I. INTRODUCTION. The delivery of calories and protein to a critically ill patient should be considered a high priority that is integral to care, rather than a supportive afterthought. The goals of nutrition therapy include prevention of infectious morbidity, preservation of muscle mass, and prevention of metabolic complications. Choosing the appropriate route, timing, and dose of nutrition are crucial to achieving these goals.
II. PATHOPHYSIOLOGY OF NUTRITION IN CRITICAL ILLNESS. Postsurgical and critically ill patients are at risk for calorie–protein malnutrition. The response to illness includes increased energy expenditure, increased secretion of certain hormones (glucagon, glucocorticoids, catecholamines, vasopressin), and increases in inflammatory mediators (cytokines, acute-phase proteins).
A. Fluid shifts occur due to water retention and increased vascular permeability.
B. Increased glycogenolysis, gluconeogenesis, and insulin resistance result in hyperglycemia.
C. Skeletal muscle protein is catabolized for gluconeogenesis, resulting in protein depletion.
D. Resting energy expenditure remains elevated, and catabolism can continue for up to 3 weeks, even after resolution of the initial insult and despite adequate nutrition support.
III. ASSESSMENT OF NUTRITIONAL STATUS. The patients’ medical history, nutritional intake history, physical exam, and laboratory data must be taken into account.
A. Clinical History. Preexisting conditions such as unintentional weight loss, chronic disease, and alcohol abuse increase a patient’s risk for protein–calorie malnutrition. The severity of the current illness, including the presence of fevers, burns, sepsis, or trauma, is associated with varying degrees of hypermetabolism and increased nutritional requirements.
B. Body Weight Measurements
1. Body mass index (BMI) = weight (kg)/height (m2)
2. Ideal body weight (IBW)
a. Men (kg) = 50 + 2.3 (height [inches] − 60)
b. Women (kg) = 45.5 + 2.3 (height [inches] − 60)
3. Adjusted body weight (ABW) = IBW + 0.4 (actual weight − IBW)
a. Calculate ABW if actual body weight is >30% of IBW
C. Nutritional Laboratory Indices
1. Baseline electrolytes, glucose, and liver function tests should be obtained prior to initiation of nutrition therapy.
2. Albumin is a long-term marker of nutritional status with a half-life of approximately 21 days.
3. Prealbumin and transferrin are serum proteins with short half-lives (2–3 days and 8 days, respectively).
4. It is increasingly recognized that low albumin and prealbumin are more reflective of acute inflammation in the critically ill patient. For this reason, initially low levels should be interpreted with caution and should always be measured concomitantly with C-reactive protein.
5. Trends over time are much more informative than single data points.
D. Resting Energy Expenditure. Energy requirements are difficult to estimate in the critically ill population. Predictive equations are commonly used but may over- or underestimate caloric requirements. Difficulties inherent in the surgical critically ill population include inaccurate weight assessments secondary to fluid resuscitation-related weight gain, inability to obtain an accurate nutrition history, and fluctuating metabolic needs throughout the course of illness.
1. Indirect calorimetry, also referred to as metabolic cart, measures the ratio of carbon dioxide eliminated (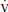 CO2) to the oxygen consumed (
CO2) to the oxygen consumed (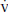 O2) by the body. This is measured over a 10-to-30-minute period to calculate a respiratory quotient (RQ). Patients must be intubated, breathing low FIO2 (<50%), and without chest tube leaks for this to be accurate. They also should be hemodynamically stable and on a stable nutrient regimen.
O2) by the body. This is measured over a 10-to-30-minute period to calculate a respiratory quotient (RQ). Patients must be intubated, breathing low FIO2 (<50%), and without chest tube leaks for this to be accurate. They also should be hemodynamically stable and on a stable nutrient regimen.
2. Indirect calorimetry is recognized as more accurate than predictive equations and is recommended by the Society of Critical Care Medicine (SCCM) and the American Society of Parenteral and Enteral Nutrition (ASPEN) to determine caloric need.
3. A recent study (TICACOS) demonstrated superior outcomes when caloric prescription was guided by indirect calorimetry measurements, and a recent review concluded that “appropriately performed indirect calorimetry is really the only true means to set accurate goals for nutrition therapy.”
4. With the use of noninvasive cardiac output monitors, indirect calorimetry may be obtained at minimal incremental time, effort, and expense. Best practice should include CO2 production measurement for all mechanically ventilated patients at the initiation of enteral nutrition, weekly thereafter, and as clinically indicated to more accurately match caloric delivery to caloric needs.
5. The Harris–Benedict equation estimates basal metabolic rate (BMR) using a calculation based on gender, weight (kg), height (cm), and age (years).
E. Protein Requirements. Nitrogen balance can be calculated by measuring 24-hour urine urea nitrogen (UUN) to assess adequacy of protein intake.
1. Nitrogen loss (g/d) = 1.2 [UUN (g/dL) × urine output (mL/d) × (1 g/1,000 mg) × (1 dL/100 mL)] + 2 g/d.
2. Nitrogen balance (g/d) = [total protein intake (g/d)/6.25 (g protein/nitrogen)] − [Nitrogen loss (g/d)]
3. The goal is a positive nitrogen balance (anabolic state). Negative nitrogen balance indicates muscle breakdown (catabolic state), and protein intake should be increased.
4. An estimate calorie-to-nitrogen ratio is 150:1 and is provided in most isotonic enteral formulas.
IV. COMPONENTS OF NUTRITION. Caloric requirements should be fulfilled with protein, carbohydrates, and fat.
A. Protein provides 4 kcal/g. Protein intake is critical for muscle anabolism and maintenance of a positive nitrogen balance. It should consist of both essential and nonessential amino acids. The following are some general guidelines when calculating protein requirements.
1. Nonstressed patient = 0.8 to 1.0 g/kg/d
2. Postsurgical, mild trauma = 1.0 to 1.5 g/kg/d
3. Severe trauma, sepsis, organ failure = 1.5 to 2.0 g/kg/d
4. Burn (>20% TBSA) or severe head injury ≥2.0 g/kg/d
B. Carbohydrates should fulfill between 40% and 60% of total caloric needs. They provide 3.4 kcal/g.
C. Fat should provide 20% to 30% of total calories. Fat provides 9 kcal/g. Polyunsaturated fats are essential fatty acids and must be obtained from a dietary source.
V. INDICATIONS AND TIMING OF NUTRITIONAL SUPPORT
A. In previously healthy, well-nourished patients, nutritional therapy should be initiated if the expected illness duration without nutrition is expected to exceed 7 days or if the patient has gone without nutrition for 7 days.
B. In critically ill patients, nutrition should be initiated earlier due to the hypermetabolism and the catabolic consequences of the disease process. In the absence of contraindications, enteral feeding should be initiated within 24 to 48 hours of ICU admission.
1. Early enteral nutrition has been shown to be beneficial in patient populations in whom it was previously thought to be harmful: severe acute pancreatitis, hemodynamically unstable requiring vasopressor support, and open abdomen.
2. Current evidence suggests that enteral nutrition is safe and beneficial in these settings as luminal nutrients have been demonstrated to enhance splanchnic perfusion.
3. Feeding in patients with cardiogenic shock or rapidly escalating vasopressor requirements is more controversial, as increased splanchnic blood flow may compromise systemic perfusion. There patients should be monitored closely for hemodynamic and gastrointestinal tolerance. Caution is advised.
4. Patients with preexisting malnourishment or severe weight loss will also benefit from early nutritional support utilizing supplemental parenteral nutrition (PN) if unable to deliver >80% of nutrient requirements by ICU day 3.
VI. ENTERAL NUTRITION VERSUS PARENTERAL NUTRITION
A. Enteral nutrition (EN) is preferred over parenteral nutrition (PN) in patients with a functional GI tract. EN reduces infectious complications, improves wound healing, decreases GI mucosal permeability (and bacterial translocation), and is more cost effective than PN.
B. Even in patients unable to tolerate enteral nutrition at sufficient rates to fulfill their caloric needs, “trophic” tube feeds (10–20 cc/h) can provide non-nutritional benefits such as maintaining muscosal integrity, immune function, and preventing ileus.
C. For patients who cannot tolerate enteric feeds (i.e., prolonged ileus, intestinal obstruction or perforation, high-output enterocutaneous fistula) parenteral nutrition may be required. For previously well-nourished patients with these conditions, late initiation of PN (day 8 vs. day 3) has been shown to be associated with fewer infections, enhanced recovery, and decreased cost compared to early initiation.
VII. DELIVERY ROUTE OF ENTERAL NUTRITION. Nasogastric or nasoenteric tubes may be used for short-term enteral nutritional support (<1 month). Postpyloric tubes may be helpful if gastroparesis prevents tolerance of gastric feeds, but have not been associated with lower rates of aspiration or delayed gastric emptying when compared with gastric feeding.
A. Placement of postpyloric tubes may be done blindly, endoscopically, or with fluoroscopic guidance.
1. Blind placement of stiletted postpyloric tubes must be done cautiously and by experienced clinicians. When inserted in sedated, mechanically ventilated patients, these soft and sharp-tipped tubes may enter the airway instead of the GI tract. Complications include pneumothorax and lung infection/abscess secondary to infusion of tube feeds into the airway.
2. Confirmatory radiographs must always be performed prior to initiation of enteral nutrition through a newly inserted feeding tube, as other methods confirmation, such as aspiration of gastric contents and auscultation are insufficiently accurate.
3. Surgical placement of a gastric or jejunal feeding tube (i.e., nonoral access) is indicated for long-term nutritional support (>1 month) and may be achieved endoscopically, fluoroscopically, or surgically.
VIII. IMMUNONUTRITION is the modulation of the immune system through the supplementation of select nutrients. Several nutrients have been studied; however, data have been conflicting regarding the use of immune-enhancing formulas in critically ill patients.
A. Glutamine: the most abundant nonessential amino acid. It is synthesized predominantly in skeletal muscle and is involved in numerous essential functions such as nitrogen transport, neutrophil function, acid–base balance and is the preferred fuel source for rapidly dividing cells in the intestinal tract and the immune system. In a randomized trial (REDOX study), early provision of glutamine was associated with an increase in mortality in patients with multiorgan failure. Data on glutamine supplementation in specific patient subsets have been conflicting. For these reasons, supplementation with glutamine in critically ill patients is not recommended.
B. Arginine: a conditionally essential amino acid in periods of stress and has important roles in nitrogen metabolism and formation of nitric acid. Arginine may exacerbate the systemic inflammatory response syndrome (SIRS) and is not recommended in septic patients.
C. Fatty Acids: although some fatty acids have been hypothesized to have anti-inflammatory or proinflammatory effects, there is no high-quality evidence supporting the routine use of specific lipids in critically ill patients.
D. Micronutrients: administration of trace elements (selenium, copper, manganese, zinc, iron, vitamins E and C) has been proposed to reduce oxidative cellular damage. Standard enteral formulas and parental preparations are usually sufficient, and additional supplementation is rarely required.
IX. PARENTERAL NUTRITION (PN) is indicated in patients who require aggressive nutritional support but cannot tolerate enteral nutrition.
A. Peripheral parenteral nutrition (PPN) provides partial nutritional support and can be administered through a peripheral vein due to lower osmotic solutions (<900 mOsm/L). Dextrose, lipids, and proteins can all be given peripherally, but require a large volume of infusion and will not meet total metabolic demands. PPN is rarely utilized in the SICU.
B. Total parenteral nutrition (TPN) contains higher concentrations of dextrose and amino acids to meet total metabolic demands and is therefore hypertonic (requires central venous access).
C. The risk–benefit ratio of PPN has been questioned because it is associated with the same risk of immunosuppression and increased risk of infections as TPN, but without the benefit of total nutritional support.
D. Components of TPN include the following:
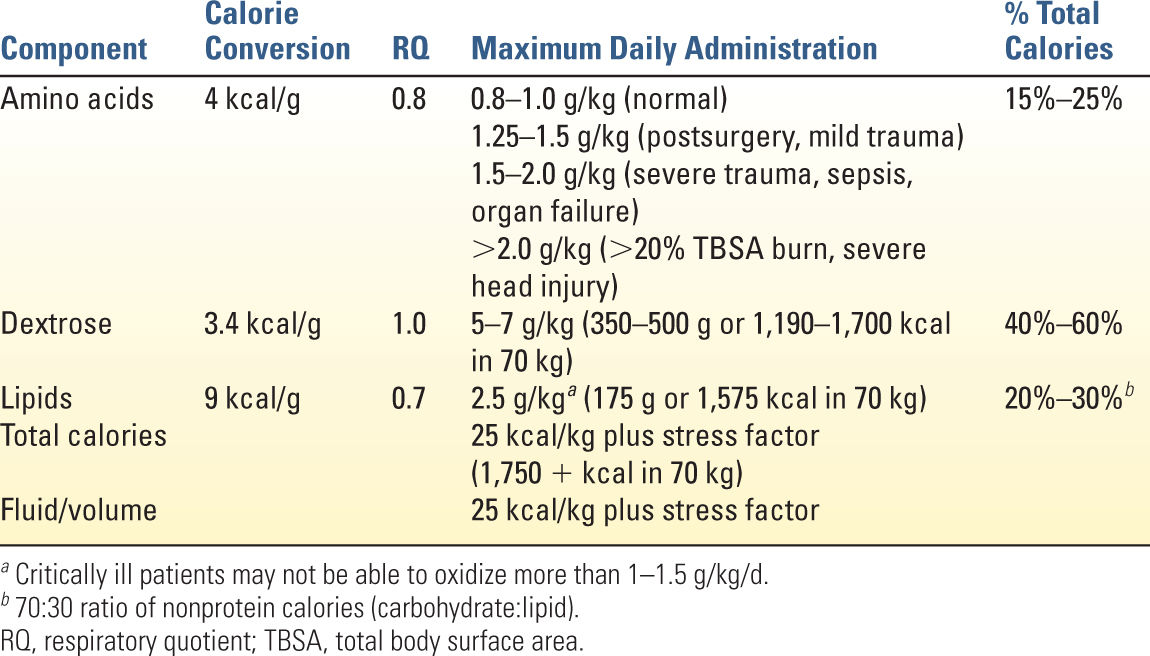
1. D-glucose (dextrose) the major source of non-protein calories (3.4 kcal/g). Maximum daily administration is 5 to 7 g/kg/d. Exceeding this maximum rate of glucose oxidation may result in lipid synthesis with CO2 accumulation and hepatic steatosis.
2. Lipid emulsion is another source of nonprotein calories (9 kcal/g) and also provides essential fatty acids (ω-6 and ω-3 polyunsaturated fats). Maximum administration of lipid emulsion is 2.5 g/kg and should comprise <30% of total calories. Lipid emulsions provide 1.1 kcal/mL (for 10% IV emulsion), 2.0 kcal/mL (for 20% IV emulsion), and 3.0 kcal/mL (for 30% IV emulsion). Avoid infusion >110 mg/kg/h because of neutrophils and monocytes impairment and worsening gas exchange.
3. Amino acids (essential and nonessential) are needed to build muscle and maintain a positive nitrogen balance, but also are a source of calories (4 kcal/g). As in EN, protein requirements should be calculated on the basis of stress level and monitored with weekly UUN and nitrogen balance.
E. Calculation of TPN Formulation (Table 11.1)
1. Calculate total calorie requirements.
2. Estimate protein requirements.
3. Calculate maximum carbohydrate and lipid amounts and provide these “nonprotein calories” in a ratio of 70:30 (ideal).
4. Fluid/volume requirements average 30 mL/kg/d, but daily weight and strict measurement of fluid losses (urine, stool, insensible losses) help monitor fluid status.
5. Electrolytes, minerals, vitamins, and trace elements should be added according to usual or recommended doses (Table 11.2). Adjustments may be needed in critical illness, or with certain disease states (i.e., renal failure or high-output fistula), and serum levels should be monitored routine.
6. Insulin can be added directly to TPN solution (up to half of daily sliding scale insulin requirement)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree