Chapter 29 Performance of Rigid Bronchoscopy
I Historical Background
Rigid bronchoscopy is an invasive surgical technique that is used to visualize the oropharynx, larynx, vocal cords, trachea, and proximal pulmonary branches. The origins of rigid bronchoscopy, like many aspects of modern medicine, can be traced back to Hippocrates (460-370 BC), who advised introducing a pipe into the larynx in a suffocating patient to assess the airways.1 Because of limitations with lighting, the field was largely dormant until the 1800s, when incandescent light bulbs were invented. The development of local anesthesia in 1880 also made bronchoscopy more tolerable. In March of 1897, Gustave Killian passed an endoscope through the larynx and removed a piece of pork bone from the right main stem bronchus using cocaine anesthesia.1 Chevalier Jackson further advanced the field of rigid endoscopy by developing improved lighting, auxiliary instrumentation for removal of foreign objects, and rigid bronchoscopy training programs.2 He is recognized as the father of contemporary rigid endoscopy.3 Since Jackson’s time, many changes have been made to the bronchoscope, so that current rigid instrumentation and ventilation systems allow for more precise and accurate assessment of the aerodigestive tract.3 H. H. Hopkins of England invented the first conventional lens system by using glass rods instead of small lenses, which produced a significantly brighter image, occupied less space, and allowed for greater visualization of an object in a single field.1 This provided the basis for fiberoptic and modern rigid bronchoscopy.
II Indications and Contraindications
The tracheobronchial tree can be directly observed with either rigid or flexible bronchoscopy. Although the advent of flexible bronchoscopy has given physicians improved access to more distal portions of the tracheobronchial tree with a more rapid learning curve and less patient discomfort compared with rigid bronchoscopy,2 there are still several situations in which rigid bronchoscopy is more appropriate. The larger working port and rigid structure make rigid bronchoscopy useful for surgical interventions within the airway, such as the removal of foreign bodies or polyps (see “Foreign Body Removal”).4 At the same time, the bronchoscope can be used in establishing and maintaining airway control in patients with difficult ventilation, such as those with an acute airway obstruction or massive hemoptysis.4 The major advantage of the rigid bronchoscope compared to the flexible one is that it has a ventilation port that can be directly connected to the anesthesia ventilator, allowing for maintenance of an airway and external ventilation while airway procedures are performed. In fact, the latest practice guidelines for the management of a difficult airway (DA) include the use of rigid bronchoscopy because it “reduces airway-related adverse outcomes.”5
Other instances in which rigid bronchoscopy is indicated include deeper diagnostic biopsies when a fiberoptic specimen would be inadequate; dilatation of strictures; bronchial stenting; fracture reduction; application of laser therapy or cryotherapy; tumor removal; and diagnosis of vascular rings. Some of the indications for pediatric rigid bronchoscopy are listed in Box 29-1.
Box 29-1
Indications for Rigid Bronchoscopy
Foreign body evaluation and management
Interval evaluation after laryngotracheal reconstruction
Management of severe laryngotracheal infections
Assessment of toxic inhalation or aspiration
Evaluation of laryngeal pathology
Management of mass lesions of the airway, including recurrent respiratory papillomatosis
Adapted from Hartnick CJ, Cotton RT: Stridor and airway obstruction. In Bluestone CD, Stool DE, Alper CM, et al (eds): Pediatric otolaryngology, ed 4, Philadelphia, 2003, Elsevier.
There are few contraindications to rigid bronchoscopy. In practice, most of the factors limiting rigid bronchoscopy are related to the general anesthesia used, such as an unstable cardiovascular or respiratory status.6 One absolute contraindication to rigid bronchoscopy is an unstable cervical spine, because of the hyperextension of the head required during the procedure. In this instance, flexible bronchoscopy is indicated to avoid any further spinal injury during bronchoscopy. Other contraindications include laryngeal stenosis that prevents passage of the bronchoscope; limited range of motion of the mandible; severe kyphoscoliosis; uncontrolled coagulopathy; and extreme ventilatory/oxygenation demands.7,8
III Instrumentation
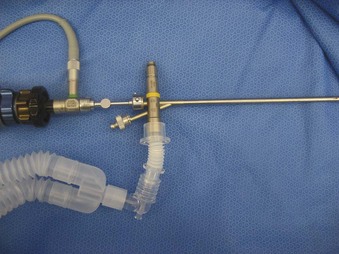
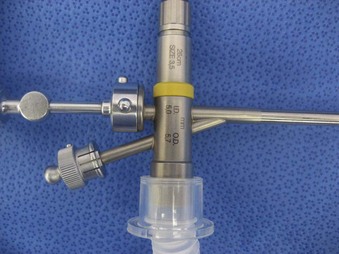
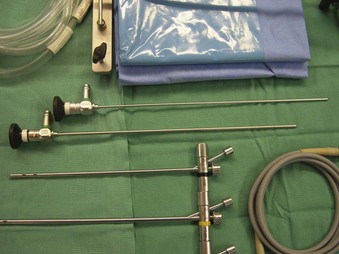
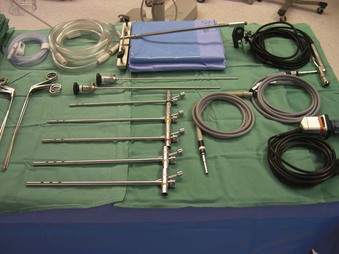
The Storz endoscope with the Hopkins rod-lens optical system, introduced in 1966, is the most widely used rigid bronchoscope and has largely replaced the Jackson and Holinger endoscopes.9 As described earlier, the rod-lens system provides better illumination, greater visualization, and angled views. The bronchoscope is a hollow, rigid metal tube that is tapered and beveled on the distal end and has a series of different-sized ports on the proximal end that serve different purposes. There are side holes at the end of the bronchoscope to allow for ventilation proximal to the tip.10 The beveled edge of the tip is used to facilitate introduction of the bronchoscope into the airway and for resection of tumors. The inferior port is usually the ventilating port connected to the anesthesia circuit. The superior port connects to the prismatic light deflector and the light source (Figs. 29-1 and 29-2). The main port is the working channel for aspiration and for insertion of instruments such as telescopes, biopsy forceps, laser fibers, balloon devices, cryotherapy probes, and stents. The port can be left open to allow room air into the system or it can be closed to prevent leakage of air or anesthetic gases, depending on the ventilation and type of anesthesia being used.
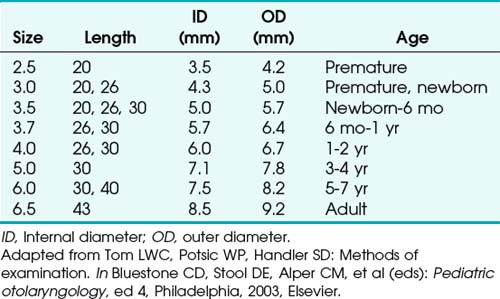
Bronchoscopes vary in size, in length, and in internal and external diameter. Table 29-1 depicts the different sizes of Storz bronchoscopes used by patient age, although this choice needs to be tailored to the individual patient. The size chosen should maximize the surgeon’s view while causing the least trauma to the airway.9 Telescopes with different angles (0, 30, and 70 degrees), lengths, and diameters can be inserted through the main working port to visualize areas that are difficult to see with the rigid bronchoscope alone, namely the right and left upper bronchial orifices and the right middle bronchial orifice.9 Illumination can be provided either through a light carrier rod that goes down a channel in older endoscopes, through the telescope, or by means of a port that attaches to a prismatic light deflector which subsequently attaches to a light source in newer endoscopes (Figs. 29-3 and 29-4). The last configuration has the advantage that it provides both proximal and distal illumination.
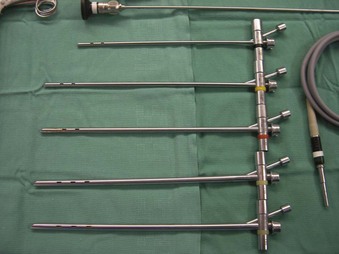
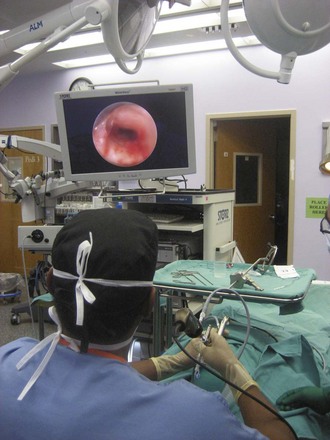
Rigid bronchoscopy should be performed either in an operating room (OR) or an endoscopy suite. As with all procedures that require general anesthesia, the room should be equipped with equipment for oxygen (O2) saturation monitoring, carbon dioxide (CO2) monitoring, blood pressure monitoring, and electrocardiography. A full set of ventilating bronchoscopes (see Table 29-1 for sizes) and a backup light source should be available. The standard adult size rigid bronchoscopy is 8 mm in diameter and 40 cm long. Other instruments such as graspers, biopsy forceps, optical forceps, and suction devices should also be available to the surgeon (Fig. 29-5). Optional items include a flexible bronchoscope that can be passed through the rigid bronchoscope for further examination of the lower tracheobronchial tree. Video capability is also desirable but not required (Fig. 29-6).
IV Preoperative Considerations
A thorough history should be taken to assess previous cardiac disease, such as a recent myocardial infarction or any documented dysrhythmias, or pulmonary disease (e.g., asthma) that may require bronchodilators before bronchoscopy.4 To minimize the risk of bleeding, the patient should have a normal platelet count, prothrombin time, and partial thromboplastin time. Unless a bleeding history is known, a preoperative coagulation workup is not necessary. Because patients with uremia have qualitative platelet abnormalities that can predispose to excessive bleeding, creatinine and blood urea nitrogen values should be relatively normal.4 Patients should also be questioned about aspirin or anticoagulant use and timing of the last intravenous dose. Additional laboratory studies and tests may be ordered depending on the history.
Pulmonary function tests are no longer indicated as routine preoperative assessment of respiratory disease but may be useful for determining the postoperative plan.11 For example, patients with a forced vital capacity (FVC) of less than 20 mL/kg is at an increased risk for pulmonary complications postoperatively and may benefit from extended postoperative monitoring.10 Also, patients with forced expiratory volumes of less than 50% are at risk for hypercarbia and hypoxia.12 The clinical predictors of cardiac complications perioperatively or postoperatively with major risk are unstable or severe angina, decompensated congestive heart failure, significant dysrhythmias, and severe valvular disease.13 Cardiac conditions should be evaluated and the medical therapies optimized before the surgery. Perhaps the simplest way to assess the patient’s overall cardiopulmonary status is to inquire about exercise tolerance.
Often, rigid bronchoscopy is performed emergently with limited preoperative evaluation or testing in order to secure an airway, and knowledge and assessment of risk factors for cardiopulmonary mortality, difficult intubation (DI), and difficult mask ventilation (DMV) may be of importance for perioperative and postoperative management. DI may be predicted by weight, head and neck movement, jaw movement, receding mandible, “buck” teeth, Mallampati classification, thyromental distance, sternomental distance, and a history of DI.5,14,15 There are five independent risk factors for DMV: age greater than 55 years, body mass index greater than 26 kg/m2, presence of a beard, lack of teeth, and a history of snoring.14 A careful evaluation of the patient’s jaw and neck mobility should be made, because severe cervical spine disease is a contraindication to rigid bronchoscopy.
V Anesthesia for Bronchoscopy
Traditionally in the pediatric population, inhalational anesthesia with spontaneous respiration has been used during rigid bronchoscopy, often with switching to a total intravenous anesthetic technique after induction. Disadvantages of inhalational anesthesia with spontaneous respiration include difficulty in maintaining an adequate plane of anesthetic depth and the anesthetic pollution of the surgical environment from a gas leak around the bronchoscope.16 Malherbe and colleagues proposed total intravenous anesthesia with propofol and remifentanil and spontaneous respiration as a reasonable alternative to deep inhalational anesthesia that carries no major complications.16 In the spontaneous respiration technique, the patient is not given an NMB agent and is allowed to breathe spontaneously. The ventilating port of the bronchoscope is attached to the anesthesia circuit with 100% O2 flow (see Fig. 29-1). The advantage of spontaneous ventilation is that it allows for continuous ventilation during the procedure; the main disadvantage is that the depth of anesthesia required for the procedure itself may suppress both cardiac output and the respiratory drive, making spontaneous respirations difficult.17 Another potential disadvantage is that the instrumentation required for the procedure itself provides increased airway resistance and may reduce the efficacy of spontaneous respirations.18
When PPV is used, it is typically achieved by first relaxing the patient with an NMB agent and subsequently connecting the ventilating port of the bronchoscope to the anesthesia circuit. Positive pressure is administered by squeezing the bag or by using the anesthesia ventilator, forcing gas pressure through the rigid bronchoscope.19 Advantages include the prevention of atelectasis, improved oxygenation, and the ability to overcome the increased airway resistance caused by the instrumentation within the rigid bronchoscope.18 A disadvantage to PPV is that a proximal eyepiece must be in place, which hinders the use of suction or other instrumentation during ventilation.20 There is also usually some gas leak during PPV due to the absence of a tight seal, decreasing efficiency. Finally, the size of the bronchoscope and the presence of a telescope in the lumen increases resistance to airflow and increases dead space ventilation, making ventilation more difficult.
In apneic oxygenation, there are periods of time when the anesthesiologist withholds ventilation during which the surgeon works. The patient is first hyperventilated to produce a profound hypocarbia. Under direct visualization, a catheter is then passed to the carina, and the flow rate of O2 is set. The period of time in which the surgeon can work is determined by the rise in CO2, after which the patient is allowed an equivalent amount of time during the next ventilation cycle to return to baseline. The partial pressure of CO2 (PCO2) is expected to rise continuously at a rate of 3 mm Hg per minute.10 This technique is risky when it is used in patients who have a history of CO2 retention (e.g., COPD), and intraprocedural awareness is also reported more frequently with this technique.
Jet ventilation is usually used during laser procedures, and it is provided by use of a jet-ventilator with a jet injection cannula (JIC), also known as a Saunders injector.21 This technique is used to overcome the hypoventilation caused by air leaks during PPV.22 The cannula has three lumens, two of which open proximally and deliver the jet ventilation; a distal lumen measures airway pressure.21 The JIC is 3 mm in diameter, can have various lengths, and is made of stainless steel and therefore has a lower risk of endotracheal ignition.21,22 The JIC is usually introduced distal to the lesion being accessed to provide optimal ventilation. High-pressure O2 (50 psi) is delivered to the airway at high velocities, creating a negative pressure and causing a Venturi effect.16 The negative pressure caused by the high velocity of air entering the airways induces a column of air from outside the injector to flow into the airways and expand the lungs. Exhalation is passive and relies on the collapse of the airway once the pressure is removed. Sufficient time must be allotted for air egress during this technique to prevent stacked breaths and progressive hyperinflation. This method is contraindicated during foreign body removal, because the high-flow air may dislodge the foreign body and cause a complete obstruction.18 Of note, jet ventilation does not prevent acidemia or hypercarbia and may increase the risk of air embolism, pneumothorax, and particle dissemination into the lungs and distal airways.20 Also, in patients with poor lung compliance, ventilation may be inadequate with this technique.10
NPV, compared with spontaneous assisted ventilation, reduces the administration of opioids, shortens recovery time from anesthesia, and prevents hypercarbia.20 It can be accomplished by a poncho-wrap connected to a negative-pressure ventilator, with a 2-L/min O2 flow, negative pressure of −25 hectopascals (hPa), a respiratory rate of 15 breaths/min, and an inspiration/expiration ratio of 1 : 1. NPV may be safer in patients with poor tolerance to hypercarbia, such as patients with myocardial ischemia.20
Intravenous and topical medications can be used provided the patient’s history reveals no prior adverse reactions or if the potential benefit outweighs the expected risks. Opioids are often used for analgesia, for sedation, and to decrease the cough reflex. However, because they depress the respiratory drive, they should be withheld from patients who have signs of airway obstruction or a foreign body. A recent study by Yoon and colleagues comparing propofol sedation to a combination of both propofol and alfentanil showed that the propofol-only group had a higher average oxygen saturation as measured by pulse oximetry (SpO2; 97.8 ± 1.6 versus 96.4 ± 1.1, P < 0.01). The degree of cough (measured using a 100 mm visual analogue scale), however, was not different between the groups (73.4 ± 22.7 versus 72.2 ± 18.5, respectively). This may be partially attributed to the intrinsic antitussive properties of propofol. Therefore, narcotics may not be required if the patient is being induced with propofol.23
Anticholinergics are often used to reduce airway secretions and have been shown to enhance the absorption and prolong the analgesic action of topical analgesics such as lidocaine which are administered to the airways to reduce reactivity, prevent bronchospasm, and dampen the systemic reaction to airway manipulation.24 Intravenous anticholinergics are also commonly used to prevent the parasympathetic-mediated bradycardia and hypotension that can occur during rigid bronchoscopy.11 Their routine use remains debated.25 A steroid such as dexamethasone can be used to decrease intraoperative and postoperative edema that can result from airway instrumentation.
For emergence from anesthesia, all anesthetic gases are turned off, NMB agents are reversed, and the patient is ventilated until spontaneous respirations resume. Topical lidocaine may again be administered to prevent laryngospasm and diminish coughing.12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree