I. QUALITY IMPROVEMENT (QI)
A. Background: The current health care environment places increasing emphasis on improving the quality of medical care. Almost half of patients do not receive recommended care while hospitalized, for a variety of reasons. The complexities of care present in the critical care environment make it particularly prone to medical error, and the patient population is particularly susceptible to experiencing morbidities related to their hospitalization. With better clinical studies, process improvement, and focus on quality and safety, ICU mortality rates [in the U.S.?] have decreased 35% between 1988 and 2012, despite an increase in age and severity of illness in the ICU population. Ensuring that all patients receive the best evidence-based treatment, proactively preventing morbidity and eliminating the occurrence of medical errors is the goal of all quality programs. Delivering safe, high-quality care remains a moral imperative: Do No Harm.
B. Definition of Quality Care: The Institute of Medicine (IOM) has benchmarked quality care using six metrics: care that is safe, timely, effective, efficient, equitable, and patient centered.
C. Financial Implications: Reimbursement for care is increasingly linked to performance, as measured by quality metrics and the prevention of patient harm. For example, Medicare no longer reimburses hospitals for certain preventable, hospital-acquired infections. Financial incentives are now a tool to enforce desired behavior and decrease unwanted practice variance.
D. Components. There are three standard quality of care components: structure, process, and outcome.
1. Structure. The structure of intensive care units varies between individual units, hospitals, and regions. Elements of the structure of an intensive care unit include the training and experience of care providers, staffing organization, and the diagnostic and treatment technology available. Changing unit structure is challenging and requires coordination with hospital and health care system infrastructure. There are insufficient data to recommend which of many existing models is best.
2. Process. Process refers to both the actual care provided to patients (and how well this care complies with best practices) as well as certain nonclinical issues that may have an indirect effect on patient care. Examples of processes of care including number of patients utilizing a central line as well as use of a checklist for line placement. Hundreds of process studies are published annually to guide consensus on ICU best practice.
3. Outcome. Outcome refers to the results realized and has been the traditional metric to judge performance of critical care clinicians and researchers. The usual benchmark is 28-day, risk-adjusted mortality. Length of stay, hospital-acquired infections, and quality-adjusted life years (QALY) serve as other outcome measure in clinical trials. However, advances in patient- and family-centered ICU care increasingly argue for use of patient-related outcome measures (PROMs), such as early, full functional recovery and return to work.
4. Focus on Process in QI. While we should continue to address outcome measures, quality improvement that focuses on process has certain advantages. In addressing the process, we can concentrate on different aspects of care, there is less need for risk adjustment as with outcome measures, sample sizes are smaller, and less time is needed to acquire results. Additionally, it can provide direct feedback to providers. Regardless, the quality measures we use should be meaningful, scientifically sound, generalizable, and interpretable.
E. Leadership and Culture: For any quality effort to take hold, there must be an enthusiastic and motivated core of people dedicated to the hard work of sustained improvement. In the ICU, this requires a multidisciplinary team that has insight into many different facets of care. A leader in quality improvement needs to be amenable to change, able to foster a culture of safety, and willing to resist the argument “because we’ve always done it that way.” An environment that fosters the “ground” team at the point of care is critical to understand issues and implement improvement process. Improving the safety culture may be needed before any improvement project is undertaken; there are various measures to assess the safety climate of a unit.
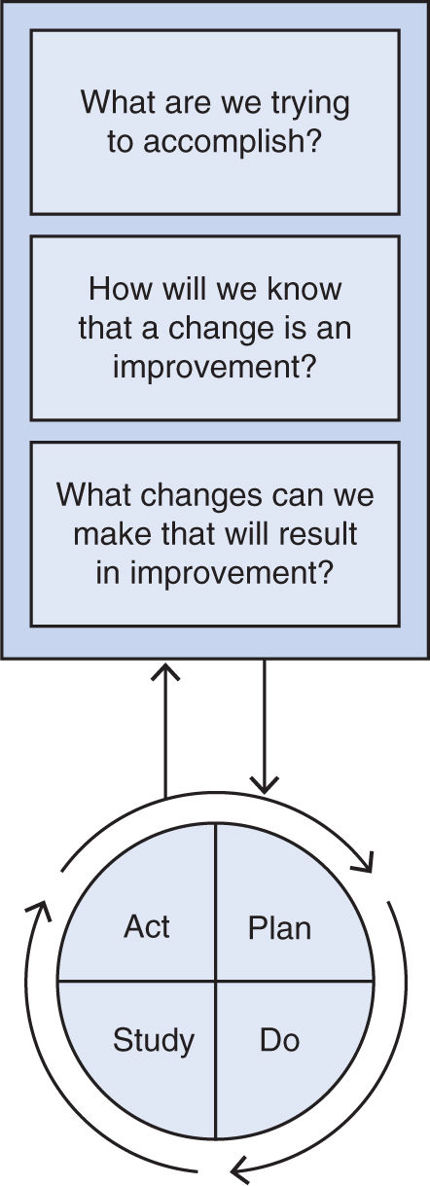
F. Model for Approaching Quality Improvement. The Institute for Healthcare Improvement (IHI) recommends a model for improvement developed by the Associates in Process Improvement (see Fig. 41.1).
1. Formulate the right questions. As seen in the Figure, three fundamental questions are used to focus the quality improvement effort, moving from a general concept to a specific target.
2. Target measure and goal. The target measure must be carefully defined and should use discrete, measurable components. The ability to quantify the target measure should be easily extracted from existing databases both to establish a baseline and to continue to monitor over time. A specific improvement goal should be explicitly stated.
3. Plan-Do-Study Act (PDSA cycle). The PDSA cycle involves creating a course for change as well as methods for evaluating the impact of these changes. It is often initially instituted in a limited or test population. The first step is developing an approach to institute an improvement (Plan). The most effective process change strategies are tailored to the environment and the improvement they are hoping to create. They rely on audit and feedback of recent performance and are augmented by a variety of educational modalities including interactive teaching methods, brief informal discussion, and more structured lectures and dissemination of written information. After an intervention is initiated (Do), the results on the target measure should be evaluated (Study), as well as feedback as to what worked and what did not. This information should be incorporated into the improvement effort (Act) and the intervention modified appropriately, restarting the plan–do–study–act cycle. Once an intervention has been optimized, it should be expanded and more universally applied.
4. Impact of quality improvement: The potential impact of a quality improvement effort can be measured in terms of five dimensions. As individual providers, hospitals, and health care systems institute quality improvement programs, they should address most, if not all, dimensions.
a. Reach: How much of the target population participated?
b. Efficacy: What is the success rate if implemented as intended?
c. Adoption: What proportion of target institutions and practitioners adopt the intervention?
d. Implementation: To what extent is it implemented as intended?
e. Maintenance: How is the intervention sustained over time?
II. STANDARDIZATION
A. Checklists. As the number of quality improvement initiatives increase, with both individual items and bundles, ensuring that each patient receives all appropriate interventions is increasingly a challenge. The use of a checklist, often incorporated as part of daily rounds, brings attention to these interventions and helps coordinate care. Checklists have been shown to increase implementation of best practices and are strongly associated with improved outcomes. Checklists may also be used to ensure that all best practice elements in a particular process are completed, like central line insertion. Figure 41.2 shows the central line insertion checklist at our hospital. Importantly, the existence of a checklist per se is not sufficient to insure quality care; in most studies, compliance rates reported in ICUs are low (30%–70%), indicating opportunities for improvement.
B. Care Bundles: Care bundles are aggregated, evidence-based interventional practices that are designed to improve outcomes by delivering structured, measureable processes of care. They consist typically of three to six separate elements supported by evidence from clinical trials. These elements are more successful when implemented together than when used individually and should be delivered to every patient every time. Bundles should decrease unwanted practice variance while still allowing for individualized care when indicated.
1. Compliance. Measurement of compliance with bundles is integral to their success, and completion of a bundle is an all-or-nothing event. Bundles should be dynamic and evolve over time as new evidence changes best practice. The greatest effect on outcomes occurs when >90% bundle compliance is reached.
2. Patient care. Bundles have been shown to have success in reducing ventilator-associated pneumonia (VAP), reducing central line–associated blood stream infections (CLABSI), and improving the management of sepsis and septic shock. A new example is the ABCDE bundle, which links Awakening and Breathing Coordination of daily sedation and ventilator removal trials, Delirium monitoring and management, and Early exercise and mobility. ABCDE has been reported to minimizing sedative exposure, reducing duration of mechanical ventilation, and help manage ICU-acquired delirium and weakness (see Fig. 41.3). Patients cared for following implementation of this bundle spent 3 more days breathing without assistance, experienced less delirium, and were more likely to be mobilized in their ICU stay. There were no significant differences in self-extubation and reintubation rates.
3. Multiple examples of checklists and care bundles are provided under the section on quality metrics.
III. QUALITY METRICS, MORBIDITY, AND SAFETY IN THE ICU
A. Background. The ICU is an high-stakes environment. Critical illness itself, the treatments we provide, and the methods of monitoring we use can each be associated with significant morbidity and mortality. Here we discuss some of the key elements to prevent ICU-related harm.
B. Central Line–Associated Bloodstream Infection (CLABSI). ICU patients often require central venous access to allow for stability of access, the ability to deliver certain medications or nutrition (e.g., vasopressors, TPN), and central monitoring. However, these benefits are balanced by risks, including CLABSI and thrombus formation. The high attributable costs of CLABSI have led federal agencies to base a significant fraction of annual reimbursement on better outcomes (that is, low CLABSI rates compared with peer institutions).
1. Costs. Estimates vary from $6,000 to $45,000 per CLABSI due to attributed costs of care related to central line infections, with annual health care costs exceeding $1 billion.
2. Prevention. The most direct way to reduce the occurrence of CLABSI is to restrict line use and to remove the line as soon as is prudent.
3. Infectious sources. Line contamination can occur at time of placement, either extraluminally or intraluminally. Therefore, strict sterile technique should be employed, using full-barrier precautions. Bacteria that enter extraluminally from the skin surface are often responsible for infections that occur with short-term use (3–7 days). Infection from intraluminal spread is increasingly being recognized as a continued cause of CLABSI, especially with longer term use (>7 days).
4. Central venous catheter (CVC) bundle of care. Below are process of care recommendations for reduction of CLABSI. They are frequently included in bundles and checklists for insertion. Daily review of indications for catheter use is indicated.
a. All associated equipment should be maintained in a central line cart.
b. Educate health care personnel on insertion, care, and maintenance of CVCs, with periodic assessment of this knowledge.
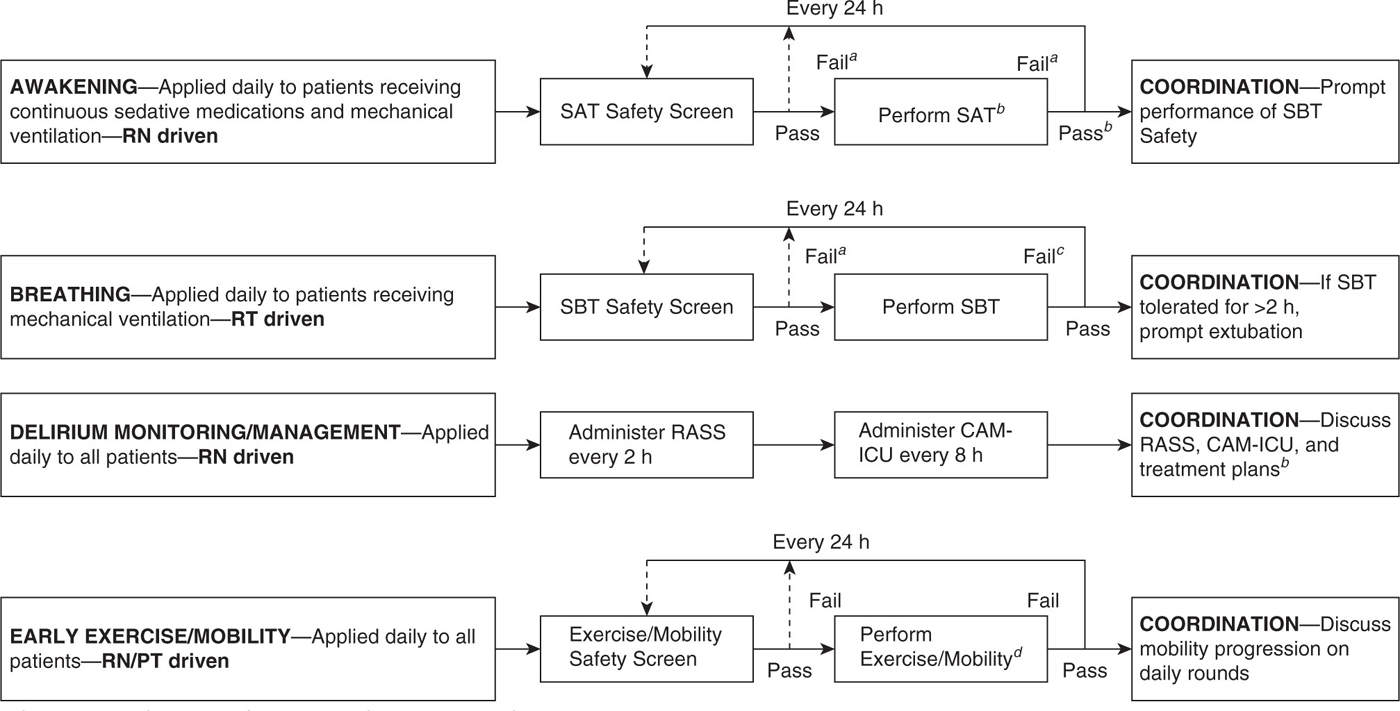
FIGURE 41.3 ABCDE bundle. (From Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med 2014;42(5):1024–1036.)
aContinuous sedative medications maintained at previous rate if SAT safety screen failure. Mechanical ventilation continued, and continuous sedative medications restarted at half the previous dose only if needed due to SBT safety screen failure. bContinuous sedative infusions stopped, and sedative boluses held. Bolus doses of opioid medications allowed for pain. Continuous opioid infusions maintained only if needed for active pain. cContinuous sedative medications restarted at half the previous dose and then titrated to sedation target if SAT failed. Interdisciplinary team determines possible causes of SAT/SBT failure during rounds. Mechanical ventilation restarted at previous settings and continuous sedative medications restarted at half the previous dose only if needed if SBT failed. dSAT pass if the patient is able to open his/her eyes to verbal stimulation without failure criteria (regardless of trial length) or does not display any of the failure criteria after 4 hr of shutting off sedation.
c. Perform hand hygiene prior to insertion and any manipulation of CVC.
d. Wear cap, mask, gown, and sterile gloves for insertion.
e. Use maximal sterile barrier precaution with drapes during CVC insertion.
f. Avoid the femoral vein as an access site in adult patients if able.
g. Use >0.5% chlorhexidine-based antiseptic skin preparation in patients >2 months old.
h. Use ultrasound guidance to place catheters to reduce cannulation attempts and mechanical complications.
i. Use a chlorhexidine-impregnated sponge dressing if CLABSI rates remain high despite implementation of other guidelines.
j. Consider use of antimicrobial-impregnated catheters in high-risk populations or if CLABSI rates remain high despite instituting other guidelines.
k. Disinfect catheter hubs, needleless connectors, and injection ports before accessing catheters.
l. Change administrations sets used for blood, blood products, or fat emulsions every 24 hours. Change other continuous-use administration sets no more frequently than every 96 hours but at least every 7 days.
m. Change dressings sterilely when soiled or loose, and at least every 7 days even if clean, dry, and intact.
n.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree