemia
Hemolytic: Excessive erythrocyte destruction
Iron deficiency: Nutritional deficiency (↓ MCV, ↓ MCH, ↓ MCHC)
Pernicious (aplastic): Bone marrow failure (↓ RBCs)
Apheresis
(see Apheresis in Part 2, p. 76)
Blood Compatibility
(see Transfusions: Compatibility, p. 294)
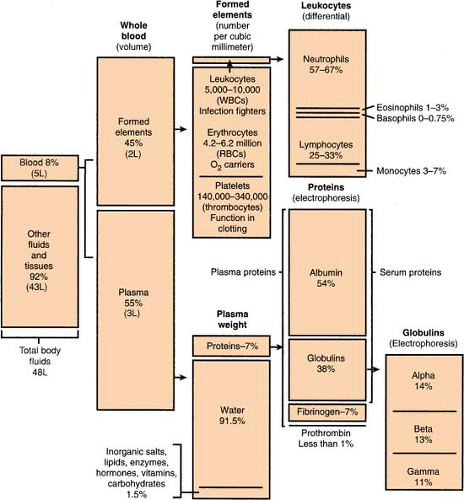
Blood, Composition Of
(see also Body Fluid Compartments, p. 280)
A person’S actual amount of blood varies with height and weight, but an average man has 12 pints and an average woman has 9 pints (or an average person has 4.5–6.0 L). Approximately 8% of body weight is accounted for by blood.
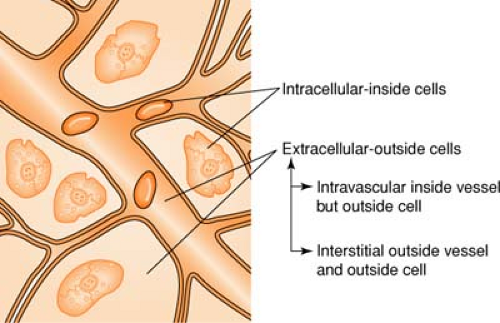
Body Fluid Compartments
(see also Blood, Composition of, p. 279)
Body fluid averages about 70% of total body weight, divided as follows:
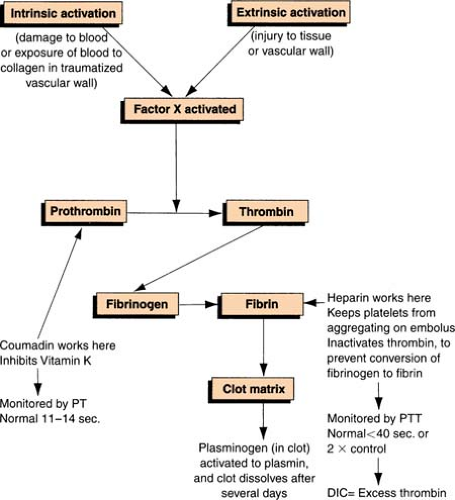
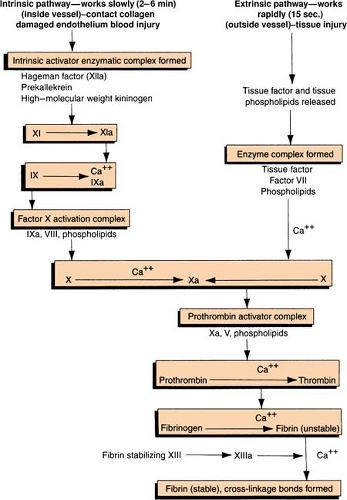
Clot Formation
Clotting Cascade
Clotting Factors
Clotting Factors
| Official Number | Synonym | Contemporary Version |
|---|---|---|
| I | Fibrinogen | I (fibrinogen) |
| II | Prothrombin | II (prothrombin) |
| III | Tissue thromboplastin | III (tissue factor) |
| IV | Calcium | IV (calcium) |
| V | Labile factor | V (labile factor) VI PF3 (platelet coagulant activities) VI PF4 |
| VII | Stable factor | VII (stable factor) |
| VIII | Antihemophilic factor | VIII AHF (antihemophilic factor) VIII VWF (von Willebrand’S factor) VIII RAg (related antigen) |
| IX | Christmas factor | IX (Christmas factor) |
| X | Stuart-Prower factor | X (Stuart-Prower factor) |
| XI | Plasma thromboplastin (antecedent) | XI (plasma thromboplastin antecedent) |
| XII | Hageman factor | XII HF (Hageman factor) XII PK (prekallikrein, Fletcher) XII HMWK (high–molecular-weight kininogen) |
| XIII | Fibrin stabilizing factor | XII Fibrin stabilizing factor |
| The Roman numerals and synonyms designating each clotting factor accepted by the International Committee on blood clotting factors are located in the left-hand columns. Note the absence of factor VI. The version in the right-hand column incorporates more recently recognized clotting factors but is not officially recognized. (Green, D. General considerations of coagulation proteins. Ann Clin Lab Sci 8[2]: 95–105. Copyright 1987 by the Institute for Clinical Science, Inc. Reprinted with permission.) | ||
Cold Agglutinins
Cold agglutinins are reported as a critical value on an antibody screen. Agglutinins are antibodies that cause RBCs to clump together. Since cold agglutinins are active at cold temperatures, any patient with a positive cold agglutinin screen will need to have the blood warmer used when transfusing red blood cells. (See Hotline Fluid Warner; Ranger Fluid Warmer; Level 1 Volume Infuser in Part 2)
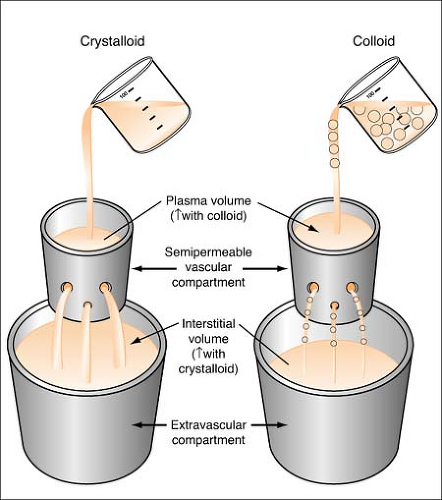
Colloids
Colloids are also known as plasma expanders. These substances contain large insoluble molecules and result in an increase in osmotic pressure. This osmotic pressure will draw fluid from the interstitial compartment into the intravascular space and will cause an increase in the total vascular blood volume. (See Figure on page 280.) Colloids cause an increase in plasma volume, while crystalloids cause an increase in interstitial volume. Colloids made from blood products include albumin and plasmanate, while artificial colloids are dextran and hespan.
Remember:
Even though colloids are called plasma expanders, they do not carry oxygen like red blood cells and are not the same thing. Also, the effect of colloids is limited, lasting only 24 to 48 hours.
Consumptive Coagulopathy
(see also Disseminated Intravascular Coagulation, p. 286)
A group of disorders characterized by the abnormally regulated activation of “procoagulant” pathways, resulting in decreased levels of hemostatic components. The prototype in this category is disseminated intravascular coagulation (DIC).
Types of consumptive coagulopathy include:
Type I DIC plus secondary fibrinolysis—acute DIC
Type II DIC (circulating thrombin)—compensated/chronic DIC
Type III Primary fibrinolysis (circulating plasmin)
Cryoprecipitate
Cryoprecipitate is a component of blood obtained by freezing and thawing plasma. It is used to correct deficiencies of factor VIII, factor XIII, and fibrinogen and occasionally used to control bleeding in uremia, and in DIC.
Volume
Usually given as a pool of 10 to 20 units
Infusion Rate
Use Y infusion set
1 to 2 mL/min (10 mL diluted component per minute)
Special Considerations
Cryoprecipitate contains a small volume of plasma and no RBCs. Plasma compatibility is preferred but not required.
Crystalloids
Crystalloids are aqueous solutions composed of water-soluble molecules (electrolytes and water) that easily diffuse across the vascular membrane into the interstitial spaces (resulting in only 10%–25% of the crystalloid solution remaining in the vascular space). (See figure on page 283.) They are used to maintain or replenish body fluids, or correct electrolyte imbalances. There are three types of this solution:
Isotonic—solute concentration equals the solute concentration of the plasma; most common is LR.
Hypotonic—solute concentration is less than the solute concentration of the plasma; most common is 0.45 NS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree