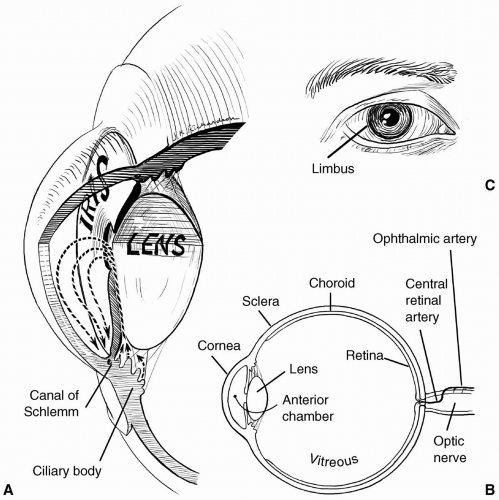
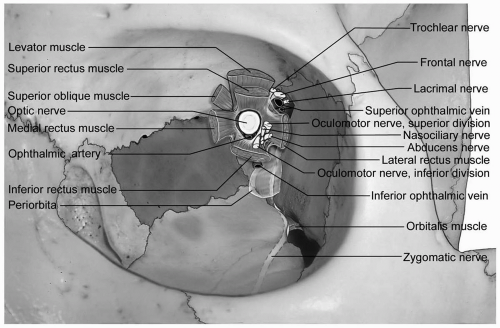
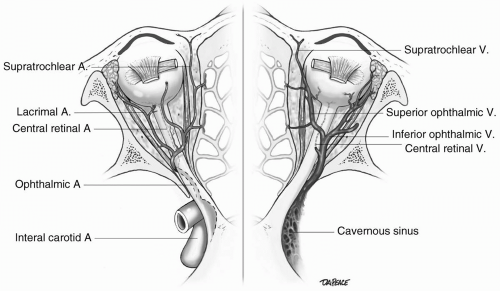
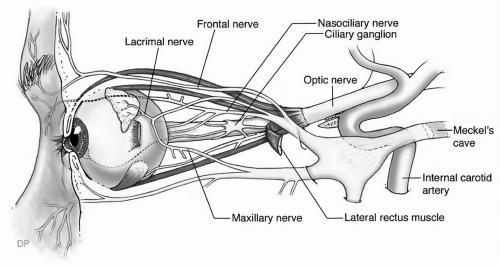
transparent and avascular. The sclera consists primarily of collagen, and is white and opaque. The several gaps found in the sclera are the locations for the optic nerve, and the various nerves, arteries, and veins of the eye. The conjunctiva, a nonkeratinized layer of epithelium, lines the ocular surface and extends over the eyelids to form the upper and lower eyelid fornices. Tenon capsule (fascia bulbi) covers the eye from the cornea to the optic nerve. The extraocular muscles travel from the orbital apex to the eye through openings within Tenon capsule.
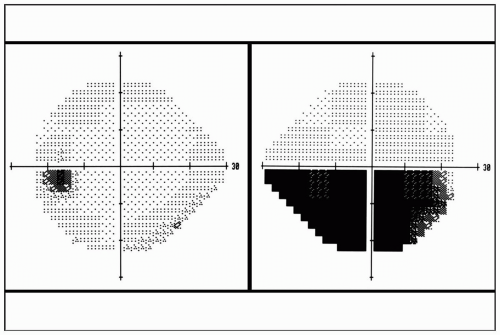 FIGURE 29.1 Automated visual field perimetry demonstrating an inferior visual field defect in the right eye. |
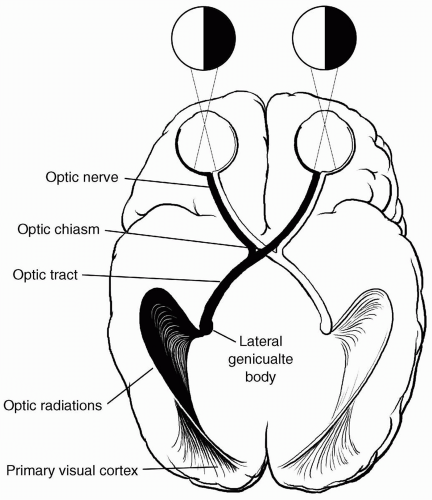
axons of each optic tract synapse at the lateral geniculate nucleus. The retrogeniculate pathway begins with the optic radiations located within the temporal and parietal lobes, traveling to and synapsing in the primary visual area of the occipital lobe or striate cortex. The integrative process of vision is accomplished by the transmission of visual impulses from the striate cortex to various vision-associated regions within the occipital, parietal, and temporal lobes.
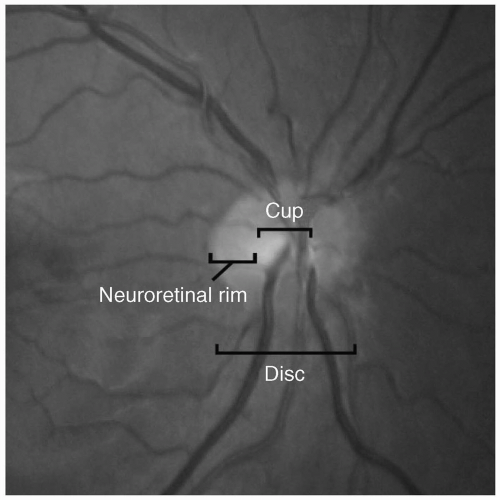
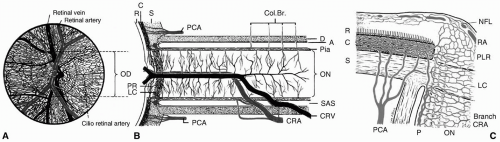
(see Fig. 29.7).3 From anterior to posterior, the optic nerve head can be divided into four regions: The optic disc (surface of optic nerve head), prelaminar, LC, and postlaminar. The optic disc is supplied by the retinal arterioles. Segmental centripetal branches from the peripapillary choroid supply the prelaminar portion of the optic nerve head. The arterial supply of the LC region of the optic nerve head is derived from the centripetal branches of the short posterior ciliary arteries, which also contribute to the circle of Haller and Zinn (CHZ). Arterial branches from CHZ, the peripapillary choroid and, infrequently, the central retinal artery (CRA) supply the retrolaminar portion of the optic nerve head. The arterial supply of the intraorbital portion of the optic nerve is derived from pial branches of the ophthalmic artery.4 These vascular networks have watershed zones and are vulnerable to occlusion due to small vessel disease, hypoperfusion, and, rarely, embolic disease, all of which can result in ischemic disorders of the optic nerve, known as ischemic optic neuropathy. Depending on the vascular network affected, the clinical presentation can be either an AION or a posterior ischemic optic neuropathy (PION).
(IOP). Blood flow to the optic nerve head can be calculated by the formula:
resistance (R = 1/C). C represents the outflow facility, which is influenced by F and IOP (C = ΔF/ΔIOP). An increase in IOP results in a collapse of Schlemm canal and thereby a decrease in outflow facility. Increases in IOP also influence the production of aqueous humor by decreasing the inflow rate.
transplantation), strabismus surgery, glaucoma surgery (trabeculectomy or filtering surgery), oculoplastic surgery, orbital surgery, vitreoretinal surgery, and traumatic open globe repair.21
TABLE 29.1 Anesthetic Implications of Assorted Conditions with Ocular Pathology | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In many cases, a combination of an agent with quicker onset, such as lidocaine, and a longer-acting agent, such as bupivacaine, is given in a 1:1 mixture. Also in many cases, a premixed local anesthetic solution with epinephrine is used to reduce bleeding, prolong the duration of the anesthesia, and improve the effectiveness of the anesthesia desired.24 Only a small amount of epinephrine is required, and the final concentration should not be greater than 5 µg/mL (1:200,000). The total dose should not exceed 0.1 mg.
TABLE 29.2 Summary of Nonocular Interactions Between Glaucoma Medications and Systemic Drugs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 29.3 Effects of Anesthetic Agents, Techniques and Adjuvant Drugs on Intraocular Pressure | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
TABLE 29.4 Advantages of Regional and General Anesthesia | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
recent advances in intraocular lens design and microsurgical techniques, topical anesthesia (with or without intracameral application) is becoming a popular choice in the surgical management of patients requiring cataract extraction.30 In a 2003 survey of cataract and refractive surgeons, topical anesthesia was used in 61% of cases, an increase from 8% in 1995.31 Topical anesthetic agents used in modern day cataract surgery include 0.75% bupivacaine, 0.5% tetracaine, and 2% or 4% lidocaine. The main advantage of topical anesthesia is avoiding the risks associated with injectable orbital regional anesthesia (explained further in the text). Other benefits of performing surgery with topical anesthesia are:
TABLE 29.5 Contraindications to Regional and General Anesthesiaa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Its safe use in anticoagulated patients
Decreased need for intravenous anxiolytic medications
Rapid onset of action
Intraoperative patient cooperation
Immediate return of vision
Lack of periorbital bruising
Absence of postoperative diplopia and
Avoidance of a postoperative eye patch
TABLE 29.6 Complications of Orbital Regional Anesthesia | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
detachment following subconjunctival anesthesia.46 To date, no systemic complications have been reported with this technique.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree