Cardiovascular System
Ablation
(see also Electrophysiology Studies, p. 106; Wolff-Parkinson-White Syndrome, p. 165)
Based on results of electrophysiology studies, ablation therapy may be used to eliminate an accessory pathway in the electrical system of the heart. When this circuit is eliminated, fast heart rates should not occur. For this procedure, a specially designed catheter is positioned next to the extra pathway. A form of energy (radio frequency waves) is then delivered into the catheter, heating the heart tissue under the catheter tip and causing the normal cells to no longer function, thus resulting in elimination of the pathway. Much of the success of this approach depends on the pathway location. If the path is on the left side of the heart, the success rate is 90% to 95%. If the pathway is in the center or on the right side of the heart, the success rate is 85% to 90%. In approximately 5% to 10% of patients, even though the extra pathway appears to have been successfully eliminated, the pathway function may return later, requiring a repeat procedure.
Action Potential
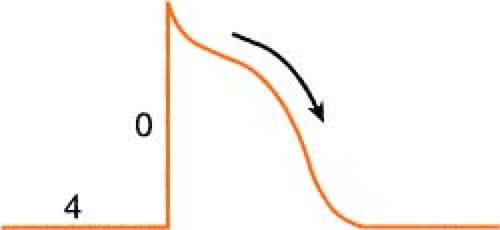
Heart muscle cells and nerve cells share many things in common, one of which is the fact that they are both capable of rapidly reversing their resting membrane potential from negative to slightly positive values. This is known as the “action potential” and is brought on by a change in membrane permeability by certain ions. The cardiac action potential, which is associated with contraction, has five distinct phases (numbered 0–4) as described below.
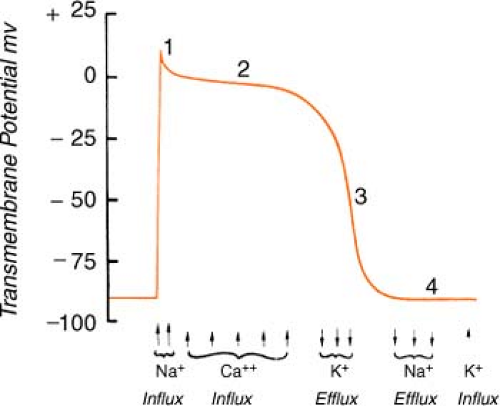 Figure. Action potential of human ventricular myocardium together with probable electrolyte movements. (Schlant, R. C., & Alexander, R. W. [1994]. Hurst’s the heart [8th ed.]. New York: McGraw-Hill.) |
Phase 0: Rapid depolarization (sodium in)
On depolarization, the cell becomes permeable to sodium through “fast” channels, and sodium, previously outside the cell, rushes inside. This causes the initial rapid upstroke of
the curve and a reversal of potential (resting cell was negative on the inside and becomes positive on the inside when depolarized).
the curve and a reversal of potential (resting cell was negative on the inside and becomes positive on the inside when depolarized).
Phase 1: Initial repolarization (sodium stops, chloride in)
The brief, rapid start of repolarization is believed to be due to the inactivation of the inbound sodium as well as a secondary influx of chloride.
Phase 2: Repolarization plateau (calcium in, potassium out)
The repolarization slows as a result of a complex interaction between a slow influx of calcium entering the cell and a slow exiting of potassium. Phases 1 and 2 are periods of absolute refractoriness.
Phase 3: Repolarization Continues (calcium stops, potassium out)
The influx of calcium ceases, whereas the outward flow of potassium continues. Phase 3 is a period of relative refractoriness.
Phase 4: Resting (sodium/potassium pump)
Most cardiac cells are -70 to -90 mV at rest. The net negative electrical charge is restored by a sodium/potassium exchange across the membrane. The slow diastolic depolarization is caused by a time-dependent fall in outbound potassium. This, combined with an increase in the sodium influx, causes the threshold to be reached.
Acute Coronary Syndrome
Acute coronary syndrome is an umbrella term used to cover a group of three clinical entities:
Unstable angina (USA)
Acute non-ST segment elevation myocardial infarction (N-STEMI)
Acute ST segment elevation myocardial infarction (STEMI)
The initial diagnosis of ACS is based on the patient’s clinical history (including risk factors), on clinical presentation, and changes on serial ECG tracings. The following table summarizes the similarities and differences of each diagnosis.
Acute Coronary Syndrome
| Cause | Signs Symptoms | Diagnostic Findings | LV Function | Complications | |
|---|---|---|---|---|---|
| USA | Thrombus partially or intermittently occludes CA |
|
|
|
|
| N-STEMI | Thrombus partially or intermittently occludes CA |
|
|
|
|
| STEMI |
|
|
|
|
|
| Treatment | |||||
| USA | O2 NTG/MSO4 to control pain Beta-blockers, ACE inhibitors, statins, glycoprotein llb/llla inhibitors, antiplatelet therapy (ASA, plavix) unless contraindicated, heparin | ||||
| N-STEMI | O2 NTG/MSO4 to control pain Beta-blockers, ACE inhibitors, statins, glycoprotein llb/llla inhibitors, antiplatelet therapy (ASA, plavix) unless contraindicated, heparin, cardiac cath, possible PCTA | ||||
| STEMI | O2 NTG/MSO4 to control pain Beta-blockers, ACE inhibitors, statins, glycoprotein llb/llla inhibitors, antiplatelet therapy (ASA, plavix) unless contraindicated, heparin PCTA within 90 minutes of medical evaluation Fibrinolytic therapy within 30 minutes of medical examination | ||||
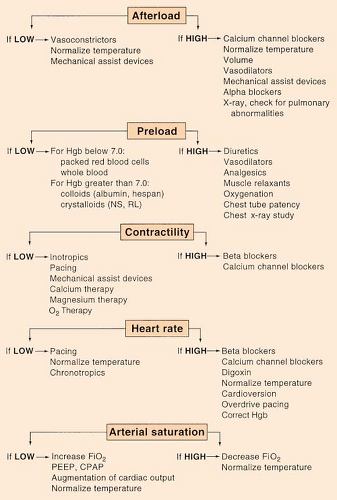
Afterload
(see Swan-Ganz: Hemodynamic Algorithm, p. 154)
Aicd
(see Automatic Implantable Cardiac Defibrillators, p. 79)
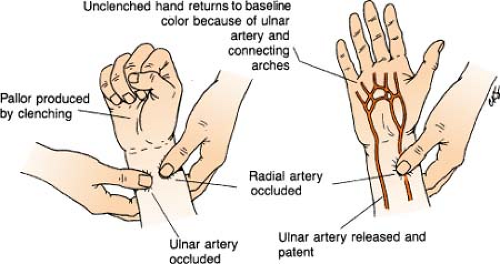
Allen’s Test
Allen’s test is a quick bedside procedure performed specifically to assess ulnar arterial circulation to the hand and evaluate the patency of the ulnar artery prior to radial arterial puncture. Elevate patient’s hand with fist clenched while both radial and ulnar arteries are compressed to occlude arterial blood flow. Pressure is then released over only the ulnar artery. Color should return to the hand within 6 seconds, indicating a patent ulnar artery and adequate collateral blood flow to the hand.
Aneurysm: Aortic
An aortic aneurysm is an abnormal dilation of a weakened aorta arterial wall that expands the diameter of the vessel >1½ times normal. (Normal aorta diameter range = 1.4–3 cm.)
Development: At first, pressure increases from the aneurysm cause the aorta lumen to widen and blood flow to slow. An aneurysm typically expands on an average of 0.3 to 0.4 cm per year, with larger aneurysms expanding faster. As the growth progresses, hemodynamic compromise ensues, creating pulsatile stresses on the weakened wall and resulting in the aorta becoming bowed and torturous. Without surgical intervention, the aneurysm may ultimately rupture or tear suddenly and cause possible death.
Sites: The abdominal aorta is the most common site for aortic aneurysms, with 80% developing below the bifurcation of the renal arteries.
Signs/Symptoms: Most aneurysms do not cause any symptoms and are usually discovered incidentally. Unfortunately, asymptomatic aneurysms have a higher risk for rupture. When symptoms do occur, they typically include flank pain (because of the pressure placed on other organs by the expanding size of the aneurysm) or back pain (between the shoulder blades and chest), bradycardia, different pressures in the right and left arms, bruit, jugular venous distention, and unequal carotid and radial pulses. An aneurysm must be at least 5 cm in diameter to be detected on a routine physical exam, and is definitively diagnosed by ultrasound, MRI, or CT.
Treatment: Elective surgical repair is considered for aneurysms that exceed 5.5 cm in diameter, or when small aneurysms increase 0.5 cm within a 6-month period.
Postoperative care focuses on strict blood pressure control in the first 24 to 48 hours to prevent bleeding, tearing of suture lines, and formation of a pseudoaneurysm.
Dissection: This occurs when there is a progressive tear in the aorta, allowing blood to collect and form a hematoma between the intimal and medial layers of the aortic wall.
Dissections are classified according to location and severity and defined by two systems; see table below.
Dissections are classified according to location and severity and defined by two systems; see table below.
Aortic Aneurysm Dissection Classifications
| System | Type | Involves |
|---|---|---|
| Daily System | A B | Ascending aorta Descending aorta |
| DeBakey System | I II III (a) III (b) | Ascending aorta, extending beyond aortic arch Ascending aorta, extending from the aortic valve to the innominate artery Descending aorta, extending from the aortic arch to the level of the diaphragm Descending aorta, extending from the aortic arch to below the level of the diaphragm |
Treatment of dissection is based on the extent and location of the dissecting segment. Daily Type A and DeBakey Type I or II are treated as surgical emergencies, and the dissecting segment is replaced with a synthetic graft or endovascular placement of a stent. Daily Type B and DeBakey Type III are generally treated medically unless hemorrhage is suspected, although grafting is an option.
Angiography, Coronary
(see also Closure Devices, p. 86; Stents, p. 149)
Coronary angiography, also called an angiogram, a heart catheterization, or a ventriculogram, is the main diagnostic test used to pinpoint where obstructions are in the coronary arteries and to determine their severity. Conscious sedation is administered, and access is accomplished via the femoral artery. A catheter is then threaded through the entry site, up the artery, through the aorta, and into the openings of the left and right coronary arteries. Contrast dye is injected, and under x-ray, the coronary arteries are viewed in motion, making it possible to visualize plaques. After visualizing the coronary arteries (sometimes before then), another catheter is threaded into the heart’s left ventricle, which allows visualization of the wall motion, wall thickness, chamber size, and determination of ejection fraction. (This is known as a ventriculogram.) Throughout the procedure, heart pressures are monitored to determine if any pressure gradients exist. Because there are no nerve endings in the arteries, the patient is unaware that the test is being done (except for a warm sensation following injection of the contrast dye). (See Schematics on next page.)
Angioplasty
(see also Atherectomy, p. 78; Closure Devices, p. 86; Stents, p. 149)
Coronary angioplasty is accomplished using a balloon-tipped catheter inserted through the femoral or brachial artery (and threaded to the coronary artery) to widen a vessel that is narrowed due to stenosis or occlusion. Also known as percutaneous transluminal coronary angioplasty (PTCA), if successful, can alleviate myocardial ischemia, relieve angina, and prevent myocardial necrosis. The indications for PTCA have expanded with the advent of improved equipment and techniques; however, the procedure is generally reserved for patients with at least a 50% narrowing of vessels. There are a number of different balloons that can be used; a discussion of the most common follows.
Saline Balloon
Typically, a coaxial catheter system with an outer diameter of 6 to 10 F is introduced via the cephalic or femoral vein into the coronary artery tree. The tips of the guiding catheters have curves that are preshaped for selective access to either the right or the left coronary artery and are carefully advanced into the area of coronary stenosis. A balloon attached to the catheter is then inflated with saline solution, increasing the luminal diameter and improving blood flow through the dilated segment. Exact placement of the dilation balloon within the stenosis is facilitated by fluoroscopy. The most common complication of the procedure is peripheral arterial injury at the site of the catheter insertion, leading to thromboembolism, hematoma, arterial perforation, pseudoaneurysm, or arteriovenous fistula. Other complications include stroke, myocardial infarction (MI), and arrhythmias. However, even though this is an invasive procedure, morbidity is minimal, and clinical success ranges from 80% to 95%. Unfortunately, long-term restenosis of the dilated artery occurs in 30% of patients within 3 months.
Cutting Balloon
Another option when performing coronary angioplasty is the use of a cutting balloon, where three or four microsurgical blades are mounted longitudinally on the balloon’s outer surface. The balloon is positioned across the lesion and is inflated. Tiny cuts are made within the surface of the plaque, and the balloon is deflated, rotated, and then inflated again. The process is repeated several times. It is believed that the cuts in the plaque allow the balloon to enlarge the narrowed lumen using less pressure (and thus lessening the chance of restenosis). This technique is most often used for “old” plaque, which may be resistant to traditional angioplasty, or for restenosis.
Cryoplasty
More recently, a technique called cryoplasty has evolved. In effect, it is angioplasty on ice. The procedure is performed much the same way as the traditional angioplasty, but when the balloon is inflated, nitrous oxide is used instead of saline. The nitrous cools to a temperature of -10°C, freezing the plaque and inducing a process called apoptosis (programmed cell death). This newer technique appears to be less traumatic to blood vessels and boasts a lower rate of restenosis.
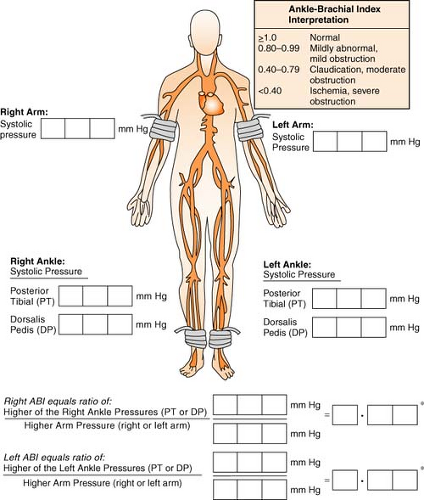
Ankle-Brachial Index
The ankle-brachial index is a noninvasive examination that provides baseline data regarding circulation in the lower limbs. A blood pressure cuff is put around the ankle above the malleolus. When the dorsalis pedis pulse has been located via Doppler, the cuff is inflated
in the usual manner until the Doppler signal is no longer heard. As the cuff is slowly deflated, the systolic pressure is recorded when the signal returns. The same procedure is done by using a Doppler on the posterior tibial pulse. The higher pressure of the two pressures is used for the calculation of the index. The brachial systolic pressure is then measured in the same manner and is divided into the ankle systolic pressure. The lower of the two numbers (right vs. left) results in the overall ABI.
in the usual manner until the Doppler signal is no longer heard. As the cuff is slowly deflated, the systolic pressure is recorded when the signal returns. The same procedure is done by using a Doppler on the posterior tibial pulse. The higher pressure of the two pressures is used for the calculation of the index. The brachial systolic pressure is then measured in the same manner and is divided into the ankle systolic pressure. The lower of the two numbers (right vs. left) results in the overall ABI.
Annuloplasty Ring
An annulus is a ring of tissue around the opening of a valve. When the annulus is dilated, it allows blood to leak back through the valve after it has closed. Most commonly used on the mitral valve, the implantation of an annuloplasty ring (either rigid or flexible) will bring the annulus back to a more normal position, facilitate the meeting of the valve leaflets, and aid in eliminating the regurgitant blood flow.
Aortic Aneurysm
(see Aneurysm: Aortic, p. 70)
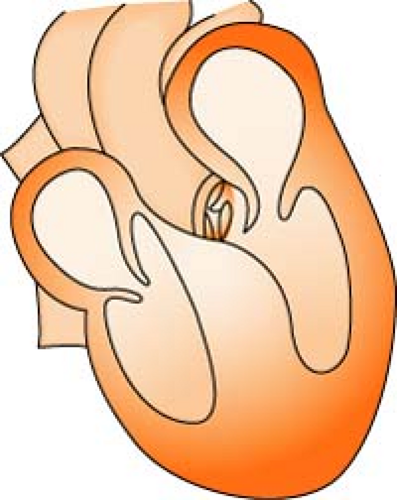
Aortic Regurgitation/Insufficiency
Aortic regurgitation/insufficiency is a condition in which there is a weakening or ballooning of the aortic heart valve, causing the valve to fail to close tightly, leading to backflow of blood into the ventricle. This reduces the amount of blood available to perfuse the coronary artery and leads to myocardial ischemia. Over time, it also causes dilatation and hypertrophy of left ventricle.
Causes:
Most commonly caused by rheumatic fever (fibrous infiltrates on the valve cusps, which causes malalignment)
Endocarditis (tissue destruction of leaflets, leading to cusp perforation or prolapse)
Myxomatous changes (enlarged, thickened, floppy, gelatinous leaflets and elongated chordae tendineae)
Aortic root disease (aortic annulus becomes so large that the valve cusps no longer approximate)
Congenital abnormalities
Assessment:
Murmur (louder and longer as severity increases)
Widening pulse pressure (may be greater than 60 mm Hg)
Decreased diastolic pressures
PMI may be displaced laterally
Quincke’s sign (pulsatile flushing/blanching of nail bed when pressure applied)
DeMusset’s sign (bobbing of head with each pulse)
Corrigan’s or Waterhammer pulse (sharp systolic upstroke and diastolic collapse of pulse)
Diagnosis:
2-D echo (shows changes in the valve, left ventricular end-diastolic volume, ejection fraction, and mass)
Transesophageal echo (TEE, shows images in both ascending and descending aorta)
EKG (left axis deviation, patterns of left ventricular strain, i.e., Q waves in I, AVL, V3–V6)
CXR (shows left ventricular enlargement, signs of CHF)
Cardiac catheterization (visualizes extent of disease)
Management:
ACE inhibitors (reduces afterload)
Vasodilators (reduces afterload)
Nifedipine (reduces left ventricular size and mass)
Antibiotic prophylaxis
Surgery (aortic valve replacement)
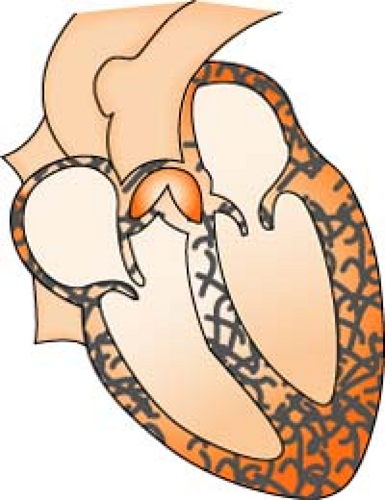
Aortic Stenosis
Aortic stenosis is a narrowing or constriction of the opening of the aortic valve, which results in a decrease in the outflow of blood from the left ventricle to the aorta. This causes a pressure overload in the left ventricle, an increase in left ventricular systolic pressure, and hypertrophy. As the left ventricular pressure continues to increase, the pulmonary system becomes congested, and the right side of the heart begins to fail. Stenosis reduces stroke volume, and without a compensatory increase in heart rate, cardiac output falls and perfusion of the coronary arteries is reduced.
Causes:
Age-related degenerative calcific changes
Rheumatic fever (scars the valve, causing fibrosis, calcification, and fusion of the leaflets)
Congenital abnormalities
Assessment:
Midsystolic murmur (rough, low pitched, loudest at second right intercostal space, radiating up neck)
Low systolic blood pressure with a normal diastolic pressure; pulse pressure may be narrowed
Indicators of left ventricular failure (increased wedge pressure)
Diagnosis:
2-D echo (defines extent of leaflet thickening, determines left ventricular function and hypertrophy, measures aortic valve pressure gradient, determines left atrial enlargement)
EKG (QRS amplitude changes associated with hypertrophy, ST/T wave changes due to left ventricular strain, possible LBB, left axis deviation, atrial fibrillation in late stages)
CXR (shows left ventricular enlargement, rounding at apex and slight backward displacement of the heart)
Cardiac catheterization (visualizes extent of disease)
Calcification of aortic valve area:
Normal
3.0 to 3.5 cm2
Mild AS
1.0 to 1.5 cm2
Moderate
0.85 to 1.0 cm2
Severe AS
Less than 0.85 cm2
Management:
No preventative measures; surgical replacement only effective for long-term treatment
Mild stenosis, echocardiography every 2 years
REMEMBER: Use meds cautiously! Medications can lower preload, and the left ventricle is very dependent on preload for adequate functioning. If preload is too low, cardiac output will be too low, and there will not be enough pressure generated to open the noncompliant valve.
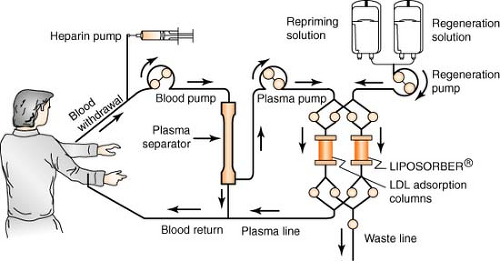
Apheresis
Apheresis is a process using the Liposorber system, indicated for use in removing LDL cholesterol from the plasma of the following high-risk patient populations for whom diet has been ineffective and maximum drug therapy has either been ineffective or not tolerated:
Patients with LDL-C >300 mg/dL
Patients with LDL-C >200 mg/dL and documented coronary heart disease
The patient’s blood is withdrawn via a venous access and is pumped to a plasma separator, where it is separated and pumped into one of the two LDL absorption columns. As the plasma passes through the column, LDL, Lp(a), and VLDL are selectively absorbed by dextran sulfate cellulose beads within the column. There is minimal effect on other plasma components such as HDL and albumin. The LDL-depleted plasma leaves the column and is recombined with the blood cells exiting the separator, all of which are returned to the patient via a second venous access. When the first column has completed absorbing the LDL, the computer-regulated machine automatically switches the plasma flow to the second column. The plasma remaining in the first column is returned to the patient. The column is then regenerated, eluting the LDL, Lp(a), and VLDL to the waste lines. After elution, the column is reprimed completely and is ready for the next cycle of absorption, allowing continuous treatment. A typical treatment takes about 2 to 4 hours to complete and may be repeated every 2 to 3 weeks.
Arterial Pressure
(see Intra-arterial Pressure, p. 123)
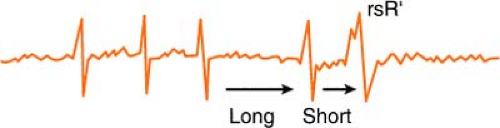
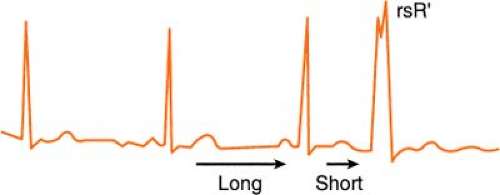
Ashman’s Phenomenon
Ashman’s theory states that aberrant conduction is more likely to complicate atrial fibrillation when a longer cycle is succeeded by a shorter one.
The phenomenon may also occur when sinus bradycardia is complicated by a premature atrial contraction (PAC). The cycle of sinus bradycardia is long, but PAC
shortens cycle and is conducted aberrantly (usually in right bundle branch [RBB] pattern, rsR′):
shortens cycle and is conducted aberrantly (usually in right bundle branch [RBB] pattern, rsR′):
Therefore, according to Ashman, the beat that follows a long/short cycle favors aberrancy. This, however, is not a hard-and-fast rule, because by the rule of bigeminy, a longer cycle also tends to produce a ventricular ectopic beat equally as many times.
Atherectomy
(see also Angioplasty, p. 73; Stents, p. 149)
Atherectomy is indicated in conditions not well treated by standard angioplasty, such as when the plaque in the coronary arteries is too large or the plaque is hardened to a degree that it is no longer possible to flatten it. Atherectomy creates a smoother surface by debulking the vessel and is sometimes followed by angioplasty or stent placement. In theory, the process permits a more controlled vascular injury and minimizes the degree of arterial stretch.
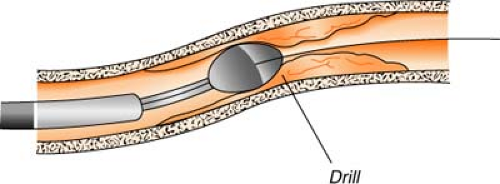
Rotational atherectomy (Rotablator) uses a catheter with a high-speed drill (up to 200,000 rpm) to grind away plaque. The abrasive tip of the drill is elliptical in shape, coated with diamond chips, and comes in a variety of sizes. Rotational speed is controlled by air pressure, which is controlled by a foot pedal. Atherosclerotic material is shredded into millions of tiny particles (much smaller than red blood cells) and is then washed away in the bloodstream by passing through the capillaries. This process is most useful for treating plaque that has hardened with calcium.
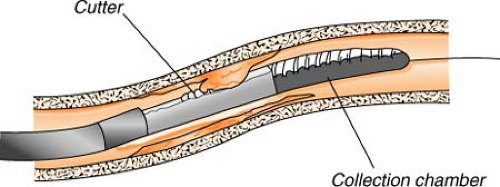
Directional atherectomy uses a catheter with a small cutting device to actually “shave off” tiny pieces of plaque. The tiny plaques are then stored in a collection chamber attached to the catheter and, at the completion of the procedure, are removed from the body when the catheter is withdrawn. This process is most useful for treating atherosclerotic deposits that are bulky, not hardened with calcium, and limited to one side of the arterial wall.
Potential complications in both procedures include perforation of the coronary artery, abrupt closure, embolization distal to the lesion site, and myocardial infarction. The rate of restenosis and other complications is comparable to that of angioplasty.
Automatic Implantable Cardiac Defibrillators
An automatic implantable cardiac defibrillator is a device implanted transvenously with local anesthetic, using a simple surgical procedure (the patient is usually able to be discharged with 24 hours) to treat ventricular arrhythmias with pacing and shock therapy. Current versions also have the capability to treat and suppress atrial arrhythmias, resynchronize the ventricles to manage heart failure symptoms, or pace the heart with all the features of a dual-chamber pacemaker. Originally indicated for patients who have survived two cardiac arrests, the devices have recently been shown to improve survival in all patients with prior myocardial infarction and an ejection fraction of 30% or less (identifying them as high risk for sudden death).
Axis Deviation
The axis is the mean direction (vector) in which electrical activity spreads across the heart. The strength of the vector is indicated by recording a small deflection for a weak vector and a large deflection for a strong vector.
Normal axis is described by some authors to be in the narrow bounds of +30° to +60° and by others to be between 0° and +90°. Using the range from -30° to +110° allows easier definition of hemiblock.
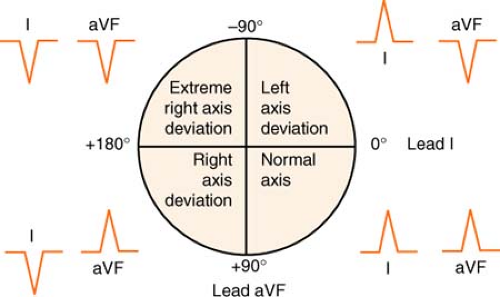 Figure. Determining electrical axis. (Adapted from Morton, P. G., & Fontaine, D. K. [2009]. Critical care nursing [9th ed.]. Philadelphia: Lippincott Williams & Wilkins.) |
Quick Method for Determining Axis
Examine deflections in leads I and aVF.
If they point toward each other on the 12-lead electrocardiogram (EKG) (lead I negative, aVF positive), the deviation is to the right.
If they point away from each other on the 12-lead EKG (lead I positive, aVF negative), the deviation is to the left.
Remember:
The axis is “right together” or “left apart.”
If leads I and aVF both deviate upward, the axis is normal.
Remember:
It’s good to be up!
If leads I and aVF both deviate downward, the axis is in “no man’s land,” or indeterminate (usually because of a junctional rhythm).
More Exact, Hexaxial Method for Determining Axis
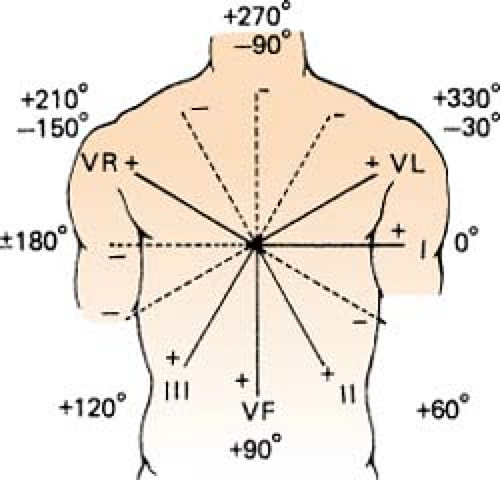 Figure. Hexaxial figure to pinpoint axis. (Goldman, M. J. [1996]. Principles of clinical electrocardiography [12th ed.]. Los Altos, CA: Lange Medical Publications.) |
Find the most equiphasic lead (lead that equals zero when the values of the positive and negative deflections are added) using leads I, II, III, aVR, aVL, and aVF (no V leads!).
Use the hexaxial figure to determine which lead is perpendicular to the equiphasic one.
Check to see whether the QRS of this lead is a negative or positive deflection. This gives the direction of the vector.
If there is no equiphasic deflection present in the six limb leads:
Look at leads I and aVF to place the axis in one of the quadrants.
Determine which of the other two leads in that quadrant has the largest complex, and place the axis closest to that vector.
Hints:
V tach commonly has an axis in no man’s land, giving a clue to the differential diagnosis between ventricular ectopy and aberration.
Axis shifts to the left with age. An axis of -30° is not necessarily a cardiac disorder.
ALL complexes in the V lead deflecting either up or down are definitive of V-tach.
Axis deviates toward bundle branch block (RBBB = R axis deviation).
Axis deviates away from MI.
Left axis deviation caused by:
Advanced age, obesity, pregnancy
Left ventricular hypertrophy
Inferior MI
Wolff-Parkinson-White (WPW) syndrome
Ascites, tumors
Hypertension
Ischemic heart disease
Right axis deviation caused by:
Youth, tall, and thin body form
Right ventricular hypertrophy
Chronic obstructive pulmonary disease (COPD), pulmonary emboli, lateral myocardial infarct
Dextrocardia
Right bundle branch block
Bnp
(see Heart Failure, p. 110; see also BNP in Part 9, p. 341)
Body Mass Index
(see Bariatrics in Part 4, p. 217)
Brachytherapy, Vascular
Many technologies are currently under investigation for the treatment and prevention of restenosis following angioplasty and stenting, but the most promising appears to be brachytherapy. This technique, approved by the FDA in 2002, delivers either beta or gamma radiation to the blocked site (via a catheter, postangioplasty) utilizing a miniaturized x-ray emitter. Once delivered, the radiation “beads” remain in place from 3 to 20 minutes before being withdrawn. No radioactive material is left in the body. The radiation is not felt, and the normal healing process is unaffected. The procedure works on the principle that the radiation interacts with vessel walls, destroying their capability for cell division and regrowth.
Brugada Syndrome
Brugada syndrome is a condition associated with sudden cardiac death, idiopathic ventricular fibrillation, or self-terminating polymorphic ventricular tachycardia in an otherwise structurally normal heart. Characterized by a right bundle branch block pattern and ST segment elevations in leads V1 to V3, the syndrome is seen more frequently in males than in females and primarily in a mean age group of 35 to 40 years. It is consistent with a chromosomal mutation and predisposes individuals to a lifetime risk of sudden cardiac death. There is no effective pharmacologic treatment, and genetic counseling is advised.
Cabg
(see Cardiac Surgery, p. 82)
Cardiac Index
(see also Swan-Ganz: Hemodynamic Normals, p. 157)
Cardiac index = cardiac output ÷ body surface area Normal: 2.5 to 4.5 L/min/m2
Cardiac Markers
(see Cardiac Markers in Part 9, p. 342)
Cardiac Output
(see also Swan-Ganz: Hemodynamic Normals, p. 156)
Cardiac output = stroke volume × heart rate Normal: 4 to 8 L/min
Cardiac Surgery
Cabg (Coronary Artery Bypass Graft)
Developed in 1954, CABG is a procedure used to reroute or bypass blood around blocked coronary arteries and to improve blood supply to the myocardium. Cardioplegic solution is used to cause intentional cardiac arrest and to provide a bloodless operating field and a motionless heart. The solution helps to protect the heart from ischemia by providing a substrate for ongoing cellular metabolism during the time on the bypass pump. The body is cooled to reduce metabolic demand.
Opcab (Off-Pump Coronary Artery Bypass)
The heart continues to beat through OpCAB. No cannulation is needed, and the body is not cooled. A specialized stabilizer is used, resulting in suction to minimize heart movement only at the site of anastomosis. Shunts are placed in the coronary vessel to be anastomosed and to continue blood flow through the vessel until the anastomosis is complete. The shunt is removed just prior to securing the suture. A mister is utilized to keep the site free of blood for better visualization for the surgeon.
Midcab (Minimally Invasive Direct Coronary Artery Bypass)
MIDCAB is similar to OpCAB, except this procedure is done with a short incision in the left chest cavity, rather than a sternotomy. The left anterior descending artery and the internal mammary artery are sutured together in the front of the heart.
Comparing Types of CABG
| Features | On-pump CABG | OPCAB | MIDCAB |
|---|---|---|---|
| Access site | Breastbone severed for heart access | Breastbone severed for heart access | Incision made between ribs for anterior heart access, no bones cut |
| Indications | Suitable for multivessel disease, any coronary artery | Suitable for multivessel disease, any coronary artery | Used only for one-vessel diseases in anterior portions of heart, such as left anterior descending artery, or some portions of the right coronary and circumflex arteries |
| Graft types | Combination of artery and vein grafts | Combination of artery and vein grafts | Arterial grafts (better long-term results) |
| Complications | Highest risk of postoperative complications | Reduced blood usage, fewer rhythm problems, less kidney dysfunction than on-pump CABG | Reduces blood usage, fewest complications, fastest recovery |
| Intubation | Up to 24 hours | Up to 24 hours | Usually 2 to 4 hours |
| Incisions | Leg incisions for vein grafting, possibly arm incisions for radial artery grafting | Leg incisions for vein grafting, possibly arm incisions for radial artery grafting | No leg incisions, possibly arm incision for radial artery grafting |
| Heart and lung function | Heart and lung circulation bypassed mechanically, affecting blood cells | Drugs and special equipment used to slow heart and immobilize it; cardiopulmonary and systemic circulation still function | Drugs used to slow heart; cardiopulmonary and systemic circulation still function |
| From Visual Nursing: A Guide to Diseases, Skills, and Treatment. Lippincott, Williams & Wilkins, 2008. | |||
Cardiac Tamponade
(see Tamponade, p. 159)
Cardiogenic Shock
(see Shock, Cardiogenic, p. 146)
Cardiomyopathy
Cardiomyopathy is an irreversible primary disease of the heart muscle, most commonly affecting the myocardial layer but occasionally affecting the endocardial, subendocardial, and/or pericardial layers of the heart. Cardiomyopathies are divided into the following three types:
Dilated cardiomyopathy REMEMBER: Heart thins and enlarges. Most cases of dilated cardiomyopathy are idiopathic (no exact cause is known), although viral infections, myocarditis, heredity, and use of toxic substances have been linked to the disease. It is characterized by damaged heart muscle, which causes an increase in size of the right and left ventricles. (A damaged heart muscle cannot pump enough blood to meet the demands of the body, so the heart compensates by enlarging the size of the ventricles.) The larger chambers temporarily move more blood, but eventually, the stretched out walls become weak. Inadequate cardiac output results, and ultimately, mitral and tricuspid insufficiency occurs.
If the cause can be determined, management is focused on treating the cause. Otherwise, treatment is directed toward maximizing heart function (digoxin, dobutamine, vasodilators, heparin, diuretics, etc.). Fifty percent of all deaths occur suddenly and are usually caused by ventricular arrhythmias and, secondarily, clots.
Hypertrophic cardiomyopathy REMEMBER: Heart muscle thickens. Also known as idiopathic hypertrophic subaortic stenosis (IHSS), hypertrophic cardiomyopathy occurs without any systemic precipitating factors. The cause is thought to be genetic, with as high as 60% to 80% of the cases being inherited through autosomal dominant transmission. It is characterized by thickened walls of the left ventricle and septum. The thicker muscle walls cause the chambers to hold less blood, and the heart muscle remains stiff in diastole. The primary goals of treatment center on decreasing the risk of sudden cardiac death and treating symptoms of angina, fatigue, dyspnea, and syncope.
Restrictive cardiomyopathy REMEMBER: Heart muscle becomes hard and stiff. This type of cardiomyopathy is rare in the United States and is seen mostly in Africa. It is frequently idiopathic, sometimes genetically linked, and is often seen in amyloidosis, sarcoidosis, endomyocardial fibrosis, and other infiltrative diseases. Clinically, it presents like congestive heart failure, but the heart is small or only slightly enlarged. Systemic and pulmonary venous pressures are high, with peripheral edema and possible ascites. Atrial fibrillation is common. There is no known treatment, so therapy is supportive and includes diuretics, steroids, anticoagulants, inotropic agents, oxygen, and fluid restriction.
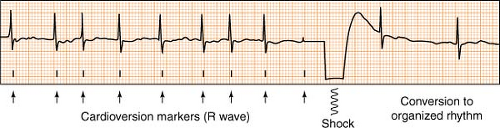
Cardioversion, Synchronized
(see also Defibrillation, p. 92)
Electrical cardioversion (also known as direct current, or DC cardioversion) is the delivery of a synchronized electrical shock through the chest wall to the heart via conducting pads. Its purpose is to suddenly and simultaneously interrupt the abnormal electrical circuit(s) in the heart, and restore a normal electrical rhythm. Most cardioversions (synchronized shocks) are performed to treat rhythms such as atrial fibrillation, atrial flutter, ventricular tachycardia with a pulse, or any other perfusing rhythm that is undesirable due to complications or symptoms.
Cardioversion differs from defibrillation in that the shock is synchronized, meaning it is timed to deliver current during the safest point of the cardiac cycle (the R wave phase). A defibrillator placed in the synchronized mode will actually delay delivery of the shock, waiting instead until it senses an R wave before firing. This avoids the delivery of energy during the electrically vulnerable downslope of the T wave, and prevents possible R on T phenomenon, where a perfusing rhythm can deteriorate to a chaotic, pulseless one.
Remember:
Most defibrillators have a safety feature designed to automatically reset the defibrillator back to the NON-synchronized (defibrillation) mode after each shock. If repeated synchronized shocks are required, the user must remember to key in the synchronous setting each time.
Central Venous Pressure
(see Swan Ganz: Hemodynamic Normals, p. 155)
Closure Devices
Closure devices assist in achieving hemostasis postfemoral artery puncture and have proven to be an efficient alternative/adjunct to manual compression. They can be divided into four categories:
Passive devices REMEMBER: “push”
Delivers compression via a device, or requires manual compression in addition to a surface pad.
Example: (devices): FemoStop, C Clamp, Safe Guard, Hemoband (surface pads): Syvek, Chito-Seal, Clo-Sur, D-Stat Dry,
Safe Seal, V + Pad, Neptune Pad
Sealing devices REMEMBER: “pull”
A mechanical seal is activated by sandwiching the arteriotomy between a suture anchor and a collagen sponge. Example: AngioSeal, VasoSeal, Duett Pro, Boomerang
Suturing devices REMEMBER: “stitch”
Delivers an intravascular suture. Example: Perclose, SuperStitch, X-Press, VasoLock
Stapling devices REMEMBER: “click”
Delivers a metal clip on surface of the procedure site. Example: Star Close, AngioLink
The number of devices available on the market is numerous and continues to expand. A short discussion of only the more “classic” models follows. In general, the principles for all devices in the same category are similar, and some universal rules apply:
Remember:
If a patient has an arterial and a venous sheath, both lines should NOT be pulled at once. Hemostasis of one line should be achieved before pulling the other, to aid in the prevention of AV fistula formation.
While parameters for safe removal are institution specific, in general, the ACT should be <170 seconds, the INR >1.5, and the platelets >50,000.
Angio-Seal
The Angio-Seal device is composed of a small collagen sponge and an absorbable anchor, connected by an absorbable suture. It stops bleeding from the puncture site by “sandwiching” the puncture between the anchor (inside the artery) and the collagen sponge (outside the artery). Bleeding is also stopped by the clot-inducing properties of the collagen, which is effective even if the patient is anticoagulated. The Angio-Seal device is completely absorbed in 60 to 90 days.
Discharge instructions:
Patient may not take a tub bath, swim, or soak in a hot tub until puncture site healed.
Patient may shower after 24 hours.
Wash site with soap and water and cover with a bandage for at least 3 days.
There may be a small olive pit-size knot over the puncture site for 5 to 7 days.
Angio-Seal is MRI compatible.
Patient needs to keep a wallet card for 90 days, then it can be discarded. (Repuncture of the same artery within 90 days is not recommended.)
D-Stat Dry
D-Stat Dry is a dry hemostatic bandage, packaged as a pad to be applied to surface bleeding from vascular sites. Utilizing the power of thrombin, D-Stat works on the clotting cascade by activating clotting factors V, VIII, and XIII while stimulating platelet aggregation and cleaving endogenous fibrinogen to fibrin. Apply shiny side down.
Duett Pro
The Duett Pro catheter is designed to be inserted at the completion of the procedure through the sheath already in place. A small balloon at the end of the catheter is inflated and pulled back against the artery. Then, the liquid procoagulant (a gluelike substance) is delivered on top of the puncture site to permanently seal it. The Duett Pro catheter and sheath are removed, and after a few minutes of light pressure, the site is sealed.
Discharge instructions:
In most cases, sitting up in bed and moving the leg is allowed 1 hour after the procedure.
Ambulation is dependent on type of catheterization procedure and the amount of anticoagulant given.
Patient may not take a tub bath, swim, or soak in a hot tub until the puncture site is healed.
Patient may shower normally.
Use routine puncture site care; keep clean and dry.
Activity may be used as tolerated, avoid heavy lifting or straining (more than 10 lb) for several days.
Femostop
FemoStop is a femoral compression system, consisting of an arch with a sterile pneumatic pressure dome, a belt, and a reusable pump with a manometer. The pressure dome is placed over the vessel puncture site in the groin. The belt is placed around the patient. The dome applies a mechanical pressure over the vessel puncture site to induce hemostasis. The pressure of the dome is controlled by the reusable pump and the manometer. The arch and the belt provide counterpressure for the dome.
Hemoband (Radial Artery Stasis)
HemoBand is an adjustable, single-use band with a molded V-shaped pressure pad to allow graft flow while controlling bleeding after sheath withdrawal (used also for removal of dialysis catheters). The band is placed into position. One person draws blood from the sheath during removal (to remove clot debris), and as the sheath exits the site, the artery is permitted to spurt slightly. Digital pressure is applied above and below the site. A sterile 4 × 4 is placed over the site so that pressure is applied over the skin entry site and the actual arteriotomy site. If the radial artery is not compressed this way, retrograde flow from the ulnar artery will occur. The band is tightened until bleeding is stopped. The ulnar pulse has to be intact so that the distal wrist and the hand continue to be perfused. The band is released two “clicks” every 15 to 20 minutes over the next 2 hours. Pulses are often checked throughout the procedure. The patient is positioned in bed with the arm draped across a pillow on the upper abdominal area.
Perclose
Perclose is a suture-mediated closure (SMC) device that involves a suture and knotting technique to directly close the arterial puncture. The sutures are cut below the level of the skin. Primary healing is not dependent upon clot formation, and this device can be used with anticoagulated patients.
Discharge instructions:
In most cases, sitting up in bed is allowed immediately postprocedure, and depending on the type of catheterization, the patient may be allowed bathroom privileges.
Patient may not take a tub bath, swim, or soak in a hot tub until the puncture site is healed.
There may be formation of a small lump (1.5–2.5 cm), which may last up to 6 weeks.
Use routine puncture site care; keep clean and dry.
If steri strips are in place, allow them to remain until they fall off on their own.
Patient needs to permanently retain wallet card.
Starclose
The StarClose system utilizes a tiny circumferential flexible clip, which, when placed on the surface of the femoral artery, causes the artery to be closed securely in a matter of seconds following a catheterization. The clip is designed for delivery “through the sheath”—a feature intended to avoid contact with the skin and thus decreasing the rate of infection. The device requires only four clicks to achieve a secure closure, leaving nothing inside the artery itself but achieving stasis entirely from outside the vessel. The clip is made of nitinol, a nickel-titanium composite that “remembers” its shape once released from the StarClose device. While oozing from the site is usually minimal, if oozing increases, D-Stat should never be used to obtain stasis.
Discharge instructions:
The patient may sit up at a 45° angle and move freely in bed immediately postprocedure as long as any subcutaneous ooze is minimal. Ambulation is allowed as directed by the physician.
Use routine site care; keep clean and dry.
Patient may shower the day after the procedure, but no tub bath or swimming for at least 5 days or until the site is healed.
Normal activity may be resumed in 2 days, but discomfort should be the guide.
Syvek Patch
The SyvekPatch is made from a polymer that is isolated from microalga. Its mechanism of action is local vasoconstriction and clot formation to achieve a hemostatic effect. In addition to an adjunct to manual pressure sheath removal, it can also be used to achieve hemostasis on groin “oozes.” The patch is external and does not leave foreign matter in the subcutaneous tissue.
Discharge instructions:
Remove the dressing and SyvekPatch 24 hours after application by soaking the patch with water (may be done in the shower). Gently peel off the patch.
Use routine site care; wash site with soap and water, and cover with a bandage for 1 day.
Patient may not take a tub bath, swim, or soak in a hot tub until the site is healed.
V+Pad
The V+Pad is a small three-layer woven gauze pad covered with a D-glucosamine–enriched coating, which, when applied to the angiography site, works to accelerate platelet aggregation and enhance platelet plug formation. The pad promotes the body’s natural thrombin to convert fibrinogen to fibrin and results in a “mesh” forming around the platelet plug. More platelets continue to be trapped, and ultimately a clot is formed. Post hemostasis,
the V+Pad remains in place and is covered with a Tegaderm dressing. Patients are usually ambulatory 90 minutes post hemostasis and are able to be discharged home with instructions to remove the V+Pad within 24 hours by soaking with water and gently peeling away from the skin.
the V+Pad remains in place and is covered with a Tegaderm dressing. Patients are usually ambulatory 90 minutes post hemostasis and are able to be discharged home with instructions to remove the V+Pad within 24 hours by soaking with water and gently peeling away from the skin.
Vasoseal
VasoSeal is a closure device that enhances the body’s natural method of achieving hemostasis by delivering collagen extravascularly to the surface of the femoral artery. The collagen (Type I, produced from bovine tendons) attracts and activates platelets in the arterial puncture, forming a coagulum at the surface of the artery and resulting in a seal at the puncture site. The collagen reabsorbs over a 6-week period.
Discharge instructions:
Decreased bedrest time and greater flexibility of movement (head of bed may be elevated).
There may be a knot under the skin at the groin site for 5 to 7 days.
Use routine site care; keep clean and dry.
Patient may not take a tub a bath, swim, or soak in a hot tub until the site is healed.
Coarctation of the Aorta
Aortic constriction, usually distal to the left subclavian artery, stresses the left ventricle and causes an increase in afterload.
Signs/symptoms:
Headaches, dizziness, syncope (exercise intolerance)
Cold legs and feet, with leg pain or cramps after exertion
Shortness of breath, nosebleeds
Chest pain or palpitations
Blood pressure noted higher in arms than in legs (related to upper extremity hypertension). Femoral pulse may be noted weak, or even absent.
Treatment: Usually corrected by surgery or by nonsurgical balloon dilation.
Congestive Heart Failure
(see Heart Failure, p. 110)
Cor Pulmonale
Cor pulmonale is a right ventricular enlargement secondary to a lung disorder that produces pulmonary artery hypertension (i.e., pulmonary embolism, COPD, loss of lung tissue related to surgery or trauma). The following hallmarks indicate that the right ventricle is feeling strain:
Chest x-ray shows RV and pulmonary artery enlargement.
EKG exhibits:
Right axis deviation (by >30%)
Inverted, diphasic, or flattened T waves in leads V1 to V3
ST depression in leads II, III, and aVF
P waves are tall and peaked (>2.5 mm) in leads II, III, and aVF
P waves are inverted in lead aVL
P waves in V1 to V3 are sharp and pointed
Low-voltage QRS is common
Coronary Angiography
(see Angiography, p. 71)
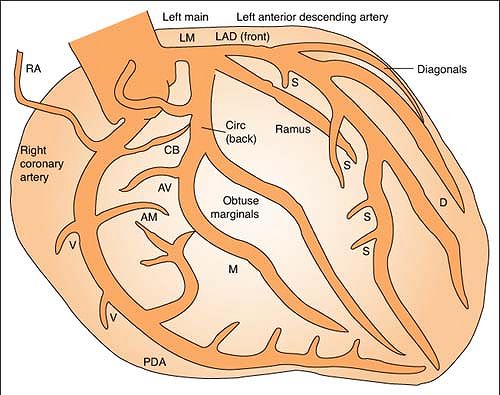
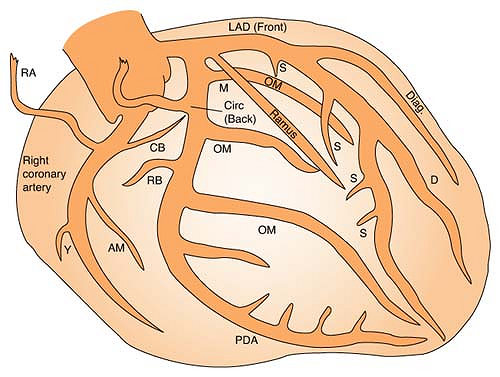
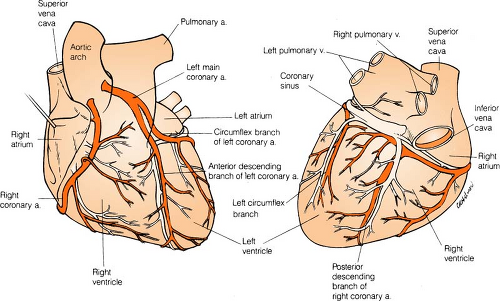
Coronary Arteries
Right coronary artery (RCA) perfuses:
SA node in 60% of population
AV node in 80% to 90% of population
Bundle of HIS
Part of left bundle branch
Posterior third of septum
Right atrium and ventricle
Inferior wall of left ventricle
Left anterior descending (LAD) artery perfuses: (Branches are “diagonals”):
Anterior wall of left ventricle
Two thirds of septum
Right bundle branch
Part of left bundle branch
SA node in 40% of population
Left circumflex artery (LCA) perfuses: (Branches are “obtuse marginals”):
Part of the left bundle branch
Lateral wall of left atrium and ventricle
Coronary Artery Bypass Graft
(see Cardiac Surgery, p. 82)
Coronary Stents
(see Stents, p. 149)
Cryoplasty
(see Angioplasty, p. 73)
Cvp
(see Swan-Ganz: Hemodynamic Normals, p. 155)
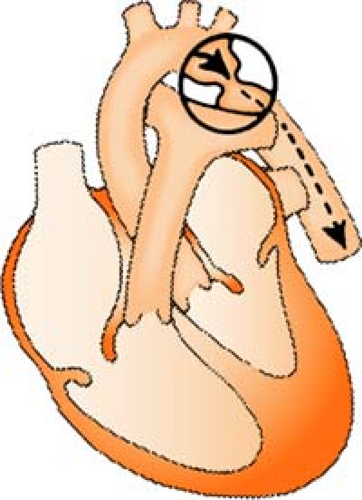
Defibrillation
Defibrillation is the discharge of energy (joules) in the form of a nonsynchronized shock, generally delivered to the heart via transthoracic paddles or pads for the purpose of reestablishing normal electric conduction in the presence of VF or pulseless V-tach.
Defibrillator Hints
Energy stored in the defibrillator is constant, so the only variance to the delivered current results from the operator and the impedance of the individual patient’s chest. To minimize this variance:
Use the hands-free adhesive defibrillation pads (always preferred) rather than the hands-on paddles. The hands-free pads provide the lowest resistance and greatest amount of safety.
If paddles are required, 25 lb of pressure per paddle must be applied.
Use the proper pad (paddle) placement so the heart (primarily the ventricles) is in the path of the current.
Placing the defibrillation pad over a medication patch can decrease the effectiveness of the electrical therapy. REMEMBER: Remove any transdermal medication patches from the patient’s chest before defibrillating.
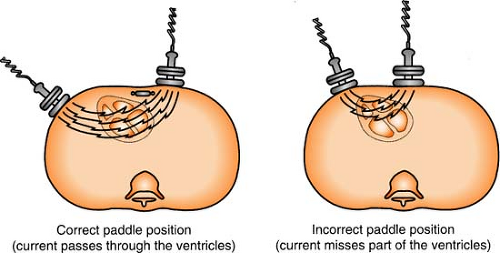 Figure. Correct and incorrect paddle placement for defibrillation. (Crockett, P. [1991]. Defib: What you should know. Redmond, WA: Physio-Control. Reprinted with permission.) |
The sternum/apex position is most frequently used, with sternum pad on upper right side of chest to right of sternum, below clavicle, and apex pad on lower left side of chest over cardiac apex, to left of nipple in midaxillary line.
Defibrillation of A Patient with an Implanted Pacemaker
Place the pads as far away from pulse generator as possible. Standard anterior/lateral placement is most convenient, but it delivers defibrillation energy in the same direction as the pacemaker’s sensing vector and may damage the pacemaker. Place pads in the anterior/posterior position so that energy is delivered perpendicular to sensing vector.
Defibrillation of A Patient with an Implanted Defibrillator
If an unconscious patient receives shocks from an implanted defibrillator but V-tach or ventricular fibrillation (V-fib) persists, external countershock should be delivered. It is possible that the limited energy output of the implant is insufficient to defibrillate the heart. If initial attempts at external defibrillation are unsuccessful, change the placement of the pad electrodes. For example, if the sternum/apex position fails, try the anterior/posterior position.
Monophasic Versus Biphasic Defibrillation
Defibrillators are classified according to two types of waveforms: monophasic and biphasic. Monophasic defibrillators were introduced first, and although they are no longer being manufactured, many remain in use today. They deliver current in one direction only. Biphasic defibrillators deliver two-way current. During the first phase of the shock, the current flows in one direction. During the second phase, the current reverses, and travels backwards.
Defibrillation Energy Levels
Research indicates that lower-energy biphasic defibrillation has equivalent or higher success for termination of VF than does monophasic defibrillation which delivers escalating energy (200–300–360 J) with successive shocks. Although the optimal energy for biphasic waveform defibrillation has not been conclusively determined, shocks with relatively low energy (<200 J) have been shown to terminate VF with equivalent or higher success rates than do monophasic shocks of equivalent or higher energy.
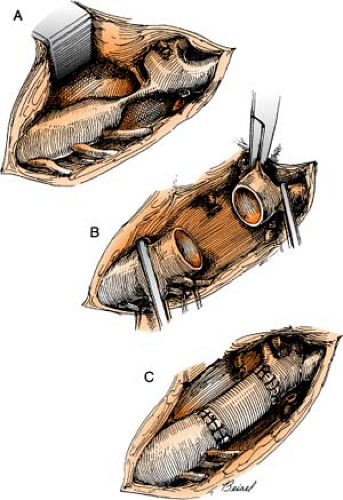
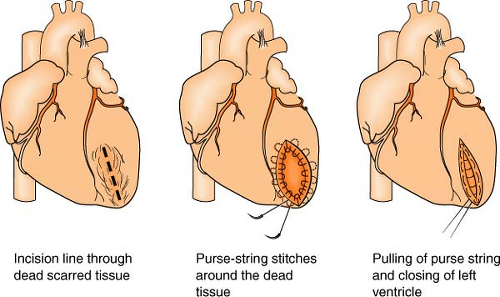
Dor Procedure
(see also Ventricular Restoration, p. 164)
The Dor procedure, also known as left ventricular reconstructive surgery, or EVCPP (endoventricular circular patch plasty), is a surgical approach that is sometimes used after an aneurysm forms following a heart attack. By restoring the normal elliptical geometry of the left ventricle, the ability of the ventricle to contract is improved, resulting in improved heart function.
The heart is placed on heart-lung bypass, and a small incision is made into the left ventricle to find the exact location of the scarred tissue. Two or more rows of circular stitches are then placed around the border of the dead tissue to separate it from healthy muscle tissue and then are pulled together like a purse string to permanently separate the dead tissue from the rest of the heart. (Sometimes an area of scar tissue is removed first before the stitches are pulled together, of if there is a lot of dead tissue to remove and the stitches are not enough to isolate the area, a patch must be placed.) Lastly, the outside of the ventricle is closed and reinforced with another row of stitches. Often, other surgeries, such as CABG or valve surgery, are often done at the same time.
Dressler’s Syndrome
A pronounced form of pericarditis that develops in some patients weeks or months after an MI, characterized by pleuritic chest pain, fever, pericardial friction rub, and mild to moderate pleural effusion, Dressler’s syndrome is rarely serious, although it often recurs several times before finally resolving spontaneously.
Ecmo
(see Extracorporeal Life Support, p. 108)
Edema Grading Scale
(see also Third Spacing in Part 7, p. 293)
Pitting >1 inch = 4+
Pitting ½ to 1 inch = 3+
Pitting ¼ to ½ inch = 2+
Pitting <¼ inch = 1+
Eecp
(see Enhanced External Counterpulsation, p. 107)
Einthoven’s Triangle
(see EKG Lead Placement, p. 101)
Ejection Fraction
First-pass angiocardiography is used to show end-diastolic and end-systolic images, shunts, and details of heart wall motion. From these values, an ejection fraction (EF) can be determined, indicating the percentage of ventricular chamber emptying. It is determined by this formula:
Normal ejection fraction is 60% to 65% (±8%); lower values indicate ventricular dysfunction, whereas an ejection fraction of <35% indicates serious ventricular problems.
Ekg: Fusion Beats
Fusion beats occur when two opposing electrical currents meet and collide within the same chamber at the same time. The ensuing EKG complex is often narrower and of lesser amplitude than an ectopic beat alone.
Remember:
With a fusion beat: The P to P is regular, the R to R is regular, and the P does bear a relationship to the QRS (the PR interval may be shorter than that of the sinus rhythm, but never by more than 0.06).
Ekg: Interpretation Parameters
(see also Myocardial Infarction: EKG Patterns, p. 131)
EKG Interpretation Parameters
| Rhythm | P Wave | PR Interval | QRS Rate and Rhythm | Comment |
|---|---|---|---|---|
| Sinus | Before each QRS | 0.12–0.20 | 60–100; regular | |
| Sinus arrhythmia | Before each QRS | 0.12–0.20 | 60–100; phasic variation with respiration | |
| Sinus bradycardia | Before each QRS | 0.12–0.20 | <60; regular | Not often below 40 |
| Sinus tachycardia | Before each QRS | 0.12–0.20 | >100; regular, but may vary a little | Usually 100–160; may be higher in children |
| Sinus arrest | Pause does not march out | R to R regular except for missed beat | ||
| Sinus block | Pause marches out | 2nd or 3rd degree may follow | ||
| Wandering pacemaker | Morphology changes; may be hard to see | Varies as site changes | R to R varies as site changes | |
| Premature atrial contraction (PAC) | Premature P may have different configuration; P may be buried in T | May be <0.12 or >0.20 | QRS may be normal or widened; irregular | No compensatory pause, usually; frequent PACs can lead to A fib |
| Premature atrial tachycardia (PAT) | Before each QRS; may be buried in preceding wave or T fused P & T | May be <0.12 or >0.20 | 160–250; absolutely regular, usually | QRS normal, usually; abrupt onset and termination; carotid pressure may terminate attack |
| Atrial flutter | Atrial rate is 200–300; Ventricular rate varies; Sawtooth pattern | Constant or variable | 75–300, depending on amount of AV block; regular or irregular | Carotid pressure produces temporary slowing, if any; Watch for emboli |
| Atrial fibrillation (A fib) | Atrial rate 300 or more; Ventricular rate varies; Irregular, undulant baseline (“F” waves) | Variable; R → R never march out; exception: CHB | 50–250, depending on degree of AV block; irregular | “F” waves may be shown better in V2 than lead II; Watch for emboli |
| PJC | Single premature beat interrupts rhythm; slight delay before next | |||
| Junctional | Before, after, or in QRS Often inverted | Usually <0.12 | 40–100 | |
| Premature ventricular contraction (PVC) | None preceding the premature QRS | Usually normal, can occur at any rate | Compensatory pause, usually; QRS configuration different, >0.12, usually | |
| Ventricular tachycardia (V tach) | Usually not seen; if present, not related to QRS | Variable | 100–220, usually regular or nearly regular | QRS broad, different, >0.10, usually Can be with or without pulse |
| Ventricular fibrillation (V fib) | None | No well-defined QRS complexes | No palpable pulse and no audible tones | |
| 1st-degree AV block | Before each QRS | 0.21 or more | Regular | May be a warning that 2nd- or 3rd-degree block will follow |
| 2nd-degree AV block (Type 1, Mobitz 1, or Wenckebach) | Before each QRS except for blocked P | Lengthens until one beat is dropped; first of the PR series is usually >0.20 seconds | Normal | “Group beating” is obvious; usually a transient rhythm |
| 2nd-degree AV block (Type II or Mobitz II) | Before each QRS except for blocked P | Normal or prolonged but constant | Normal if block is at bundle of HIS, wide if at the level of the bundle branches | Is often irreversible and progresses into 3rd degree; may need pacemaker; REMEMBER: “Out of the blue, Mobitz II drops a Q (wave)” |
| 3rd-degree AV block (complete heart block) | Occurs regularly but without relationship to QRS | Variable | Below 60, usually; regular, usually | Spells of syncope common (Adams-Stokes attacks); pace-maker is in the ventricles (idioventricular rhythm) |
Ekg Lead Placement
(see also ST Segment Monitoring, p. 148)
Depending on the monitoring system, the number of EKG electrodes (wires) positioned on the patient will differ. But remember: One electrode (wire) cannot be a “lead.” Leads are pictures, so this means that a group of electrodes is required to capture a specific view. (For example, to obtain a 12-lead EKG, there are six “chest” electrodes, and four “limb” electrodes. This is only 10 wires, but the different combinations of the wires yield 12 different views.)
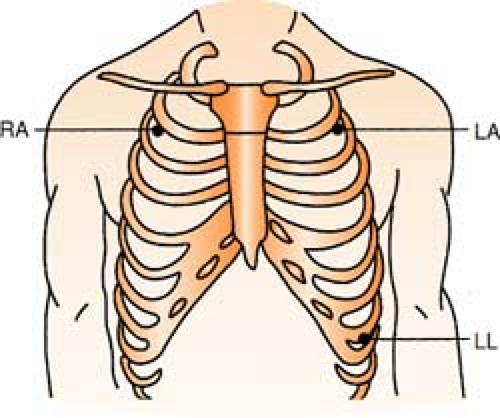
Three-Lead System
A three-lead system can only give you a choice of limb leads: I, II, III, aVR, aVL, and aVF.
Position:
RA (white) Below right clavicle, 2nd ICS, MCL
REMEMBER: White is right
LA (black) Below left clavicle, 2nd ICS, MCL
LL (red) Left lower rib cage, 8th ICS, MCL
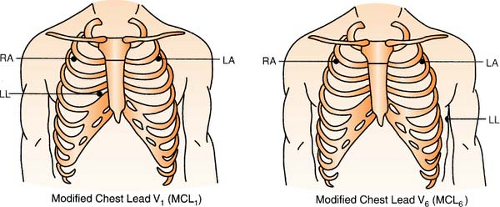
Chest leads are unavailable with a three-lead system; however, changing the lead selector on the monitor to lead III and repositioning an electrode can facilitate a modified chest lead (MCL) tracing. To monitor modified chest leads: LL (red) must move to the appropriate chest lead position, either:
MCL1 = 4th ICS, right sternal boarder or
MCL6 = 5th ICS, L midaxillary line to heart
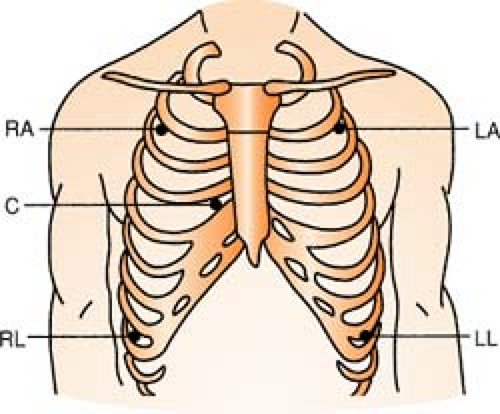
Five-Lead System
A five-lead system allows monitoring in the limb leads: I, II, III, aVR, aVL, and aVF and by using the lead select on the monitor, a chest lead can be obtained as well.
Position:
| RA (white) | Below right clavicle, 2nd ICS, MCL |
| RL (green) | Right lower rib cage, 8th ICS, MCL |
| LA (black) | Below left clavicle, 2nd ICS, MCL |
| LL (red) | Left lower rib cage, 8th ICS, MCL |
| CHEST (brown) | Any V lead position, usually V1 |
Remember:
Clouds over grass, smoke over fire.
(White electrode above green electrode; Black electrode above red electrode)
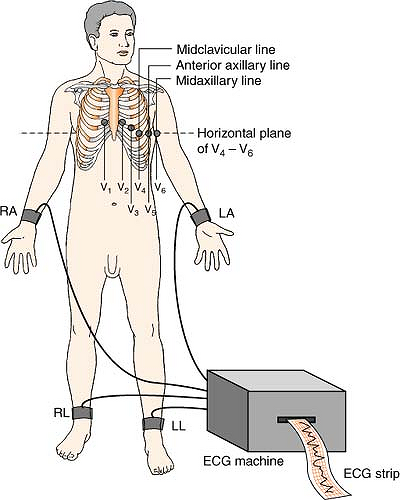
Twelve-Lead System
The 12-lead EKG views six limb leads in the frontal plane of the body, and six chest leads in the horizontal plane.
Position:
| RA (white) | Anywhere on right arm (below shoulder) |
| LA (black) | Anywhere on left arm (below shoulder) |
| LL (red) | Anywhere on left leg (below inguinal fold anterior; below gluteal fold posterior) |
| RL (green) | Anywhere on right leg (below inguinal fold anterior; below gluteal fold posterior) |
| V1 (brown) | 4th ICS, right sternal boarder |
| V2 (brown) | 4th ICS, left sternal boarder |
| V3 (brown) | Halfway between V2 and V4 |
| V4 (brown) | 5th ICS, left MCL |
| V5 (brown) | 5th ICS, left anterior axillary line |
| V6 (brown) | 5th ICS, left midaxillary line |
Remember:
When you think of how the LIMB leads “look” at the heart, imagine looking at the face of a clock:
| Lead I | views the heart from | 3 o’clock |
| Lead II | views the heart from | 5 o’clock |
| Lead III | views the heart from | 7 o’clock |
| aVR | views the heart from | 10 o’clock |
| aVL | views the heart from | 2 o’clock |
| aVF | views the heart from | 6 o’clock |
Remember:
When you think of how the CHEST leads “look” at the heart, imagine a vector projecting through the AV node toward the patient’s back. V1 to V6 are like the spokes of a wheel, with the center being the heart.
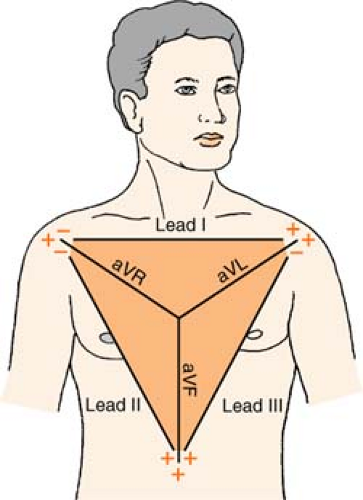 Figure. EKG planes. (From Morton, P. G., & Fotaine, D. [2009] Critical care nursing: A holistic approach [9th ed.]. Philadelphia: Lippincott Williams & Wilkins.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|