Chapter 30 Percutaneous Dilational Cricothyrotomy and Tracheostomy
I. Definitions and Classifications of Percutaneous Cricothyrotomy and Tracheostomy
IV. Indications and Contraindications for Percutaneous Dilational Cricothyrotomy and Tracheostomy
V. Percutaneous Dilational Cricothyrotomy
VI. Percutaneous Dilational Tracheostomy
VII. Postoperative Considerations
IX. Miscellaneous Considerations
I Definitions and Classifications of Percutaneous Cricothyrotomy and Tracheostomy
A Cricothyrotomy and Percutaneous Dilational Cricothyrotomy
Cricothyrotomy is a technique for providing an opening in the space between the anterior inferior border of the thyroid cartilage and the anterior superior border of the cricoid cartilage for the purpose of gaining access to the airway. This area is considered to be the most accessible part of the respiratory tree below the glottis.1–6
Cricothyrotomy can be classified in several ways. Based on the urgency of the clinical situation, the procedure has been classified as emergent or elective. Emergent cricothyrotomy may be done in the prehospital setting, emergency room, intensive care unit (ICU), or operating room. Elective cricothyrotomy is usually done before surgery in the operating room. It also may be performed in critically ill patients in the ICU at the bedside.7 Depending on the technique used, the procedure may also be classified as nonsurgical or surgical. The nonsurgical approach can be achieved by needle puncture or percutaneously over a guidewire, with or without a cricothyroid membrane (CTM) incision.8
A practical and clinical classification of cricothyrotomy techniques includes three categories. The first category includes techniques that use a needle or over-the-needle catheter placed directly into the cricothyroid space. The needle technique is used for transtracheal catheter ventilation or, more properly, transcricoid ventilation.9 The cricothyrotomy needle (Fig. 30-1) and the Ravussin cannula (Fig. 30-2) are examples of these devices. Transtracheal catheter ventilation cannulas are also available, but they are inserted as described for the second category (Fig. 30-3).
The third category is surgical cricothyrotomy, which involves the use of a scalpel and other surgical instruments to create an opening between the skin and the cricothyroid space. It is discussed in Chapter 31.
B Tracheostomy and Percutaneous Dilational Tracheostomy
1 Open Tracheostomy
Surgical tracheostomy, as described by Chevalier Jackson,10 is a surgical procedure that provides an airway through the cervical trachea. It remains the standard against which all other procedures with the same aim must be compared in terms of success and complications rates. Classically, the procedure is performed in the operating room under general or local anesthesia, as dictated by the clinical situation. An open tracheostomy may be performed at the bedside in the ICU or in the emergency room in urgent situations. After an initial skin incision, sharp dissection is carried out to the thyroid isthmus, which is divided. The cervical trachea is then incised and a tracheostomy tube inserted.
2 Percutaneous Tracheostomy
Percutaneous tracheostomy is performed by means of a skin puncture into the trachea that is subsequently dilated to form a stoma, rather than creating a stoma by surgical incision.11 Although there are many techniques for performing a percutaneous tracheostomy, the initial part of the procedure always involves a puncture through the skin into the trachea, which is then enlarged by dilation or with forceps to spread the puncture to a size that allows placement of an appropriate tracheostomy tube. These techniques are typically used in patients with an established airway (i.e., endotracheal tube [ETT] or laryngeal mask airway [LMA]) and are mostly used for intubated ICU patients.
Emergency situations were traditionally considered absolute contraindications to the use of this technique, but in the past few years, some reports have supported its safety and feasibility in selected emergent cases.12,13 The Ciaglia technique, first described in 1985, is the original technique now described as percutaneous dilational tracheostomy (PDT). It involves making a very small skin incision, introducing a needle into the trachea, and dilating the opening with sequentially larger dilators to allow insertion of a tracheostomy tube of the selected size. As originally described, this procedure was performed blind, but it is increasingly performed under continuous endoscopic guidance.
3 Percutaneous Dilational Cricothyrotomy and Tracheostomy in Airway Control
a the Problem of Airway Control
Adverse outcomes related to respiratory events account for one of the two largest classes of injury in the American Society of Anesthesiologists (ASA) Closed Claims Project. As reported by Caplan and colleagues and Cheney and associates, the two major categories of anesthesia-related events or mechanisms causing death or brain damage between 1975 and 2000 were respiratory and cardiovascular difficulties, which together made up 68% of damaging events.14,15 Three mechanisms of injury were responsible for most of the adverse respiratory events: difficult ETT placement (23%), inadequate ventilation (22%), and esophageal intubation (13%).
In an analysis of claims against the National Health System in England between 1995 and 2007,16 airway and respiratory claims accounted for 12% of anesthesia-related claims, 53% of deaths, 27% of cost, and 10 of the 50 most expensive claims in the dataset. These claims most frequently described events at induction of anesthesia, involved airway management with a tracheal tube, and typically led to hypoxia and the patient’s death or brain injury.
In the operating room, in the ICU (or in other hospital areas), and in the prehospital setting, three difficult scenarios have been repeatedly observed during attempts to control the airway: (1) the airway can be easily controlled by mask ventilation, but endotracheal intubation is not possible; (2) the airway cannot be mask ventilated but can be intubated; and (3) rarely, the airway cannot be mask ventilated or intubated. It is every anesthesiologist’s nightmare to encounter a true difficult airway as depicted in the third scenario.17 Five to 35 of 10,000 patients (0.05% to 0.35%) reportedly cannot be endotracheally intubated, and approximately 0.01 to 2.0 of 10,000 patients are difficult to mask ventilate and intubate.18,19
b Roles of the Anesthesiologist, Otolaryngologist, and Emergency Medicine Physician
The anesthesiologist may be called immediately or after other physicians have attempted unsuccessfully to secure the airway or failed to recognize the futility of standard intubation techniques. In rare instances, the availability of a physician skilled in the technique of cricothyrotomy or percutaneous tracheostomy may be life-saving. This individual should be the anesthesiologist. Appropriate equipment for cricothyrotomy should be available throughout the hospital or as part of an emergency airway kit. No data exist regarding how frequently anesthesiologists are called to secure an airway outside the operating room, but in many hospitals, this responsibility seems to be handled more frequently by other physicians.20–23
Each institution should have a clear plan for alerting qualified individuals when emergency airway support is required in different areas of the hospital.24 Many publications describe the use of various types of advanced airway equipment, report the availability of such devices, and explain the use of simulators to teach difficult airway management skills, and many articles identify the need to educate residents in advanced airway techniques.
Organizing resources and staff to manage a difficult airway and maintaining appropriate training are important for patient safety and clinical quality.25,26 In 2000, Showan and Sestito proposed that the components of a successful airway management system include personnel, training, an emergency response system, an oversight process, standardized equipment, and patient education. As with any type of emergency, preparedness is the key when planning for response.27–30
In 1996, a comprehensive airway program was introduced at Johns Hopkins.26 The core components of the comprehensive difficult airway program were communication and electronic medical record information (including airway documentation), equipment, personnel, and education. Investigators based their implementation on the causes that required a surgical airway and the inability of an anesthesiologist to intubate and ventilate. The causes were an inability to access the written medical record, resulting in a lack of preoperative information about the patient’s airway; lack of immediate access to equipment and supplies necessary to manage a difficult airway; and lack of availability of trained personnel to help manage and secure the airway.
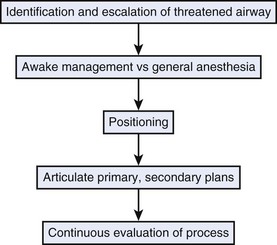
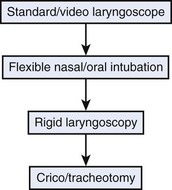
A threatened airway protocol has been proposed for implementing an escalation-based model at The University of Texas Medical School at Houston.31 The model is based on seven general principles to guide physicians in the identification and management of situations in which hospitalized patients may have rapid deterioration of a condition affecting the upper airway that requires immediate intervention to maintain or reestablish ventilation and oxygenation. These seven principles are concerned with appropriate communication among providers, maintenance of oxygenation, avoidance of sedation until the patient is in a safe environment, complete airway assessment, maintenance of spontaneous ventilation as long as possible, and avoidance of rapid-sequence induction (i.e., administration of a muscle relaxant without a prior attempt to ventilate), unless an easy airway and a full stomach are expected. Four main features constitute the cornerstones of management of a patient with a threatened airway: identification of the airway emergency and escalation of the approach to management, choice of appropriate sedation or anesthesia technique, positioning, and articulation of plans for intervention. A progressive algorithm (Fig. 30-4) that guides the progression of the necessary steps has been inspired by others’ work.31,32 The use of specific airway devices or tools is mandated in the primary and secondary plans by the success in securing the airway or depending on changes in airway viability (Fig. 30-5).
The otolaryngologist plays a critical role in airway management by contributing a skill set that is different from but complementary to that of the anesthesiologist. Circumstances may range from well-controlled elective situations to near-panic, last-ditch attempts to establish an airway when all else has failed.33 The otolaryngologist possesses an excellent knowledge of the three-dimensional anatomy of the upper aerodigestive tract and the variations encountered in pathologic circumstances. This knowledge and expert endoscopy skills can assist the anesthesiologist in determining a difficult airway.
II Historical Perspective
In 1909, Chevalier Jackson,10 a laryngologist at the Jefferson Medical School in Philadelphia, described the technical details of surgical tracheostomy and standardized the procedure. Jackson’s technique was for years considered the preferred method of surgical airway management. Jackson later published the results of 30 years’ observation of his own tracheotomized patients and reported a very high incidence of laryngeal and subglottic stenosis in patients who underwent a procedure that he referred to as high tracheostomy, involving division of the cricoid or thyroid cartilage.34 As a consequence, the high tracheostomy technique was abandoned for many decades.
In 1969, Toye and Weinstein described a technique for percutaneous tracheostomy based on the premise that a functional tracheal airway could be more rapidly and safely achieved percutaneously than with Jackson’s method of surgical dissection.35 The technique involved inserting a needle into the trachea and dilating the resultant needle tract to allow placement of a breathing catheter.
Cricothyrotomy, which differed from high tracheostomy because it involved opening of the CTM instead of dissection of the cricoid cartilage, was proposed again in 1976 by two Denver cardiothoracic surgeons, Brantigan and Grow. They published the results of 655 consecutive cricothyrotomies in which there were minimal complications and no reported incidence of subglottic stenosis. Subsequently, other clinical and experimental series have been reported, and cricothyrotomy has become generally accepted. The procedure was found to be faster, simpler, less invasive, and less likely to cause bleeding than tracheostomy. It is associated with lower morbidity and mortality rates than emergency tracheostomy, making it desirable as an emergency technique for gaining immediate airway control. Various modifications of the original technique have been developed. The use of the Seldinger technique for insertion, as described by Corke and Cranswick in 1988, enhances the safety of the procedure.8
The Ciaglia technique, first described in 1985, is the original technique now described as PDT. In this technique, insertion of the guidewire is followed by serial dilations performed with multiple, progressively larger dilators.36 The Rapitrach method, proposed in 1989 by Schachner and coworkers, entails the use of dilating forceps and a single-step dilating technique.37
In 1990, Griggs and colleagues presented the guidewire dilating forceps method, which was similar to the Rapitrach method and based on a one-step dilating techinque38 that used a modified forceps. In 1997, Fantoni proposed the translaryngeal tracheostomy technique based on retrograde dilation of an initial tracheal puncture by means of a conic cannula inserted through the oral cavity.39
The Ciaglia Blue Rhino, a modified version of the Ciaglia technique, was introduced by Cook Medical (Bloomington, IN) in 2000.40 In this technique, the series of sequentially larger dilators of the original Ciaglia technique is replaced by a single, curved dilator with a hydrophilic coating, the Blue Rhino, that progressively dilates the stoma in one step.
In 2002, Frova and Quintel described the Percutwist tracheostomy technique. A “rotating dilation” is performed in a single step by means of a screwlike, rotating device.41 In 2008, a further development of the Ciaglia technique was presented by Cook Medical: the Ciaglia Blue Dolphin balloon percutaneous tracheostomy introducer. This device combines balloon dilation and tracheal tube insertion into one step.
Many of the techniques proposed for PDC and PDT are performed over guidewires. Although anesthesiologists are familiar with the Seldinger technique for the insertion of vascular catheters, many are unacquainted with airway management techniques that use airway devices based on the same technology and concept.27,42
III Anatomy and Physiology
Safe and rapid performance of cricothyrotomy requires a thorough knowledge of cricothyroid space anatomy (Fig. 30-6) and its relation to other structures in the neck.1,6,43–47 The CTM ligament is 10 mm long and 22 mm wide and is composed mostly of yellow elastic tissue. It covers the cricothyroid space and is located in the anterior neck between the thyroid cartilage superiorly and the cricoid cartilage inferiorly. The cricothyroid space can be readily identified by palpating a slight dip or indentation in the skin immediately below the laryngeal prominence.
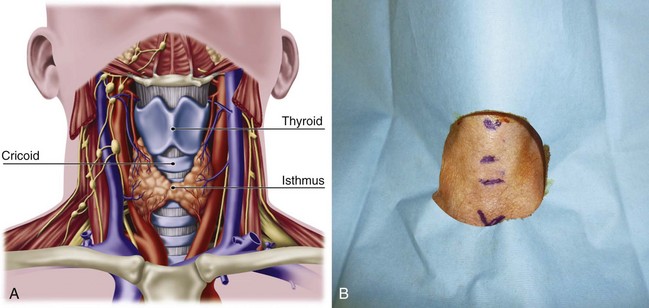
Figure 30-6 A, Dissection anatomy. B, External landmarks.
(A, From De Leyn P, Bedert L, Delcroix M: Tracheotomy: Clinical review and guidelines. Eur J Cardiothorac Surg 32:412–421, 2007.)
The CTM consists of a central anterior triangular portion (i.e., conus elasticus) and two lateral parts. The thicker and stronger conus elasticus narrows above and broadens below, connecting the thyroid to the cricoid cartilage. It lies subcutaneously in the midline and is often crossed horizontally in its upper third by the superior cricothyroid vessels. To minimize the possibility of bleeding, the CTM should be incised at its inferior-third portion. The two lateral parts are thinner, lie close to the laryngeal mucosa, and extend from the superior border of the cricoid cartilage to the inferior margin of the true vocal cords. On either side, the CTM is bordered by the cricothyroid muscle. Lateral to the membrane are venous tributaries from the inferior thyroid and anterior jugular veins. Because the vocal cords usually lie 1 cm above the cricothyroid space, they are not commonly injured, even during emergency cricothyrotomy.48 The anterior jugular veins run vertically in the lateral aspect of the neck and are rarely injured, but tributaries may occasionally course over the cricothyroid space and be damaged during the procedure. Characteristically, the CTM does not calcify with age and lies immediately underneath the skin.
Conscious effort to identify these landmarks reduces the possibility of committing this preventable error (see Fig. 30-6B). When the normal anatomy is distorted, identification of these landmarks is difficult. In these cases, the suprasternal notch may be used as an alternative marker. The small finger of the right hand should be placed in the patient’s suprasternal notch, followed by placement of the ring, long, and index fingers adjacent to each other in a stepwise fashion up the neck, with each finger touching the one below it. When the head is in the neutral position, the index finger is usually on or near the CTM.
IV Indications and Contraindications for Percutaneous Dilational Cricothyrotomy and Tracheostomy
A Cricothyrotomy
Cricothyrotomy is considered by many to be the standard approach to airway management when orotracheal or nasotracheal intubation and fiberoptic approaches have failed.5,18,49 In the emergency room or prehospital setting,50,51 cricothyrotomy is indicated for immediate airway control in patients with maxillofacial, cervical spine, head, neck, and multiple trauma and in patients in whom endotracheal intubation is impossible to perform or contraindicated. It is also used for the immediate relief of upper airway obstruction. In the operating room and in the ICU, the technique is indicated when conventional methods of intubation fail, such as in patients with traumatic facial injuries in whom other techniques of airway access are difficult or impossible to perform. Cricothyrotomy can also be used as an alternative to tracheostomy in patients with recent sternotomy who need airway access because the incision does not communicate with the mediastinal tissue planes. A needle-size cricothyrotomy with a Luer-Lok connection (for jet ventilation) or an anesthesia circuit–size connection is used for thoracic and other procedures involving the airways, especially the trachea, larynx, epiglottis, and base of the tongue.
Emergency cricothyrotomy has largely replaced emergency tracheostomy in the emergency department because of its simplicity, rapidity, and minimal morbidity, and percutaneous techniques are replacing surgical approaches.52,53 Use of emergency tracheostomy is limited and indicated only when laryngeal trauma may be accompanied by local edema, hemorrhage, subcutaneous emphysema, and damage to the thyroid or cricothyroid cartilage, precluding the performance of cricothyrotomy.
Cricothyrotomy is technically problematic to perform in the pediatric population and should be performed with extreme caution in children younger than 10 years. It should not be performed at all in children younger than 6 years unless a wire can be placed in the cricothyroid space and placement within the trachea can be verified.54 Emergency tracheostomy under controlled conditions is the preferred choice.55 Physicians who are unfamiliar or inexperienced with the technique are discouraged from performing the procedure without adequate supervision from a more senior or knowledgeable member of the medical team. Inexperience has been implicated as the most important factor contributing to cricothyroid complications.56–58 Accuracy in identifying anatomic landmarks significantly depends on the physician’s experience but is poor overall, justifying the percutaneous technique in emergency conditions but supporting the use of ultrasound or video-enhanced visualization during elective procedures.
B Percutaneous Dilational Tracheostomy
PDT is mainly indicated in adult intubated patients (Box 30-1). In this patient population, the main indications for performing a PDT are the same as those for surgical tracheostomy:
• Preventing upper airway damage due to prolonged intubation
• Facilitating pulmonary toilet
• Providing a stable airway in patients requiring long-term mechanical ventilation and oxygen support
Box 30-1 Indications and Contraindications to Percutaneous Tracheostomy in Intubated Adults in the Intensive Care Unit
Several benefits of performing a tracheostomy in patients who require prolonged ventilation have been postulated and are supported by different levels of evidence.59 Shorter ICU and hospital stays and less need for sedation are the most widely recognized benefits, whereas improved patient comfort, decreased work of breathing, improved oral hygiene, better long-term laryngeal function, faster weaning from mechanical ventilation, lower risk of ventilator-associated pneumonia, and lower mortality rates have also been reported but are supported by a lower level of evidence.59
For the population of critically ill adult patients, PDT has been recommended as the procedure of choice for performing elective tracheostomy.59,60 PDT is recommended on the basis of a lower risk of wound infection, being able to perform it at the bedside rather than transferring critically ill patients to the operating room, and better cost-effectiveness compared with surgical tracheostomy.60
Upper airway obstruction due to tumor, edema, infection, stenosis, or trauma represents the other major category of indications for tracheostomy. However, the overall safety of performing PDT in emergent situations and with unprotected airways is extremely controversial, and, in these conditions, the procedure should be reserved for selected patients and performed only by experienced providers.13 Anatomic suitability for this procedure must be determined preoperatively with the patient’s neck extended. Maximum neck extension increases the length of the cervical trachea and defines critical anatomic landmarks, such as the cricoid cartilage and sternal notch. A contraindication to the procedure is the inability to palpate the cricoid cartilage above the sternal notch. Similarly, the patient with a midline neck mass, high innominate artery, or large thyroid gland should undergo open surgical tracheostomy in the operating room. Coagulopathies should be corrected preoperatively. Ideally, the functional platelet count should be 50,000 or greater, and the international normalized ratio (INR) should be corrected to 1.5 or less. However, there have been reports of PDT safely performed in patients with severe thrombocytopenia.61
Patients requiring a positive end-expiratory pressure (PEEP) of 15 cm H2O or higher are at high risk for complications such as subcutaneous emphysema and pneumothorax, and when possible, the procedure should be postponed for these patients. PDT is relatively contraindicated in nonintubated patients with acute airway compromise and in the pediatric population. For airway compromise in nonintubated patients, the risks are related to the length of the procedure and the inability to perform the procedure under direct endoscopic visualization without an ETT. Reasons to avoid PDT in children include the different airway anatomy and dimensions and the technical difficulties of maintaining adequate ventilation with a bronchoscope within a small ETT. Selected cases may present an exception to these contraindications, depending on the experience of the providers.13
V Percutaneous Dilational Cricothyrotomy
A Principles and Planning
This chapter focuses on percutaneous dilational techniques. Surgical cricothyrotomy and transtracheal catheter ventilation are discussed elsewhere in Chapter 31.
PDC is fast and usually easy to perform, even on patients with short necks or with spinal injury. Cricothyrotomy may be performed for elective airway management in trauma patients with technically challenging neck anatomy in lieu of tracheostomy, because it does not require a surgeon’s skill to gain airway access and has fewer operative and postoperative complications.62–65 Several commercially available devices use this technology. These devices have in common the insertion of an airway catheter over a dilator, which is usually introduced over a guidewire. The guidewire is inserted through a needle or over-the-needle catheter (i.e., Seldinger technique) after making an initial skin incision. This technique, often used for the insertion of catheter introducer sheaths and central lines, is familiar to anesthesiologists. An airway over a dilator and guidewire is preferable because of the inherent safety of this technique and the ability to insert an airway of far greater diameter than the initial catheter.
The over-the-wire technique offers several advantages. Even if the over-the-needle or direct dilational technique, such as the Quicktrach, PCK, or the Melker military version, may be faster to perform, the reported difficulties and complications are greater. This is also true for the Nu-Trake device. Complications have included failure to gain airway access, multiple attempts at cannulation, mediastinal injury, pneumothorax, and severe bleeding. The wire-guided technique has the disadvantage of the wire kinking. Several clinical and cadaver-based studies have established the safety and efficacy of the percutaneous over-the-needle or -cannula, wire-guided technique.42,66–71 For some of the devices, their use, diffusion, and success seem to have been influenced by local availability, original country of manufacturing, preliminary animal studies, and marketing, despite scarce clinical evidence of efficacy.
B Insertion Techniques
1 Percutaneous Dilational Cricothyrotomy Device
The PDC device manufactured by Melker contains a scalpel blade; a syringe with an 18-G over-the-needle catheter or a thin-walled introducer needle, or both; a guidewire; a dilator of appropriate length and diameter; and a polyvinyl airway catheter with or without a cuff (Fig. 30-7). A universal kit combines open cricothyrotomy and percutaneous tools in a single tray. (Although it defeats the concept of a percutaneous approach, it may be useful in remote or austere locations.) Detailed insertion instructions for this type of device are available from the manufacturer’s Website, brochure, and CD. A description of the Melker insertion technique (Fig. 30-8) follows:
1. Position the patient supine, and if there is no contraindication, slightly extend the neck by using a roll under the neck or shoulders. If cervical spine injury is suspected, properly immobilize the head and neck, and maintain a neutral position.
2. Open the prepackaged cricothyrotomy set, and assemble the components. Whenever possible and appropriate, use aseptic technique and local anesthetic.
3. Identify the CTM between the cricoid and thyroid cartilages.
4. Carefully palpate the CTM, and while stabilizing the cartilage, make a vertical or horizontal skin incision using the scalpel blade (can also be performed after the Seldinger technique). Make a stab incision (vertical or horizontal) through the lower third of the CTM. An adequate incision eases introduction of the dilator and airway, but the incision can follow the placement of the guidewire.
5. Attach the supplied syringe to the 18-G introducer needle–plastic catheter (over the needle technique) system (same that you would use to place an angio-catheter), or alternatively attach the syringe to the introducer needle only (having removed the plastic catheter) if you prefer or are concerned the plastic catheter may kink. Insert the syringe-needle-catheter or syringe-needle only, and advance it through the incision into the airway at a 45-degree angle to the frontal plane in the midline in a caudad direction. When advancing the needle forward, entrance into the airway can be confirmed by aspiration with the syringe resulting in free air return or air bubbles in a saline-filled syringe.
6. Remove the syringe and needle, leaving the plastic catheter or introducer needle in place. Do not attempt to advance the plastic catheter completely into the airway, which may result in kinking of the catheter and an inability to pass the guidewire. Advance the soft, flexible end of the guidewire through the catheter or needle and several centimeters into the airway.
7. Remove the plastic catheter or needle, leaving the guidewire in place.
8. Advance the handled dilator inside the airway catheter (single dilation if a preincision was made), tapered end first, into the connector end of the airway catheter until the handle stops against the connector. With other sets, insert the dilator to the recommended depth, or insert the dilator over the guidewire for a preinsertion dilation (recommended if a preincision was not made). Use of lubrication on the surface of the dilator may enhance the fit and placement of the emergency airway catheter.
9. Advance the emergency airway access assembly over the guidewire until the proximal stiff end of the guidewire is completely through and visible at the handle end of the dilator. Always visualize the proximal end of the guidewire during the airway insertion procedure to prevent its inadvertent loss into the trachea. Maintaining the guidewire position, advance the emergency airway access assembly over the guidewire with an in-and-out motion.
10. As the airway catheter is fully advanced into the trachea, remove the guidewire and dilator simultaneously.
11. If a cuffed tube is inserted, inflate it with 10 mL of air with the syringe provided.
12. Fix the emergency airway catheter in place with the cloth tracheostomy tape strip in a standard fashion.
13. Using its standard 15- to 22-mm adapter, connect the emergency airway catheter to an appropriate ventilatory device.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree