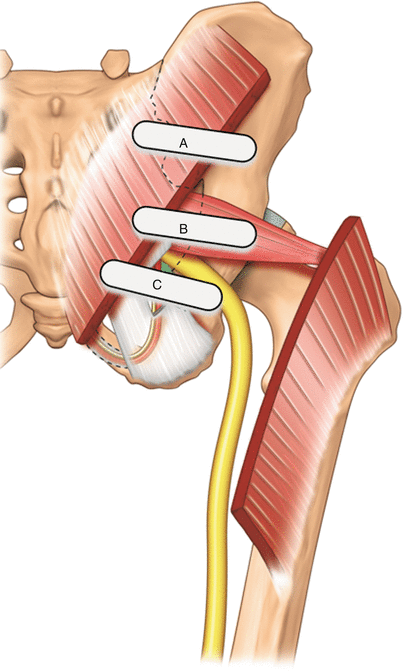
Fig. 56.1
Posterior view of pelvis, showing the piriformis muscle and the neurovascular bundle deep to it. The pudendal nerve and artery run between the sacrospinous and sacrotuberous ligaments (Reproduced with permission from Philip Peng Educational Series)
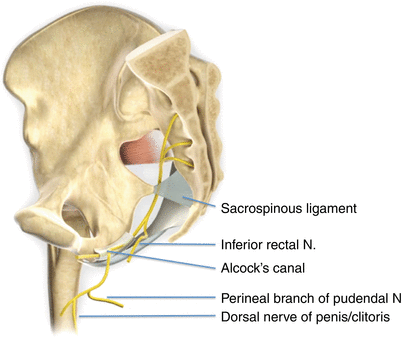
Fig. 56.2
Pudendal nerve is seen arising from S2–S4 and exiting pelvis to enter gluteal region through the greater sciatic foramen. The nerve gives rise to the inferior rectal nerve, perineal nerve, and the dorsal nerve of the penis or clitoris. The inferior rectal nerve branches from the pudendal nerve prior to Alcock’s canal. N nerve (Reproduced with permission from Philip Peng Educational Series)
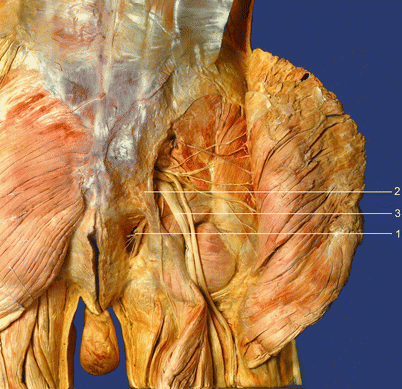
Fig. 56.3
Anatomic specimen. (1) Pudendal nerve and pudendal vessels in the ischiorectal fossa, (2) sacrotuberous ligament, (3) sciatic nerve (With permission from Dr. Danilo Jankovic)
The anatomical course of the pudendal nerve has been the subject of numerous investigations, and studies continue to report novel anatomic variations. These studies challenge the originally held belief of the pudendal nerve as a singular nerve with a consistent pathway through the pelvis. In contrast, this nerve can be quite complex with a number of well-described variations. Knowledge of these variances can aid in the appropriate management of patients requiring intervention through nerve blockade or surgery.
Nerve Roots
The ventral rami of S2, 3, and 4 commonly form the pudendal nerve, yet contributions from S1 and S5 nerve roots have been documented [4, 6–10].
Frequently, the L4 nerve root of the lumbosacral plexus is divided between the lumbar and sacral plexuses, acting as a boundary root. On occasion, the L3 or L5 nerve root may serve as the boundary root, leading to a shift in the nerve roots composition of the plexus involving upper or lower nerve roots or prefixed or postfixed plexus, respectively. These alterations could influence nerve root contributions to pudendal nerve formation. This is exemplified in an anatomic study of 20 adult cadaveric specimens that sought to clarify the nerve root formation of the pudendal nerve in these plexus variations [11].
Of 20 cadaveric specimens, 9 normal lumbosacral plexuses were found, in which the S2 and S3 nerve roots contributed to pudendal nerve formation. In 8 prefixed plexuses, S1 and S2 nerve roots predominated the formation of the nerve, while in the postfixed plexus type, S3 and S4 nerve roots predominated nerve formation. Overall, the S2 nerve root participated in pudendal nerve formation in 17 of 20 cases [11].
A separate anatomical investigation by O’Brichere revealed that the S2 and S4 nerve roots are major contributors to formation of the pudendal nerve [10]. Conversely, Robert et al. discovered that the pudendal nerve is usually formed by S3, with contributions from S2 and 4 [8]. Clearly, variation in pudendal nerve formation is common. This can influence the success of interventions aimed to affect pudendal nerve function at the level of the nerve roots.
Nerve Trunks
The notion that foundational nerve roots consistently combine to form a single pudendal nerve is mistaken. In fact, much of what has been described in anatomical studies reveals that contributing nerve roots may either combine to form a single pudendal nerve or form between two and three “trunks,” which may or may not combine to form the pudendal nerve and its terminal branches (inferior rectal, perineal, and dorsal branches) [9].
The frequency of occurrences of trunk types within the gluteal region has been investigated in several anatomical studies [2–4, 9, 10, 12–16]. Although separate research groups propose different classification schemes of the trunks, some generalizations can be drawn. The occurrence of the pudendal nerve as a single common trunk has been reported with the most frequency, occurring between 29 and 96 % of anatomical dissections. The occurrence as two trunks has been reported between 4 and 45.5 % and that of three trunks between 6 and 19.5 % in the literature [3].
Morphology and Anatomical Relationships at the Ischial Spine
A comprehensive knowledge of the nerve’s anatomy at the level of the ischial spine is important when performing pudendal nerve blocks. This is a possible site of pudendal nerve entrapment and an accessible area for pudendal nerve blockade through either blind or image-guided approaches.
After exiting the pelvis from the infrapiriform notch, the pudendal nerve crosses over the posterior aspect of either the sacrospinous ligament or the ischial spine. Pirro et al. studied the pudendal nerves of 20 cadaveric specimens and observed that the nerve crosses the sacrospinous ligament in 80 % of cases, while in 15 % of cases it crossed the ischial spine. The remaining specimens contained multi-trunked nerves crossing both the ischial spine and the sacrospinous ligament [3].
The relationship of the pudendal artery to the nerve has also been examined. In 80 % of cases, the pudendal nerve lay medial to the artery, while in 10 % of cases, the nerve lay lateral to the artery. Remaining anatomical relationships observed include having the artery lie between two trunks, and in 7.5 % of cases, the artery crossed the nerve [3]. Knowledge of the pudendal nerve’s position relative to the pudendal artery is useful when using ultrasound to guide neural blockade as the nerve itself is typically not easily visualized through a transgluteal approach. Identification of the artery as a pulsatile structure or via Doppler imaging can help clarify the most likely location of the nerve.
The pudendal nerve’s mean diameter at the level of the ischial spine has been reported between 2 and 6 mm [3].
Nerve Branches
The pudendal nerve divides into three branches: the inferior rectal branch, the perineal branch, and the dorsal nerve of the penis/clitoris (Fig. 56.4a, b) [17].
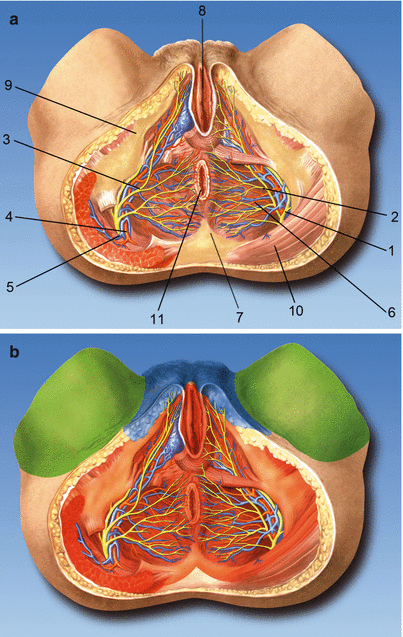

Fig. 56.4
(a) Anatomy. (1) Pudendal nerve, (2) inferior rectal nerves, (3) perineal nerves, (4) internal pudendal artery, (5) internal pudendal veins,(6) inferior rectal artery, (7) ischiorectal fossa, (8) vaginal orifice, (9) ischial tuberosity, (10) gluteus maximus muscle, (11) anus. (b) Skin innervations area of the pudendal nerve (red), ilioinguinal and genitofemoral nerves (blue), and obturator nerve (green) (With permission from Dr. Danilo Jankovic)
The inferior rectal branch descends from its origin in the pelvis to occupy the lower half of the ischiorectal fossa. It further subdivides into cutaneous branches, which supply sensory innervation to the anal canal and the skin around the anus. Sensory innervation may also include the skin of the scrotum. The inferior rectal branch provides the main motor innervation to the external anal sphincter, and investigations have also documented occasional motor innervation to the levator ani muscle as well, through an “accessory rectal nerve” [6, 18]. The inferior rectal branch has been observed to pierce through the sacrospinous ligament as it proceeds to the ischiorectal fossa, which can be a possible site of entrapment [2].
The perineal branch of the pudendal nerve divides into superficial and deep branches. The superficial branch provides sensory innervation through the posterior scrotal/posterior labial nerve, which contributes to the innervation of the posterior aspect of the scrotum or labia majora [9]. This branch may join the sensory branches of inferior rectal branch [17]. The deep branch supplies motor innervation to the muscles of the pelvic floor and the deep perineal pouch. The external urethral sphincter receives voluntary innervation from this branch. Other muscles that receive motor innervation include the transverse perinei, bulbospongiosus, and ischiocavernosus muscles [19, 20]. Motor innervation has also been reported to include the anterior part of the external anal sphincter and the levator ani muscle [20].
The dorsal nerve of the penis or clitoris is the final branch of the pudendal nerve. It supplies the erectile tissue of the corpus cavernosum and of the crus penis/clitoris. This branch supplies sensory innervation to the skin over the dorsal and lateral aspects of the penis and clitoris [9].
Alcock’s Canal
Distal to the sacrotuberous and sacrospinous ligaments, the pudendal nerve reenters the pelvis through the lesser sciatic foramen and enters Alcock’s canal [21]. This canal lies medial to the obturator internus muscle and is formed by a splitting of the muscle’s fascia into a medial and lateral layer. The medial layer covers the pudendal neurovascular bundle and fuses below with obturator fascia. The lateral layer is continuous with the obturator fascia [5]. The length of the canal has been measured from 1.4 to 1.8 cm in adults, ending at a distance of 2–3 cm from the inferior border of the symphysis pubis [5]. The canal has been described to lie 2.5–4.5 cm from the inferior border of the ischial tuberosity.
Early anatomical studies report that the pudendal nerve and its branches course together through Alcock’s canal and exit by piercing its fascial walls [5, 21]. As described by Shafik et al., the dorsal and perineal branches of the pudendal nerve exit the canal by piercing the obturator fascia at the canal’s distal end [5]. The inferior rectal nerve showed some variation in that it either did not enter the canal or entered the canal, but then pierced the proximal aspect of the canal to enter the ischioanal fossa.
A more recent cadaveric study has challenged the previous anatomical descriptions of Alcock’s canal. Furtmuller et al. performed dissections of 12 formalin-embedded cadavers, using a medial intrapelvic approach to map the course of the pudendal nerve and its branches [9]. Contrary to the classical description of the exiting pattern of the perineal and dorsal branches, they found that these branches do not exit the canal together at its distal end. Instead, it was discovered that the dorsal branch of the pudendal nerve exited 15–18 mm more anterior along the inferior pubic ramus than the perineal branch did, with its own distinct second exit from the canal. Furthermore, the dorsal nerve branch was found to take a higher course within the pelvis than the perineal branch. Rather than exiting the pelvis at the base of the urogenital diaphragm, the dorsal branch typically exited 12 mm lateral to the pubic symphysis in the parasymphyseal space [9]. This higher course of the dorsal nerve extends this branch in a horizontal fashion from the ischial spine to the parasymphyseal space, traveling separately from the perineal branch in the most superior aspect of Alcock’s canal.
Urogenital Diaphragm
The dorsal branch of the pudendal nerve pierces through the superior fascia of the urogenital diaphragm once it reaches the inferior pubic ramus. Beyond this point, the nerve travels in a pouch that is defined by the crus of the penis/clitoris anteriorly and inferiorly. The inferior pubic ramus forms the posterior and superior border. The branch may then pierce either the inferior fascia of the urogenital diaphragm or pierce above the inferior transverse pubic ligament. Once exited the pelvis at this point, the nerve travels anterior to the pubic bone in a groove known as the “sulcus nervi dorsalis penis/clitoris.” It then deflects ventrally to innervate the penis or clitoris [9, 22].
Indications
Surgical Anesthesia and Analgesia
There have been a number of investigations evaluating the utility of this block for hemorrhoidectomy, as the postoperative pain of this procedure can be very severe [23–29]. Pudendal nerve blockade has been found to confer substantial benefits for pain control over other types of analgesia, such as neuraxial blocks, general anesthesia, or nonspecific local anesthetic infiltration to the soft tissues of the perineum [23, 24, 26, 29]. Additionally, the use of this block is associated with reduced length of patient stay in hospital, reduced oral analgesic consumption, and improved patient satisfaction over other methods of analgesia [23, 24, 28, 29]. Urinary retention after hemorrhoidectomy is a common and undesirable side effect of anal surgery, as well as with neuraxial anesthetic techniques [30, 31]. The use of pudendal nerve blockade has been shown to significantly reduce this particular postoperative complication [26, 28, 32].
The evaluation of benefits of pudendal nerve blockade has also been investigated for urological procedures such as penile prosthesis surgery [33, 34], hypospadias repair, and circumcision in pediatric population [35, 36], prostate biopsy [37–39], and placement of prostate HDR brachytherapy [40].
The use of pudendal nerve blockade has been described for gynecologic surgical procedures such as placement of suburethral tape and colpoperineorrhaphy [41, 42], yet reports of its use are not as robust as for its use in obstetrical practice. Pudendal nerve blockade, however, has not proven useful to reduce pain after transvaginal pelvic reconstructive surgery [43].
Obstetrical Anesthesia and Analgesia
The use of pudendal nerve blockade during labor has typically been reserved for the second stage of labor. During this stage, pain is experienced in the perineum and becomes somatic, innervated by the S2 to S4 nerve roots and the pudendal nerves.
Pudendal nerve block was likely more commonly used prior to the introduction of epidural anesthesia techniques. However, it can still be employed when neuraxial techniques are contraindicated or if sacral sparring occurs during epidural catheter use. This nerve block has been described for facilitating instrumented deliveries, episiotomies, repair of perineal tears, and McDonald cerclages for incompetent cervices [44–48].
Pudendal Neuralgia
Pudendal neuralgia is an uncommon cause of perineal pain that may result from compression of the nerve or its trunks along its course through the pelvis. Patients will typically describe pain in the perineal area that can include the clitoris, penis, vulva, and perianal area. This may be accompanied by a foreign body sensation within the vagina or rectum. Sitting on a flat surface, which transfers pressure from the soft tissues within the perineum to the pudendal nerve, may exacerbate pain. Conversely, pain may be relieved by standing or sitting on a toilet seat, which relieves that pressure [49].
Diagnosis of this pelvic pain syndrome is challenging, given the lack of a commonly accepted diagnostic test. To assist in identification of the subset of pudendal neuralgia-pudendal entrapment neuropathy (PNE), the Nantes criteria have been proposed which list clinical inclusion and exclusion criteria. Essential criteria include: (1) pain in the anatomical territory of the pudendal nerve, (2) symptoms worsened by sitting, (3) patient is not woken at night by pain, (4) no objective sensory loss on clinical examination, and 5) positive anesthetic pudendal nerve block [50].
Pudendal nerve blockade may satisfy the last essential Nantes criterion if the pain is relieved for the duration of the local anesthetic. However, as described in the original article, a positive diagnostic block may not be specific for pudendal neuralgia, as alternative causes of the perineal pain will be anesthetized if they are situated within the nerve’s territory. The typical clinical practice is to inject both local anesthetic and steroid around the nerve. For patient with entrapment neuropathy, the duration of relief will be expected to outlast the effect of local anesthetic.
A negative diagnostic block may not rule out pudendal neuralgia if the nerve is anesthetized distal to the site of entrapment. For example, one may not achieve pain relief through a block performed at Alcock’s canal when the site of entrapment is more proximal at the ischial spine [50].
Block Techniques
Transvaginal Technique
The pudendal nerve can be blocked transvaginally through a “blind” technique using the ischial spine as an anatomical landmark (Fig. 56.5). To perform the block, the distal end of an introducer is used to guide the needle toward the pudendal nerve, which allows for infiltrating needles to be advanced 1.0–1.5 cm beyond their distal openings. Introducers described in the literature include the Iowa trumpet or Kobak needle and needle guide [51].
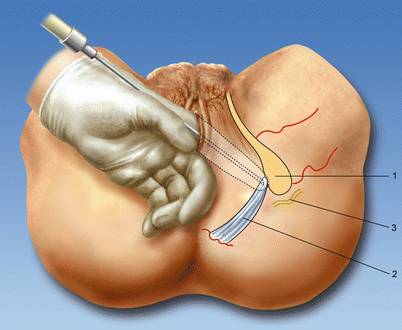

Fig. 56.5
Transvaginal access. (1) Ischial spine, (2) sacrospinous ligament, (3) pudendal nerve (With permission from Dr. Danilo Jankovic)
The introducer is first placed against the vaginal mucosa, inferior to the ischial spine. In obstetrical anesthesia literature, the guide is to be held parallel to the delivery table [51]. The needle is advanced into the vaginal mucosa and 1 mL of local anesthetic is infiltrated. The needle is then advanced further until contact is made with the sacrospinous ligament, where another 3 mL of local anesthetic is injected. Care should be taken at this point to first aspirate for blood to help exclude intravascular injection prior to injection as the pudendal vessels will be in close proximity. The needle is then passed through the ligament into the loose areolar tissue posterior to it, where another 3 mL of local anesthetic is deposited prior to aspiration. These steps are then repeated, but with the introducer placed superior to the ischial spine so as to ensure adequate spread around the pudendal nerve [52].
Transperineal Technique
This approach has been described in the literature together with the use of nerve stimulation and has mainly focused on providing analgesia for either perineal surgical procedures or for management of pudendal neuralgia.
The techniques described commonly include stimulation of the pudendal nerve adjacent to the ischial spine to elicit a contraction of the external anal sphincter and perineal muscles. The ischial spine can be localized by palpation of the ischial spine by inserting a finger through the vagina or rectum. Once this anatomical landmark is identified, a needle is guided to this point through the skin overlying the ischiorectal fossa (Fig. 56.6). The skin entry point can vary between descriptions. However, maintaining anal sphincter and perineal muscle contraction while diminishing the stimulating current to 0.5–0.6 mA is typically used to optimize the final needle tip position [25, 27, 53].
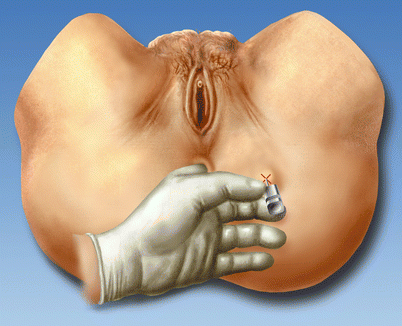

Fig. 56.6
Transperineal access. Rectal palpation of the ischial spine with the index finger. The needle is introduced into the ischiorectal fossa (With permission from Dr. Danilo Jankovic)
It should be noted that anal sphincter contraction alone might not be sufficient for a satisfactory pudendal nerve block, as this may indicate that only the inferior rectal nerve branch is being stimulated. Contraction of the pelvic floor muscles is more desirable as it indicates that the perineal branch is also being stimulated, signifying that the pudendal nerve itself, rather than individual branches, is being contacted.
Transgluteal Approach
Fluoroscopic Guided
Blockade of the nerve is accomplished by targeting the nerve within the gluteal region as it courses adjacent to the ischial spine (Fig. 56.7).
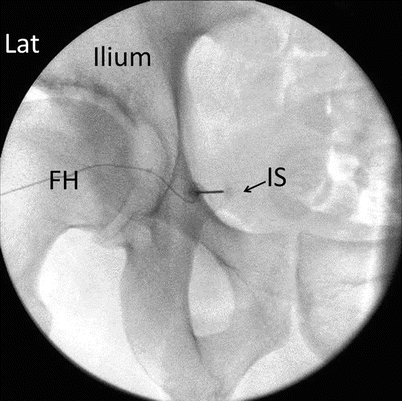

Fig. 56.7
Fluoroscopy guided pudendal nerve block. FH femoral head, Lat lateral, IS tip of the ischial spine (Reproduced with permission from Philip Peng Educational Series)
Patients are placed in a prone position. A fluoroscope is then positioned over the targeted side of blockade to obtain an oblique view 5-20° to the side to be blocked. This view exposes the ischial spine more clearly, avoiding the overlapping with the pelvic brim [54, 55]. Once the ischial spine is identified, a skin entry point on the buttock is marked at the tip of the ischial spine. After skin infiltration with local anesthetic is achieved, a spinal needle can be advanced, coaxial to the fluoroscopic beam, until it contacts the bony surface of the spine. At this juncture, a lateral fluoroscopic view can be obtained to confirm appropriate needle depth and contact with the spine. Once satisfied, 1 mL of contrast medium can be injected to confirm appropriate soft tissue spread [54, 55]. Once complete, injection of the chosen solution can take place.
Contrast spread patterns described include spread in an irregular or round pattern at the tip of the ischial spine. Additionally, spread can occur along the ipsilateral obturator internus muscle, sacrotuberous ligament, or sacrospinous ligament. Investigators have not described any particular correlation between pattern of spread and success of sensory blockade [54].
Ultrasound Guided
The use of an ultrasound-guided approach to block the pudendal nerve has been described in the literature [12, 14, 56, 57]. The use of ultrasound allows for the visualization of soft tissues, needle advancement, and live spread of injectate around the target structures. The target at the level of ischial spine is the interligamentous plane, which is defined by soft tissue, not bony, landmark.
This technique has been validated using sensory change as endpoint [56] and compared against the use of the fluoroscopic guided approach in a randomized trial [57]. The use of ultrasound has proven to be as accurate as the use of fluoroscopy, yet ultrasound guidance requires more procedure time.
Patients are placed in a prone position, and a curvilinear transducer with a low frequency (2–5 MHz) is required because of greater tissue depths. The transducer is first positioned over the ilium at the level of posterior superior iliac spine (PSIS). The ilium appears as a straight, hyperechoic line descending laterally (Figs. 56.8 and 56.9). As scanning continues caudally at the level of greater sciatic notch, the hyperechoic line of the ilium starts to regress from the medial aspect of the screen. The lateral aspect of the ultrasound screen transitions to a curved hyperechoic line revealing the posterior aspect of the acetabulum. At this point, two muscular layers can be identified: the gluteus maximus and the piriformis muscles.