Fetal Heart Rate Monitoring
Fetal Heart Rate Monitoring
I. INTRODUCTION. The obstetric patient population is in general healthy, but preexisting comorbid conditions and several pregnancy-related disorders may be associated with significant morbidity and mortality. The incidence of ICU admission for pregnant and postpartum women ranges from 0.7 to 13.5 per 1,000 deliveries.
A. When caring for the critically ill pregnant woman, it is important to consider how management affects the fetus.
B. Any pregnant patient admitted to the ICU should prompt multidisciplinary care plan formation, including potential labor and delivery plan. Preterm labor and delivery are common in setting of critical illness.
II. GENERAL CONSIDERATIONS RELEVANT TO THE OBSTETRIC, CRITICALLY ILL POPULATION
A. Physiologic Changes of Pregnancy
1. Pregnant women undergo normal physiologic changes associated with pregnancy, labor, and delivery. See Table 36.1 for details. Maintenance of these physiological changes is critical for placental perfusion and fetal oxygenation. Thus, common treatments in the ICU such as high levels of PEEP, diuretics, and vasopressors must be weighed against the risk of decreased venous return, decreased cardiac output, and changes in maternal blood flow distribution—all of which may affect placental perfusion and fetal oxygenation.
2. After 20 weeks’ gestation (uterus palpable above the umbilicus), patients should be positioned with left uterine displacement or in the lateral position to minimize aortocaval compression.
3. Physiologic changes of pregnancy can alter pharmacokinetics and pharmacodynamics.
B. Viability of the Fetus and Fetal Heart Rate Monitoring (reference video)
1. Most sources define the age of fetal viability as being about 24 weeks of gestation. After 24 weeks, fetal heart rate monitoring is recommended to evaluate fetal well-being. Normal fetal heart varies between 110 and 160 beats per minute. A heart rate that does not vary or is too low or too high may signal a potential problem with the fetus.
C. Teratogenic Agents
1. Teratogens are substances that act to irreversibly alter growth, structure, or function of the developing fetus.
2. Timing of exposure as well as the drug-dosing regimen can influence teratogenicity. The classic period of susceptibility to teratogenic agent is between 2.5 and 8 weeks after conception, or during the period of organogenesis. Later effect is more prominent on growth and/or nervous system and gonadal tissue. The US FDA issued a drug classification system with five categories (A–D and X) implying a progressive fetal risk from Category A to X. Category X drugs are contraindicated in pregnancy.
| Physiologic Changes of Pregnancy | |
Parameter | Change (Relative to Nonpregnant State) |
Blood volume | +45% |
Plasma volume | +55% |
Red blood cell volume | +30% |
Tidal volume | +45% |
Cardiac output | +50% |
Stroke volume | +25% |
Heart rate | +25% |
Systemic vascular resistance | −20% |
Left ventricular end diastolic volume | ↑ |
Ejection fraction | ↑ |
Left ventricular stroke work index | No change |
Central venous pressure | No change |
Pulmonary capillary wedge pressure | No change |
Maternal oxygen consumption | +20% |
Inspiratory reserve volume | +5% |
Expiratory reserve volume | −25% |
Residual volume | −15% |
Vital capacity | No change |
Total lung capacity | −5% |
Inspiratory capacity | +15% |
Functional residual capacity | −20% |
Minute ventilation | +45% |
Alveolar ventilation | +45% |
3. Medications commonly encountered in the ICU setting and have known teratogenic properties include antiepileptics (phenytoin, carbamazepine, phenobarbital, valproic acid), lithium, statins, ACE inhibitors, warfarin, tetracyclines, and ribavirin.
4. Multiple Internet resources are available for further drug and teratogen information, including the websites of the American College of Obstetrician and Gynecologists (ACOG) and the Centers for Disease Control and Prevention (CDC).
5. Most drugs are safe for use during lactation. Typically only 1% to 2% of the maternal dose appears in breast milk. The Drugs and Lactation database (LactMed) of the National Library of Medicine’s Toxicology Data Network is an up-to-date source of further information.
D. Vasopressor Use and Potential Effects on the Fetus
1. Little data exist regarding vasopressor use for maternal hypotension or shock due to critical illness. Traditionally, there have been concerns with vasopressor use during pregnancy regarding the potential adverse effects on uterine blood vessels and fetal blood flow.
2. When managing hypotension, it is reasonable to attempt other interventions initially, such as administering intravenous fluids and placing the patient in left lateral decubitus position to prevent compression of the inferior vena cava by the gravid uterus.
3. In the setting of persistent hypotension, restoring maternal perfusion pressure is of paramount importance, and it should override any theoretical concerns of vasopressor-induced uterine vasculature constriction. Increasing the MAP will improve perfusion pressure to organs, including the gravid uterus. Norepinephrine, an endogenous catecholamine that crosses the placenta, is often used as first-line vasopressor. Second-line vasopressors such as epinephrine or vasopressin may be considered. No good data exist regarding the use of vasopressin in pregnant women. Therefore, caution is recommended if this agent is used since it may theoretically activate uterine V1a receptors, leading to uterine contractions.
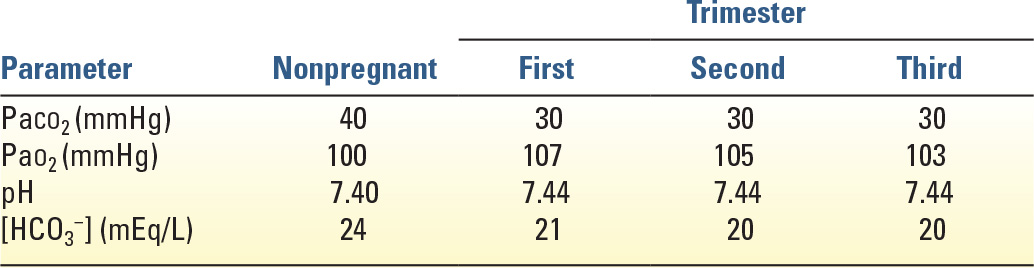
E. Ventilation and Blood Gases (Table 36.2)
1. Most aspects of mechanical ventilation are the same for pregnant and nonpregnant women. An exception is the target arterial carbon dioxide tension (PaCO2). Ventilator settings should be adjusted to maintain mild respiratory alkalosis with PaCO2 between 30 and 32 mmHg and arterial pH between 7.40 and 7.47. This replicates normal physiology during pregnancy due to respiratory stimulation by progesterone.
2. Significant respiratory alkalosis with PaCO2 <30 mmHg should be avoided because it may decrease uterine blood flow. Maternal hypercapnia (PaCO2 >40 mmHg) may result in decreased removal of CO2 from the fetus, causing fetal acidosis, in addition to increased fetal breathing movements, which may increase fetal oxygen use. Thus, it seems prudent to avoid maternal hypercapnia even though studies have not identified any adverse sequelae in fetuses that were exposed to PaCO2 levels as high as 60 mmHg during permissive hypercapnia. Maternal oxygen consumption increases in pregnancy by 20% to 30% at term, largely because of increased consumption by the fetus and placenta. To minimize fetal effects, the maternal PaO2 should be maintained at more than 60 mmHg.
III. PREECLAMPSIA is part of a spectrum of hypertensive disorders specific to pregnancy. Although the precise etiology of preeclampsia remains unknown, it is a disease that occurs only in the presence of placental tissue. The maternal manifestations are consistent with a process of vasospasm, ischemia, and changes in the normal balance of humoral and autocoid mediators. Preeclampsia is diagnosed in 3% to 5% of all pregnancies in the United States and is most common in nulliparous women. A patient meets the criteria for a diagnosis of preeclampsia if she has persistently elevated blood pressure after 20 weeks’ gestation in the setting of previously normal blood pressure, and proteinuria of greater than 300 mg in 24 hours or signs of end-organ dysfunction. The diagnosis of preeclampsia is divided into mild and severe on the basis of the presence or absence of specific signs, symptoms, and abnormal laboratory values (Table 36.3).
A. Two additional diagnoses, eclampsia and potentially HELLP syndrome, are a part of this spectrum of disease.
1. Eclampsia is defined as the occurrence of seizures or coma in a woman with preeclampsia that cannot be attributed to other causes. Eclamptic seizures may occur antepartum, intrapartum, or postpartum. Eclampsia is a cause of significant maternal and fetal morbidity and is present in approximately 50% of maternal deaths associated with preeclampsia. Eclamptic seizures are usually preceded by headache and visual disturbances. Seizures are generally abrupt and self-limited, but may be complicated by cardiopulmonary arrest or pulmonary aspiration of gastric contents.
2. HELLP syndrome (Hemolysis, Elevated Liver enzymes, and Low Platelets) involves a constellation of laboratory abnormalities and was previously regarded as a subset of severe preeclampsia; however, it is now recognized as potentially a distinct clinical entity. The diagnosis of HELLP syndrome is also associated with an increased risk of adverse outcomes including abruption, renal failure, hepatic subcapsular hematoma formation, liver rupture, and fetal and maternal death. Management is generally aimed at administering magnesium, supportive care with normalizing blood pressure in the face of severe hypertension, and delivery of the fetus with recognition of the increased risk of hemorrhage in this population. Platelet counts can fall precipitously, and platelet transfusions are indicated in any parturient with significant bleeding or with a platelet count of less than 20,000/mm3. Subcapsular hematoma, if it occurs, is an emergency and can result in shock and fulminant hepatic failure. Death is typically due to exsanguination and coagulopathy. Prompt surgical intervention and resuscitative measures have led to improvement in maternal survival.
| Diagnostic Criteria for Mild and Severe Preeclampsia | |
Blood pressure | ≥140 mmHg systolic or ≥90 mmHg diastolic on two occasions at least 4 hours apart after 20 weeks of gestation in a woman with a previously normal blood pressure |
| ≥160 mmHg systolic or ≥110 mmHg diastolic, hypertension can be confirmed within a short interval (minutes) to facilitate timely antihypertensive therapy |
and |
|
Proteinuria | ≥300 mg per 24-h urine collection (or this amount extrapolated from a timed collection) |
| or |
| Protein–creatinine ratio ≥0.3 (each measured by mg/dL) |
| Dipstick reading of 1+ (used only if other quantitative methods are not available) |
Or in the absence of proteinuria, new-onset hypertension with the new onset of any of the following: | |
Thrombocytopenia | Platelet count <100,000/µL |
Renal insufficiency | Serum creatinine concentrations >1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease |
Impaired liver function | Elevated blood concentrations of liver transaminases to twice normal concentration |
Pulmonary edema |
|
Cerebral or visual symptoms |
|
Adapted from American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Washington, DC: American College of Obstetricians and Gynecologists, 2013.
B. Management
1. Delivery. The only definitive treatment for preeclampsia, eclampsia, or HELLP syndrome is delivery of the fetus and placenta. The decision of when to deliver is made on the basis of the gestational age and the severity of the disease. Each patient and clinical situation should be individualized with a management strategy that seeks to balance and minimize both maternal and fetal morbidity.
2. Pharmacologic therapy
a. Seizure prophylaxis. Although the mechanism of action is unknown, magnesium sulfate is the medication of choice for prophylactic prevention and treatment of eclamptic seizures. Dosage of magnesium is 4 g intravenous (IV) bolus over 30 minutes followed by 2 g/h IV, but because it is renally cleared, this may need to be adjusted if severe renal dysfunction is present. The drug is administered during active labor, delivery, and for 24 to 48 hours postdelivery. Because of its relaxant effect on vascular and visceral smooth muscle, magnesium may decrease maternal blood pressure and predispose to postpartum atony and hemorrhage. It also inhibits acetylcholine release at the motor endplate, leading to potentiation of neuromuscular blocking agents.
b. Antihypertensive medications such as labetalol, hydralazine, and calcium-channel blockers are frequently administered for control of blood pressure. The goal is not to normalize blood pressure, but to keep patients from progressing to a hypertensive crisis, encephalopathy, or stroke. When administering antihypertensive medications, it is important to remember that the placenta has no ability to autoregulate flow. Thus, a sudden drop in maternal blood pressure may decrease placental perfusion and result in significant compromise to the fetus.
IV. ACUTE FATTY LIVER OF PREGNANCY (AFLP) is a rare but potentially fatal complication of pregnancy, involving microvesicular fat deposition in the liver and characterized by liver dysfunction, DIC, severe and refractory hypoglycemia, encephalopathy, and renal insufficiency. Patients usually present in the third trimester, although the disease has been described as early as 23 weeks’ gestation. More than half (50%–70%) of AFLP patients can have associated preeclampsia. Some authors consider AFLP and HELLP to be part of a spectrum of a single disease, but AFLP can be distinguished from HELLP on the basis of severe hepatic dysfunction, rather than merely elevated liver transaminases (as occurs in HELLP). The precise pathogenesis of AFLP remains unknown although believed to be due to defective mitochondrial β oxidation of fat either in the mother or the fetus, which is hepatotoxic. Mortality remains high for both mother and fetus.
A. Clinical Manifestations. Patients frequently present with nonspecific symptoms such as malaise, nausea and emesis, jaundice, epigastric or right upper-quadrant pain, headache, and anorexia. Hypoglycemia may be present and severe and accounts for some of the mortality associated with AFLP.
B. Diagnosis and Laboratory Findings. One distinct laboratory finding of acute fatty liver of pregnancy is hyperbilirubinemia with serum levels of 3 to 40 mg/dL reported. Patients also have profound elevation of alkaline phosphatase with mild to moderate transaminase elevations. Progression to hepatic failure can be rapid, and large elevations in hepatocellular enzymes may be missed. In addition to hepatic dysfunction, renal failure, coagulopathy, profound hypoglycemia, and metabolic acidosis are early complications.
1. Hyponatremia from diabetes insipidus can be present in up to 10% of patients. Liver biopsy is rarely required and should be performed only when absolutely necessary because of the concomitant coagulopathy.
C. Management. The liver dysfunction and failure associated with AFLP is reversible in most patients, and supportive care and delivery are the mainstays of treatment. AFLP is a medical emergency that requires immediate evaluation. The most important component of treatment is the delivery of the fetus. Careful attention to fluid balance is crucial due to the increased risk of pulmonary and cerebral edema secondary to low colloid osmotic pressure. There is almost always early onset of acute renal dysfunction (90%), which is usually reversible, but renal support is often required.
1. Serum glucose levels should be checked every 1 to 2 hours and hypoglycemia aggressively treated; all patients should receive an infusion of at least 5% dextrose, and many will require higher concentrations with intermittent boluses to maintain normoglycemia. Coagulation studies should be followed at regular intervals and postpartum hemorrhage anticipated. Regardless of the mode of delivery, patients should have adequate IV access and cross-matched blood products available. If the patient requires a cesarean section, improving or correcting the coagulopathy prior to incision should be considered.
2. There is often a worsening of liver function, renal function, and coagulopathy for 48 hours after delivery, but then improvement should be expected. Transplantation is generally not required for AFLP, but may be necessary in severe cases.
V. NEUROLOGIC DISEASE
A. Stroke. Pregnant women are at higher risk than nonpregnant women with the incidence of stroke being 5 to 15 per 100,000 deliveries with cerebrovascular events accounting for 5% of maternal deaths. A significant proportion of strokes occur late in pregnancy, with the highest incidence in the peripartum period. Ischemic events appear to account for one-half to two-thirds of cerebrovascular events in pregnancy, whereas hemorrhagic strokes may be slightly less common.
1. Etiologies. Ischemic cerebral infarctions may be divided into primarily arterial or venous etiologies. Arterial etiologies include vasculopathies, dissections, and embolic events; venous infarctions may result from hypercoagulable states, dehydration, or infections. Hemorrhagic events are largely a result of aneurysms, vascular malformations, preeclampsia, or trauma. Treatment may be challenging, as up to 50% of venous infarctions manifest in areas of hemorrhage, making anticoagulation difficult.
2. Clinical manifestations are similar as in nonpregnant women. Headache is the most common presenting symptom. Other symptoms include focal neurologic deficits and seizures.
3. Diagnosis. In addition to physical examination, neurologic imaging is critical for establishing the diagnosis and etiology. Attempts should be made to minimize fetal exposure to radiation, but appropriate diagnostic imaging should not be avoided. The fetal radiation exposure from noncontrast CT scan is less than 1 rad.
4. Management in the pregnant patient is similar to the treatment of nonpregnant patients. Care is supportive, and thrombolytic therapy should be considered if indicated for ischemic stroke. Thrombolysis with recombinant tissue plasminogen activator (r-tPA) has been reported during pregnancy and appears to be safe for the fetus; there is minimal transplacental passage of r-tPA. However, retroplacental bleeding with pregnancy loss has been reported. The therapeutic window (i.e., time from onset of symptoms to administration of the agent) is 4.5 hours.
a. For preservation of fetal well-being, oxygenation and intravascular volume should be maintained, and hypotension, hyperglycemia, and fever avoided. Delivery of the fetus may be complicated by the risk of aneurysm or arteriovenous malformation (AVM) rupture, thrombolytic therapy, or anticoagulation; both the route of delivery and anesthetic management should be tailored to each individual patient.
B. Seizure Disorders. Approximately 0.5% of all parturients have a chronic, preexisting seizure disorder. A third of women will experience an increase in their seizure frequency, 50% will experience no change, and the remainder will note a decrease in seizures. Because many antiseizure medications carry an elevated risk of teratogenicity to the fetus, patients and providers must weigh the risks and benefits of continuing these medications in pregnancy. Many patients begin monotherapy, and folate supplementation is a necessity. Uncontrolled generalized seizures pose serious risk to both mother and fetus. Serum levels of antiseizure medications may fall due to greater drug clearance and decreased protein binding in pregnancy. In addition, epileptic parturients appear to be at increased risk for preeclampsia, preterm labor, and placental abnormalities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree