CHAPTER 14

PAIN MANAGEMENT
Pain is a normal response to tissue injury or stimuli that can result in injury if sustained. This response results in neurochemical reactions that produce the perception of pain. In addition, there are humoral, sympathetic, and metabolic sequelae to pain. When pain is not controlled or poorly controlled, these humoral, sympathetic, and metabolic sequelae may be pathologic and produce serious side effects. In addition, without the development of adequate and powerful pain relievers, performing any major surgical procedure would be impossible, as successful surgical treatment requires that the pain produced by the procedure be adequately controlled.
Fredrich Wilhein Adam Sertürner isolated the active component of opium in 1806. He named this new constituent morphine after Morpheus, the Greek god of dreams. This discovery, among others, has allowed complex surgery to be developed. In 1884, cocaine was recognized as an effective local anesthetic. This allowed surgeons to perform surgery without producing substantial pain and obviated the need for systematic analgesics. As surgical complexity increased, the management of the resultant pain has continued to be a difficult problem to adequately resolve. In addition, postoperative pain is not the only form of acute pain that needs to be relieved. Pain from trauma, burns, and medical conditions like pancreatitis must also be managed.
From the 1950s until the late 1980s, pain management consisted of intramuscularly administered opioids on a fixed or pro re nata (prn) schedule.1 This approach was used for its familiarity and safety and did not require specialized training or personnel. Additionally, the gradual onset of action of intramuscularly administered medication allowed time to monitor for side effects. Although arguably fairly safe, we now recognize that this approach is not efficacious. Intramuscular analgesia results in wide variability in medication levels and therefore fluctuations in pain levels (Fig. 14.1). Advances in perioperative care have transformed many procedures from mandating prolonged hospital care to requiring only abbreviated hospitalization or allowing for discharge on the day of the procedure. The management of postoperative pain under these circumstances can be even more challenging. Thus, it is important that the acute care surgeon is knowledgeable about the modalities and medications available to help control acute pain.
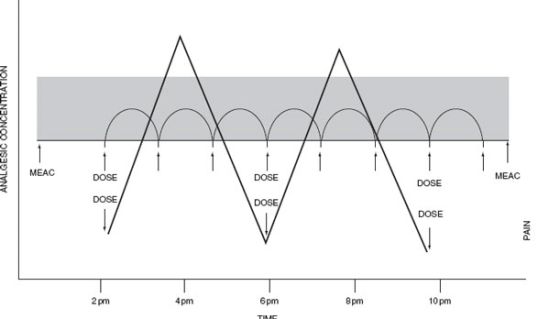
FIGURE 14.1. PCA paradigm. The relationship between plasma opioid concentration (ordinate), dosing interval (abscissa), and analgesic effect (Z axis), defining therapeutic effectiveness.
EPIDEMIOLOGY AND SCOPE
More than 73 million operative procedures are performed annually in the United States. Perhaps not surprisingly, 75% of patients report experiencing pain postoperatively, with 80% of those rating it as moderate or severe.2 Pain management has become the rallying cry of insurers and governmental regulatory organizations. The American Pain Society urges health professionals to consider pain the fifth vital sign. The Joint Commission on Accreditation of Healthcare Organizations in 2000 declared pain management a patient right, as well as an education and training issue. They emphasized quantitative aspects of pain and encouraged systematic assessment and safe management3 (Table 14.1). The United States Congress even declared 2001–2010 the “Decade of Pain Control and Research.” Despite this, a 2003 survey of postoperative patients found that 70% of patients reported having experienced moderate or severe to extreme pain at some time during their postoperative period.4
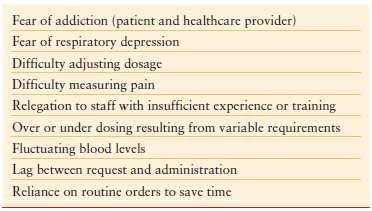
Increased attention is now being focused on assessing and controlling perioperative pain. An analysis of reasons for readmission from same day surgery demonstrated that pain was the most frequent cause (36%).5 There are a myriad of factors that lead to inadequate pain control6 (Table 14.2). One of the most important factors in the difficulty of managing pain is related to our ability to quantify it. Pain is highly subjective. Individual variation regarding tolerance to noxious stimuli is substantial. At the same time, if we are to be successful in managing pain, it is important to have a mechanism to quantify pain and to assess the efficacy of treatment of pain.
TABLE 14.2
FACTORS CONTRIBUTING TO INADEQUATE PAIN MANAGEMENT
Many physicians and nurses who care for patients in pain on a daily basis misjudge the intensity of their patients’ pain and the efficacy of medications given for treatment of that pain. Use of autonomic and behavioral responses by the clinician to determine pain intensity and to judge response to medication is error prone.7 For instance, tachycardia and hyperventilation may reflect anxiety or even the anticipation of pain. There are cultural and individual differences in the expression of pain. Some people remain impassive during even the most excruciatingly painful experiences, while others are loudly vocal and demonstrative of relatively minor pain. A nonrandomized study of patients in the emergency department of a tertiary care teaching hospital demonstrated the inconsistency between patient and caregiver perception of acute pain. In this study, caregivers consistently rated patients’ pain lower than the patients’ own rating.8
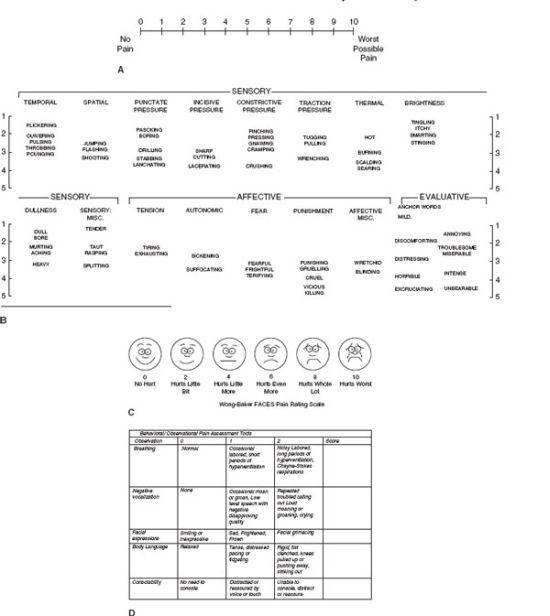
For this reason, the best estimation of a patient’s pain and its response to treatment is the patient’s perception. Several pain scales have been developed to standardize pain measurement. Visual analog scales are useful assessment tools.9 They are simple, sensitive, and reproducible instruments that allow the patient to give a numerical score to the severity of the pain they are experiencing (Fig. 14.2A). Use of such scales allows the nurse and/or physician to assess the efficacy of intervention by comparison of scores before and after intervention. If such scales are to be used, it is imperative that the patient be conscious and have the cognitive ability to express themselves. Behavioral scales are appropriately used when patients are unable to communicate verbally (Fig. 14.2D). Many factors affect the perceived intensity of postoperative pain and these include the following:
- Preexisting pain
- Anxiety
- Age
- Type of operative procedure performed
FIGURE 14.2. Pain assessment tools. A. Visual analog scale. B. Verbal pain scales Classification of words used to describe pain. (Redrawn from Burchiel K, ed. Surgical Management of Pain. Thieme, 2002.) C. Faces pain scale for pediatrics. (Redrawn from Wong DL, Hockenberry-Eaton M, Wilson D, et al. Whaley and Wong’s Nursing Care of Infants and Children, 6th ed. St. Louis, MO: Mosby; 1999:1153.) D. Behavioral pain scale recommended for use in demented patients. (Redrawn from Sinatra RS, de Leon-Cassasola OA, Viscusi ER, et al., eds. Acute Pain Management. New York, NY: Cambridge University Press; 2009:157.)
Anxiety is often an emotional reaction in anticipation of pain. Anxiety can produce autonomic stimulation such as an increased heart rate. It can also cause any stimulation to be perceived as painful.10 Although the mechanism is not fully elucidated, there is support for the notion that there is age-related decrease in pain perception and report.11
The site of surgery may also be the most important determinant of postoperative pain.12 Operative procedures associated with the highest analgesic consumption are emergency, major, and abdominal surgeries. The most painful operative procedures are as follows:
- Orthopedic procedures with major joints surgery
- Thoracic procedures
- Open abdominal procedures
Analgesic requirements can be estimated based on an assessment of the likely intensity of postoperative pain.13
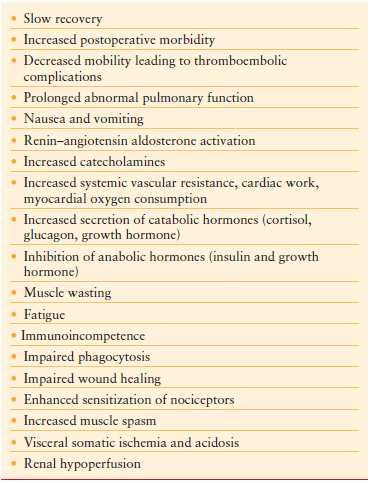
Sequelae of uncontrolled or poorly controlled pain following surgery are well documented. Many of these are listed in Table 14.3.
PHYSIOLOGY OF PAIN
Pain can be defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”14 Pain is an individual and subjective experience. It is influenced by a multitude of factors that include culture, previous pain events, mood, beliefs, and an ability to cope. Pain that is of recent onset with a presumed short duration and can be related to a distinct process is termed acute pain. In general, the injured site and its periphery are the sites of ongoing pain, and as healing progresses, pain is diminished and finally disappears. Pain that persists for more than 3–6 months beyond the time required for healing or has no identifiable cause is termed chronic pain.
Pain perception requires the integration of peripheral and central processes. Nociception links the site of tissue damage and the electrochemical events that lead to the perception of pain. At the site of injury, peripheral sensory fibers that are widely distributed throughout the body in the skin, muscle, joints, viscera, and meninges are activated by mechanical, thermal, and chemical stimulus. This stimulus is transduced into an electrical signal.
Pain is perceived when there is nociceptive afferent transmission to the spinal cord and through the dorsal horn to higher centers. The A-δ myelinated fibers transmit the location and quality of the stimulus to the neothalamus and the higher cortex.15 This prompts rapid withdrawal from the stimulus. Peripheral unmyelinated C fibers establish multiple synaptic connections within the brainstem, the midbrain nuclei, and the limbic system. When peripheral nerve injury occurs, several endogenous chemicals are released. These include bradykinin, histamine, serotonin, eicosanoids, and substance P. These substances, which are also involved in inflammation, activate receptors on sensory afferents beginning nociceptive transmission. This electrical stimulus is then transmitted to the spinal cord.
Prostaglandins, leukotrienes, and bradykinin act together to enhance the activation of the primary afferent terminal. Neurotransmitters including substance P, neurokinin A, and calcitonin gene-related peptide are released and cause increased permeability of blood vessels. Edema is produced and more vasoactive substances are released amplifying the response and spreading the pain outward from the initial locus. Thus infection, inflammation, and ischemia can lead to stimulation of an array of chemical mediators such as prostaglandins and histamines that can sensitize receptors to increase the perception of pain (peripheral sensitization).16
Peripheral sensitization can lead to central sensitization. Inhibitory modulation is also possible. The dorsal horn of the spinal cord contains μ, δ, and κ opioid receptors both pre- and postsynaptically. Inhibitory neuropeptides are also released after nociceptive stimulation. Alpha-adrenergic receptors also modulate nociceptive information. These receptors are found in high concentration in the substantia gelatinosa, and α2 adrenergic receptors are found in the dorsal horn. Centrally, multiple areas of the brain are involved in pain perception. These areas (limbic system, areas in the cortex and the thalamus) interact with the psychological and environmental factors to produce the pain experience17.
Classification
Pain is classified as nociceptive, neuropathic, or psychogenic. Nociceptive pain can be further characterized as somatic or visceral. Somatic pain results from activation of receptors in the cutaneous and deep tissues. It is usually well localized and described as throbbing, aching, or gnawing. Visceral pain occurs when organs that are innervated by the sympathetic nervous system are injured. It is often difficult to localize and described as aching, squeezing, dragging, or pressure like. It is produced by distension, mucosal irritation, traction, or torsion on mesenteric attachments or ischemia and necrosis. Pain in tissues distant from the pathologic condition (referred pain) is sometimes produced. This is thought to occur as a result of dual innervation of multiple structures or central convergence of impulses.
Neuropathic pain results from aberrant somatosensory pathways. This includes phantom limb pain associated with amputation as well as reflex sympathetic dystrophy, which is typically accompanied by vasomotor changes. Neuropathic pain is often described as dysesthetic. The pain is often a sensation that is not the usual recognizable discomfort. It may be itching, stinging, burning, squeezing, or numbness. It is decidedly uncomfortable, even intolerable. There is often a continuous component onto which is added an intermittent element. This is often described as an electric shock or jolt or lancing pain. Many patients have an abnormal sensory threshold and may have dysesthesia, hyperalgesia, allodynia, hyperesthesia, or hyperpathia.18 Psychological stress, anxiety, and depression can influence the perception of pain in ways that make it difficult to determine if they are the cause or the effect. Anxious patients have increased perceived pain and may even have more complications from surgery.19,20
Assessment
Pain can be classified in a variety of ways. It can be described by the mechanism such as nociceptive versus neuropathic. The time relative to injury as in acute or chronic may also be used to describe pain. The etiology of the pain, such as cancer or postoperative pain, may be used to classify pain. Pain reactions are individual. Strong emotions are often associated with pain. Depression, anxiety and fear may alter pain perception. Objective physiologic responses to pain do occur. These include hypertension, tachycardia, sweating, and tachypnea. Grimacing, restlessness, and immobility are other typical physical signs of pain.
The use of behavioral cues or physiologic responses, though seemly objective, is inaccurate and suffused with the biases of the observer. This method is best reserved for those who are cognitively limited or nonverbal. Observer pain scores are notoriously unreliable.
Patient self-reporting is the best tool available for assessing pain. Several tools have been developed for use in clinical practice and are valid and reliable measures of pain intensity. They are the Numeric Rating scale, the Verbal Descriptor scale, the Visual analog scale, and the Faces Pain scale (Fig. 14.2A-D). These are one-dimensional scales and are most appropriate for assessing acute pain following trauma or operation. Multidimensional tools abstract information regarding the characteristics of the pain and its effect on the patient’s life. These tools allow the emotional and physiological responses of patients to be accounted for and are more appropriate for accessing chronic pain. The McGill pain questionnaire is an example. It is a lengthy tool that incorporates categories encompassing the three major dimensions of pain: sensory, affective, and evaluative. This makes it an excellent tool to evaluate different modes of therapies and different analgesics. Its length and complexity make it a less useful clinical tool.21
Pathophysiology
Poorly controlled acute pain has the potential to produce deleterious effects that can lead to a host of complications, increased length of hospitalization, and even death. Several alterations in physiology are known to occur. Peripheral sensitization occurs at the site of injury and areas adjacent to it. This response is produced by the release of a combination of local mediators like bradykinin, serotonin and histamine, and cytokines like interleukin-1 beta (IL-1β) and IL-6. Additionally, sympathetic and sensory nerve endings release substance P and norepinephrine that further enhance pain sensitivity. Secondary hyperalgesia is the delayed alteration in noxious sensitivity seen in nontraumatized areas around the site of injury. Changes in spinal cord, brainstem, and limbic cortex pathways result in altered pain perception. Excitatory amino acids, aspartate, and glutamate act on N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors. Activation of these receptors increases the responsiveness of dorsal horn neurons to noxious input. Initially there is increased firing which is short lived followed by second longer lived period of enhanced sensitivity that does not require further noxious stimulation. This second phase of pain is much more difficult to control. This is the neurochemical basis for splinting. Pain is perceived at dermatomes above and below the site of injury and exacerbated by movement.22
Increased secretion of catabolic hormones occurs after extensive tissue injury. Cortisol, glucagon, growth hormone, and catecholamines are increasingly secreted, while insulin and testosterone are diminished. These hormones have the ability to negatively impact recovery through increased glucose, protein, and fat turnover producing hyperglycemia and negative nitrogen balance. Muscle wasting and fatigue can lead to prolonged convalescence. Immunoglobulin synthesis may be diminished leading to increase infection risk (Fig. 14.3).
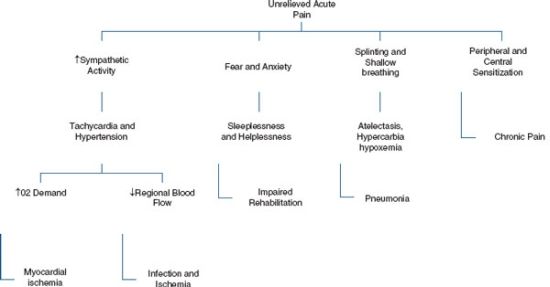
FIGURE 14.3. Sequela of uncontrolled pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree