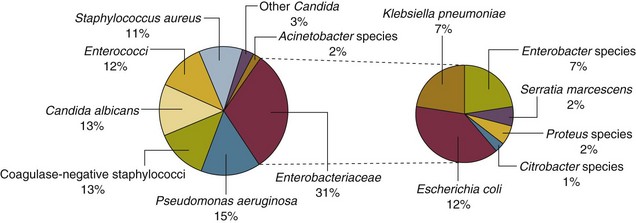
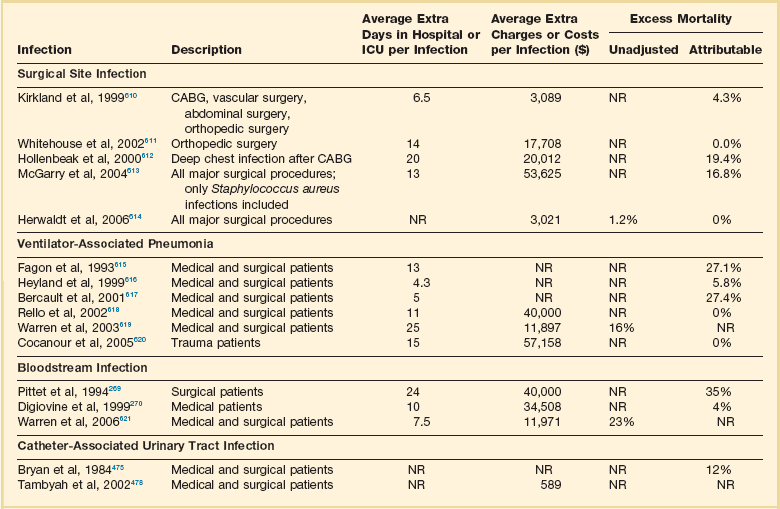
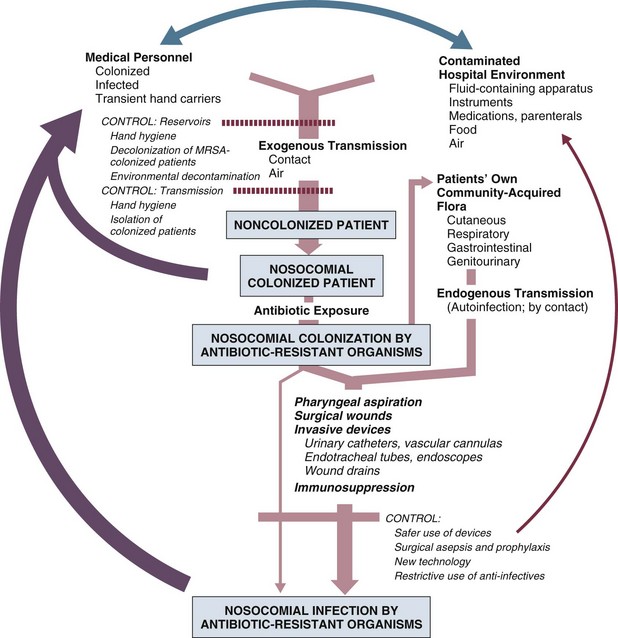
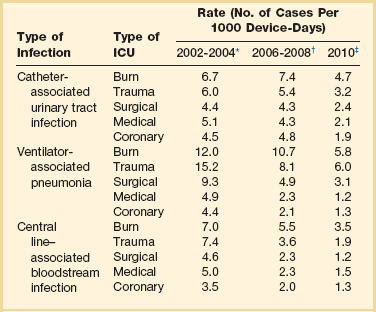
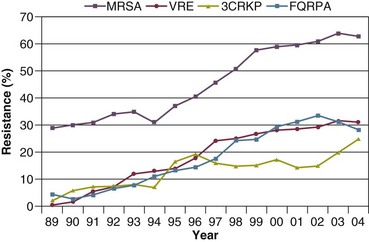
50 GENERAL INFECTION CONTROL MEASURES Hospital Infection Control Programs Role of the Microbiology Laboratory Architectural and Environmental Issues Reliable Sterilization Procedures, Chemical Disinfectants, and Antiseptics Isolation Precautions for Communicable Infections NOSOCOMIAL INFECTIONS AND SPECIFIC INFECTION CONTROL MEASURES Intravascular Device–Related Bloodstream Infection Ventilator-Associated Pneumonia Catheter-Associated Urinary Tract Infection AVANT GARDE INFECTION CONTROL MEASURES PROTECTION OF HEALTH CARE WORKERS IN THE INTENSIVE CARE UNIT Intensive care units (ICUs) have contributed greatly to the survival of patients with trauma, shock states, and other life-threatening conditions1–3 but are associated with a greatly increased risk of nosocomial (hospital-acquired) infection. Rates of nosocomial infection in patients requiring more than 1 week of advanced life support within an ICU are three to five times higher than in hospitalized patients who do not require ICU care.4–8 Infection, usually nosocomial, is the most common cause of death, directly or indirectly, of patients who survive the early period after major trauma or full-thickness burns and is the most commonly identified cause of multiple-organ dysfunction syndrome.9–11 Published guidelines for prevention are now available, based increasingly on randomized trials that have established the efficacy of specific control measures. Knowledge and technology of asepsis with regard to surgery and high-risk medical devices are now sufficiently advanced that, if applied consistently, the risk of nosocomial infection can be greatly reduced.12–15 Obtaining meaningful data on rates of nosocomial infection that can form the basis for comparisons within a hospital and, especially, among hospitals and that can also be used to monitor secular trends and document the efficacy or lack of efficacy of control measures must begin with clear, unambiguous definitions. Although there are no standardized definitions for infection at specific sites that are universally accepted by clinicians or investigators, the Centers for Disease Control and Prevention (CDC) has published definitions for the purpose of surveillance of nosocomial infection within hospitals, which most U.S. centers and an increasing number of hospitals around the world have adopted (Box 50.1).16,17 For research purposes, more stringent definitions for specific infections will usually be necessary,18 especially for pneumonia.19 The incidence of hospital-acquired infection is most commonly expressed as the number of infections per 100 patients hospitalized and is highest in burn units7,20 and surgical ICUs,5–7,20–23 with intermediate risk in medical ICUs,* and lowest risk in coronary care units.4,7,8,20 Recognizing that the risk of nosocomial infection within ICUs is heavily influenced by the length of stay and that the length of stay ranges widely among ICUs in the same hospital and among different hospitals, the CDC has advocated the use of rates expressed per 1000 patient-days to permit more meaningful intrainstitutional and, especially, interhospital comparisons.25–27 Furthermore, recognizing the powerful influence of exposure to invasive devices on susceptibility to infection28,29 and the great variation in use of devices among different ICUs in the same hospital and among different hospitals, the CDC has further recommended surveillance of device-associated nosocomial infections expressed as infections per 1000 device-days.25 Representative rates of device-associated nosocomial infection in U.S. hospitals, which can be used for intrahospital and interhospital comparisons, are shown in Table 50.1.25–27 In the future, device-associated infection rates will be sought in accreditation reviews by the Joint Commission on the Accreditation of Healthcare Organizations (JCAHO)30 as this influential organization continues to move toward measurement of patient outcomes as the most effective way to improve patient care in the United States. Table 50.1 *Data from National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32:470-85. †Data from Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009;37:783-805. ‡Data from Dudeck MA, Horan TC, Peterson KD, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control 2011;39:798-816. Approximately 40% of endemic nosocomial infections within ICUs are catheter-related urinary tract infections, and 25% are pneumonias—most associated with endotracheal intubation and mechanical ventilatory support. Up to 10% of patients hospitalized in a medical-surgical ICU for more than 72 hours acquire a nosocomial bloodstream infection, most commonly from an intravascular device.25,31,32 Postoperative surgical site infections, Clostridium difficile infection, nosocomial sinusitis, and nosocomial meninigitis account for the remainder.4–8,25,33–37 Nearly 50% of nosocomial infections in the ICU are caused by aerobic gram-negative bacilli, especially Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae, and 35% are caused by gram-positive cocci, most commonly coagulase-negative staphylococci, Staphylococcus aureus, and enterococci (Fig. 50.1).38,39 Almost 15% are caused by Candida species.38,39 Filamentous fungi such as Aspergillus and Zygomycetes are being increasingly encountered in patients with hematologic malignancy or those who received solid organ transplants.40–42 Legionella species account for up to 10% of nosocomial pneumonias in centers that make efforts to diagnose Legionella infections.43 The microbial profile of infections at individual sites in ICU patients is shown in Table 50.2.39 There has been an unrelenting increase in nosocomial infections caused by intrinsically resistant organisms, especially P. aeruginosa, Acinetobacter species, and other resistant gram-negative bacilli; coagulase-negative staphylococci, S. aureus, enterococci; and Candida.31,38,39,44,45 Moreover, the incidence of infection caused by organisms with acquired resistance, especially methicillin-resistant S. aureus (MRSA); enterococci resistant to vancomycin (VRE), ampicillin, or both drugs; and gram-negative bacilli resistant to extended-spectrum β-lactams and fluoroquinolones, has increased even more sharply over the past several decades (Fig. 50.2).39,46 The recent emergence of carbapenem-resistant K. pneumoniae has become a significant problem as there are limited therapeutic options to treat this pathogen.39,47 Table 50.2 Profile of Nosocomial Infections in the Intensive Care Unit (ICU) Modified from Maki DG: Nosocomial infection. In Parrillo JE (ed): Current Therapy in Critical Care Medicine, 2nd ed. Philadelphia, BC Decker, 1991. Figure 50.2 Temporal trends in the proportion of isolates resistant to antibiotics among pathogenically important bacteria in U.S. intensive care units (ICUs), National Nosocomial Infections Surveillance System (NNIS) 1989-2004. FQRPA, Pseudomonas aeruginosa resistant to fluoroquinolones; MRSA, methicillin-resistant Staphylococcus aureus; 3CRKP, Klebsiella pneumoniae resistant to third-generation cephalosporins; VRE, vancomycin-resistant enterococcus. (From Centers for Disease Control and Prevention: Trends in antibiotic resistance in National Nosocomial Infections Surveillance (NNIS) system hospitals, 1989-2004. Available at http://www.cdc.gov/ncidod/dhqp/pdf/ar/ICU_RESTrend1995-2004.pdf. Accessed January 15, 2007.) Nosocomial infections acquired in the ICU clearly differ from infections acquired in non-ICU patient care units within the same institutions. Overall rates are two to three times higher, and rates of ventilator-associated pneumonia (VAP) and primary bacteremia—most of which originate from intravascular devices—are 10 times higher. A far greater proportion of ICU-acquired infections are caused by antibiotic-resistant bacteria because the intensive antimicrobial therapy characteristic of modern-day ICUs grossly distorts patients’ microflora. Moreover, more than half of all nosocomial epidemics now occur among the 10% of hospitalized patients confined to an ICU.20,31 Finally, the risk of occupationally acquired infection among health care workers (HCWs), particularly by bloodborne viruses and herpes simplex virus (HSV), is highest among ICU personnel, as contrasted with those who work in non-ICU patient care units (see Protection of Health Care Workers in the Intensive Care Unit later). Nosocomial infections have a considerable impact on morbidity and mortality rates and are estimated to affect more than 2 million patients in U.S. hospitals annually.48 Table 50.3 summarizes major studies that have examined mortality, length of stay, and costs associated with the major nosocomial infections in U.S. hospitals.2–19 Nosocomial infections have been ascribed by the National Institute of Medicine to be responsible for more than 80,000 hospital deaths each year and in 1995 resulted in more than $5 billion in excess health care costs.48 Considering that nosocomial infections acquired by ICU patients account for nearly half of all infections in most hospitals, progress in reducing the incidence of infection acquired within ICUs could produce substantial economic benefits. Beginning in the late 1960s, scattered U.S. hospitals began to establish infection control programs to conduct surveillance, to develop infection control policies, and especially to try to implement control measures more consistently.116 In 1976 JCAHO added to its requirements for hospital accreditation the establishment of a formal infection control program. In the early 1970s the CDC undertook determining the effectiveness of nosocomial infection surveillance and control programs in the United States through the auspices of the Study of the Efficacy of Nosocomial Infection Control (SENIC). The goals of SENIC were to determine the extent to which infection control programs had been adopted by U.S. hospitals and to ascertain how much these programs had reduced rates of nosocomial infection. SENIC was launched by a survey of all U.S. hospitals to determine the characteristics of infection control programs and was completed in 1975-1976 by a review of more than 339,000 patient medical records in 338 randomly selected hospitals.117 The SENIC found that hospitals reduced their nosocomial infection rates by approximately 32% if their surveillance and infection control program included four components: (1) emphasis on both surveillance and an infection control program, (2) at least one full-time infection control practitioner for every 250 beds, (3) a trained hospital epidemiologist, and (4) surveillance of surgical wound infections with feedback of wound infection rates to practicing surgeons.118 However, the relative importance of each component varied for the four major types of nosocomial infections (surgical wound infections, urinary tract infections, bloodstream infections, and pneumonia).118,119 SENIC suggests that nearly one third of all nosocomial infections are in theory preventable, whereas a 1983 survey of surveillance and control programs in a random sample of U.S. hospitals found that failure to implement all essentials of the program, particularly to have an adequate number of infection control practitioners or a trained hospital epidemiologist or to disseminate wound infection rates to surgeons, was greatly limiting the potential for prevention: U.S. hospitals were estimated to be preventing only 9% of all infections.120 It is hoped that surveillance and control programs will continue to evolve. Prevention of nosocomial infections is a major priority of the U.S. Public Health Service,121 JCAHO,30 and the Institute of Medicine.122 With the shift to prospective-payment reimbursement, hospitals now have a powerful financial incentive to reduce their rates of nosocomial infection,123 and it can be anticipated that efforts to prevent hospital-acquired infections will assume ever greater importance. The occurrence of nosocomial infection reflects the conjunction in space and time of a pathogenic microbe and a vulnerable patient, catalyzed by events associated with hospitalization and the patient’s care. Many patients admitted to an ICU are intrinsically more susceptible to infection because of underlying diseases or conditions associated with impaired immunity such as cancer, trauma,49 or advanced age50 or because of immunosuppression associated with malnutrition51 or therapy with corticosteroids,52 cancer chemotherapeutic agents,53 or other immunosuppressive drugs.54 Moreover, many drugs have indirect effects that increase susceptibility to infection, such as narcotics or sedatives that impair the capacity to protect the airway, or antacids or H2-histamine receptor antagonists that neutralize gastric acidity, producing gastric overgrowth by gram-negative bacilli,55 increasing the risk of nosocomial pneumonia.56 Even transfusion therapy produces immunosuppression and increases the risk of nosocomial infection.57 Moreover, most nosocomial pathogens exhibit resistance to antibiotics (see Figs. 50.1 and 50.2),44,45,47,58–61 and many are also more virulent because of (1) their capacity to subsist or even multiply in aqueous reservoirs for prolonged periods (e.g., pseudomonads62 or Legionella pneumophila63); (2) the elaboration of endotoxins (e.g., all of the gram-negative bacilli) or exotoxins (P. aeruginosa,64 C. difficile,65 or S. aureus66); or (3) the production of adhesions67 or exoglycocalyx68 (e.g., coagulase-negative staphylococci), conferring the capacity to adhere avidly and form biofilms on biologic and prosthetic surfaces resistant to host defenses69 and even antibiotics.70 Because most patients in ICUs receive broad-spectrum antibiotics, resistant nosocomial organisms have an enormous ecologic advantage and, in darwinian fashion, predictably supplant the normal cutaneous, respiratory, and gastrointestinal flora. In most cases, colonization is the first step in the progression to nosocomial infection,71 especially if the patient is already vulnerable because of underlying disease, if the organism is more virulent or resistant to antibiotics, or if the patient has invasive medical devices that assist invasion by colonizing organisms, bypassing or further impairing host defenses. In the ICU the major reservoir of nosocomial organisms is the infected or colonized patient (Fig. 50.E1).28 Whereas Streptococcus pneumoniae,72 Mycobacterium tuberculosis,73–75 Legionella,43 Aspergillus and Zygomycetes,40–42 measles,76 rubella,77 and influenza A78 are transmitted by the airborne route, the best evidence suggests that most aerobic bacteria—particularly S. aureus,79 enterococci,29 and the enteric gram-negative bacilli80; many viruses such as hepatitis A, RSV,81 and rotaviruses82; C. difficile 83; and even Candida84—are spread in the ICU on the hands of medical personnel, who themselves are not infected or even permanently colonized. Surgery and exposure to invasive devices of all types greatly amplify transmission, colonization, and susceptibility to infection.28,85 Outbreaks of S. aureus86 or group A streptococcal infection87 usually indicate a health care provider who is a carrier of the epidemic strain. Airborne spread of gram-negative bacilli is probably rare unless unusual environmental circumstances generate massively contaminated aerosols.88 Increasing evidence suggests that many nosocomial infections acquired in the ICU derive from resistant organisms of enteric origin89–92 or that are present on skin89,90 or in the lower respiratory tract91 on admission to the ICU. This explains the failure of conventional infection control practices, based on the use of barriers, to prevent extrinsically acquired infection.93 Whereas food94 and even enteral feeding preparations95 are often heavily contaminated by microorganisms, studies have not conclusively linked such contamination to disease. Nosocomial organisms originating from colonized or infected patients are readily perpetuated and spread in contaminated medical apparatus or devices28 such as urine-collection receptacles,96 respiratory therapy equipment,97,98 transducers used for hemodynamic monitoring,99 dialysis machines,100,101 and fiberoptic bronchoscopes and endoscopes.98,102–104 Given the implicit close proximity of vulnerable ICU patients and the HCWs who have repeated contact with them each day, it is almost predictable that the ICU is a milieu within the hospital uniquely conducive to the epidemic infection, especially infections caused by antibiotic-resistant pathogens. Risk factor analysis using statistical techniques such as multivariate analysis can identify variables that put a patient at increased risk for nosocomial infection and further guide the development of preventive strategies. Risk factors based on prospectively collected data and, in most cases, the use of multivariate analysis are listed in Table 50.2 for urinary tract infection,105,106 pneumonia,107,108 surgical site infection,109 intravascular device–related bloodstream infection,110 ventriculostomy-associated meningitis,111 C. difficile infection,33,112 and candidemia.113–115 Critical care medicine is synonymous with cutting-edge, high-tech medicine; mechanical ventilatory support; hemodynamic monitoring; total parenteral nutrition; hemodialysis; intracranial pressure monitoring; innovative forms of surgery; and a huge arsenal of drugs, especially anti-infectives of every genre. This technology, more than anything else, has forced critical care medicine to accept the necessity for nosocomial infection control. In general, invasive devices of all types are far more important in determining susceptibility to nosocomial infection than underlying diseases (see Tables 50.2 and 50.E1). However, this should be viewed as welcome news: There is far more hope for reducing nosocomial infections in the coming years by innovative improvements in aseptic technique and advances in the technology of invasive devices than by breakthroughs that will reverse the ravages of chronic organ failure or degenerative diseases such as type 1 diabetes mellitus. Table 50.E1 *Relative risk or odds ratio: values >1 denote significantly increased risk of infection, and ratios <1, decreased risk, vis-à-vis a protective effect. From Craven DE, Kunches LM, Lichtenberg DA, et al: Nosocomial infection and fatality in medical and surgical intensive care unit patients. Arch Intern Med 1988;148:1161-1168. JCAHO mandates that all hospitals have an active program for the surveillance, prevention, and control of hospital-acquired infections, which begins with an institutional infection control committee with representation from the major clinical services and hospital departments including the institution’s ICUs. The most essential members of the infection control program are the infection control practitioner(s), usually registered nurse(s), and the hospital epidemiologist, usually a physician with training in infectious diseases or microbiology, who implement the policies developed by the committee, educate hospital personnel about nosocomial infection control, and investigate suspected outbreaks (Box 50.2). Surveillance of nosocomial infections is the cornerstone of an effective infection control program and offers numerous potential benefits119,124: (1) It permits determination of baseline (expected) infection rates, assisting recognition of outbreaks and evaluation of new policies and control measures; (2) it identifies institutional problems that require attention, permitting focused infection control efforts and education; (3) it provides reliable data that can be disseminated to individual departments, increasing awareness and involvement of individual staff members; (4) it increases the visibility of the infection control staff on patient care units, providing an opportunity for consultation and ad hoc education; and (5) it facilitates the earliest discovery of patients with communicable infections, permitting timely institution of isolation precautions to limit spread. Because total surveillance (of all infections) is labor intensive, most hospitals now focus their surveillance efforts on infections that are associated with high morbidity (e.g., nosocomial pneumonia), that greatly increase health care costs (e.g., post–cardiac surgery sternotomy infections), that are caused by antibiotic-resistant organisms with potential for spread (e.g., MRSA, C. difficile), or that are highly preventable (e.g., intravascular device-related bloodstream infections).119,125 The 1990s were characterized by major efforts by hospitals to apply the numerous facets of health care principles of quality improvement developed by industry. Hospital infection control programs had been working on quality improvement126 but, influenced by JCAHO, were probably too heavily focused on process, namely, policies and procedures, rather than documenting outcome vis-à-vis reduced infection rates. Infection control programs in most U.S. hospitals are now closely allied with their institutional quality improvement departments.126,127 Hospital infection control programs are also regulated by the Occupational Safety and Health Administration (OSHA) in terms of institutional standards and programs to protect HCWs from bloodborne pathogens128 and tuberculosis129; the Environmental Protection Agency130 has also published regulations in terms of disposal and tracking of medical waste—only a small fraction of which is truly biohazardous.131 Finally, it is essential that all health care personnel working in an ICU receive training in the epidemiology and control of nosocomial infections. This may be most important for house officers in teaching hospitals, who commonly enter the ICU with only the most rudimentary knowledge of asepsis but have hands-on contact with numerous patients each day. ICU physicians and nurses must be especially familiar with their hospitals’ guidelines for the management of invasive devices, particularly intravascular catheters of all types,132 urinary catheters,133,134 endotracheal tubes,135 and tracheostomies.135 Moreover, all physicians need to be made aware that broad-spectrum antimicrobial therapy greatly increases the risk of superinfection by antibiotic-resistant bacteria and Candida, as well as C. difficile. Accurate and timely diagnostic microbiology is as essential for nosocomial infection control as it is for the clinical management of patients’ infections. Although many infections can be diagnosed on the basis of clinical criteria alone, cultures and other laboratory tests allow infections to be diagnosed with much greater certainty, and certain infections such as bacteriuria, bacteremia, and fungal and viral infections cannot be diagnosed without cultures or other laboratory tests (see Box 50.1).16,17 Moreover, accurate antimicrobial susceptibility testing of clinical isolates is the only means of monitoring trends in antibiotic resistance of hospital organisms.136 Most importantly, identifying the microbial cause of nosocomial infections allows epidemiologic tracking of individual pathogens within the hospital, especially those that are commonly spread from patient to patient such as S. aureus, beta-hemolytic streptococci, enterococci, and the numerous gram-negative bacilli. From an organizational standpoint, the institutional infection control program and clinical microbiology laboratory must have a close working relationship (see Box 50.2) to assist surveillance, which must be strongly laboratory based,119,137 and to permit the detection and resolution of potential problems. The laboratory director or a senior member of the laboratory staff should be a permanent member of the infection control committee. The primary role of the clinical microbiology laboratory in any infection control program is to provide up-to-date clinical microbiologic data for use in the surveillance of nosocomial infections and identification of potential outbreaks.137 Protocols should be developed to ensure that laboratory staff immediately contact infection control personnel after the isolation of certain important pathogens such as MRSA or vancomycin-resistant enterococci (VRE) or the appearance of new resistance patterns in endemic organisms such as resistance of Klebsiella species to third-generation cephalosporins and carbapenems, or P. aeruginosa to aminoglycosides, fluoroquinolones, and carbapenems. Sifting through these data can be time consuming, and developing electronic information systems that streamline this process is essential to improving the efficiency of the infection control program. Commercial software programs that can automate this process are now available. Many of these programs automatically collate microbiologic data, provide rudimentary geographic information, and perform basic statistical analyses that can assist in the surveillance of nosocomial infections and identification of potential outbreaks.138,139 Reporting cumulative summaries of antimicrobial susceptibility data (antibiograms) is another essential responsibility of the clinical microbiology laboratory.140,141 When implemented appropriately, the timely dissemination of antibiograms helps guide the choice of empiric antimicrobials, pending the results of clinical cultures, and provides valuable data to help the infection control department monitor institutional antimicrobial resistance trends and identify potential outbreaks.142 The Clinical and Laboratory Standards Institute—formerly the National Committee for Clinical Laboratory Standards—recommends that institutional antibiograms be updated at least annually and has recently published standards for their content and format.143 Automated electronic systems for collating and disseminating nearly real-time antibiograms along with antibiotic-use decision support exist and, when implemented properly, have been effective in improving antimicrobial utilization within the hospital setting.144,145 Monitoring of sterilizers with spore tests, environmental sampling, and advanced microbiologic support for epidemiologic investigations are additional responsibilities expected of most clinical microbiology laboratories, although some university hospital programs have dedicated personnel within their infection control programs who perform these activities.137 The clinical microbiology laboratory is a key resource in the investigation of a suspected outbreak. One of the first and foremost actions when a nosocomial outbreak is suspected is to immediately retrieve all available isolates of the putative epidemic pathogen for possible subtyping.146 The need to move rapidly becomes apparent when it is realized that most hospital laboratories discard cultures as soon as the isolates have been fully characterized. All blood isolates should be routinely saved for at least 1 year.146 Laboratory personnel must be requested to save clinical isolates of any unusual organisms that are encountered for the first time or clusters of any organism and to inform infection control personnel of the findings and availability of the isolates. The rapid evolution of molecular microbiology has revolutionized epidemiologic investigation of nosocomial outbreaks. Molecular-based tests for the rapid diagnosis of bacterial,147 viral,78,148 and fungal149 infections are now routinely available in most hospital-based and reference laboratories. Modern molecular tests can reliably detect minute numbers of organisms, allowing direct testing of clinical samples without the need for culture. In modern-day clinical virology, molecular tests based on polymerase chain reaction (PCR) for amplification of the pathogen’s DNA or RNA have supplanted tissue cultures and now allow rapid diagnosis of infections that would otherwise often not be identifiable by classic methods. The availability of molecular subtyping systems has greatly strengthened investigations of outbreaks, as well as research on the epidemiology of nosocomial infections.150,151 The antimicrobial susceptibility pattern (antibiogram) or the detailed biochemical profile (biotype) is often useful for the initial epidemiologic subtyping of many bacteria and may be adequate for identifying an epidemic caused by an unusual pathogen. However, if an epidemic organism is a common species such as S. aureus, it can be difficult or even impossible to know with certainty that an outbreak derives from a common source using these techniques because they lack sufficient discriminatory power.150 The new molecular techniques of subtyping such as plasmid profile typing by agarose gel electrophoresis or the use of restriction endonuclease digests with pulsed-field electrophoresis (DNA fingerprinting)150 are now available in most infection control research laboratories but should be adaptable by many hospital laboratories. Genetic probes promise even more powerful tools for investigating outbreaks, particularly those caused by antibiotic-resistant organisms.147 Although molecular-based tests offer several advantages over traditional microbiologic techniques, they are not a panacea. A number of molecular diagnostic assays (e.g., analyte specific reagents [ASRs]) marketed for clinical practice do not require approval by the U.S. Food and Drug Administration.147 In the absence of published data on their accuracy and precision, the results of these tests must be interpreted with caution and should always undergo extensive inhouse validation before widespread adoption. Moreover, the exquisite sensitivity of many of these tests renders them more susceptible to false-positive results as a consequence of environmental contamination152,153 and mandates stringent quality control practices and procedures. The role of the inanimate environment on the transmission of nosocomial infections has been a subject of intense debate for decades. It has been shown that hospital surfaces are almost universally contaminated by potentially pathogenic bacteria such S. aureus,154 enterococcus,155 and gram-negative bacilli such as Acinetobacter baumanii.156 Prior to the 1970s, infection control personnel routinely sampled hospital surfaces. Despite this level of surface colonization, early studies found that the inanimate environment—surfaces, walls, and even air—does not contribute materially to the occurrence of most nosocomial infections,157 other than invasive infections caused by airborne Aspergillus and other filamentous fungi in seriously immunocompromised patients.40,41 Although inanimate surfaces may rarely be involved in the direct transmission of infection to patients, more recent evidence suggests that surfaces may well play an important role in the nosocomial acquisition of pathogenic bacteria, indirectly, through contact with HCWs’ hands and equipment (see Fig. 50.3). This indirect route of infection is of particular importance in the ICU, where all patients are heavily exposed to invasive devices and have a high risk of infection. In the ICU the inanimate environment may become a reservoir for the transmission of resistant nosocomial organisms such as MRSA,158,159 C. difficile,83,160 VRE,155,161 and gram-negative bacilli such as Klebsiella spp., Acinetobacter spp., and Enterobacter organisms.162,163 Studies have shown that enhanced surface decontamination with hypochlorite-containing cleaning solutions has been necessary to terminate outbreaks caused by C. difficile 164 and Acinetobacter baumanii.156 An ICU must be adequately staffed to allow the processes of care to be carried out but also assure a high level of compliance with essential infection control measures such as hand hygiene and barrier isolation. Adequate staffing cannot be overemphasized; numerous studies have found greatly increased rates of nosocomial infection when ICUs are staffed suboptimally or when staffing requirements are met with temporary personnel who are unfamiliar with ICU infection control policies and procedures.165,166 In a large nosocomial outbreak of Enterobacter cloacae infection in a neonatal ICU, Harbarth and colleagues167 found that infection rates during periods of understaffing were strikingly higher than during periods with adequate levels of staffing (relative risk [RR] = 6, 95% confidence interval [CI] = 2.2 to 16.4). The effects of understaffing are likely multiple; however, erosion of basic hygienic practices with excessive patient-to-staff ratios likely explains much of this phenomenon.168 Many of the published recommendations for ICU architectural design169 are empiric, and evidence that they reduce rates of nosocomial infection is, by and large, lacking. Although more research is necessary before specific features of ICU design achieve a level of evidence sufficient for an evidence-based guideline, certain facets of the ICU layout deserve attention: • ICUs should be located in areas that limit traffic flow to essential ICU personnel. • ICU facilities should be designed with ICU professionals in mind, ensuring appropriate space, resources, and environment for day-to-day operations.169 Recognizing the growing variety and complexity of life support equipment required for the care of many patients, each cubicle or room should provide a minimum of 11 m2 per bed.170 The area should be large enough to accommodate the bed and all equipment yet allow immediate access to the patient at all times from both sides of the bed. Adequate space must also be provided for storage of nursing supplies. Facilities for disposal of biohazardous waste (e.g., bedpan flushers); for cleaning, reprocessing, and storage of ICU equipment; and for storage of housekeeping supplies should be separate from patient care areas. Single-patient rooms may increase the likelihood of handwashing being done and improve compliance with isolation practices, reducing the risk of cross-infection. For example, Mulin and colleagues171 found that converting from an open unit to single rooms in their ICU greatly reduced rates of patient colonization with A. baumanii, and Shirani and colleagues172 found that renovation of their burn unit to include separate bed enclosures reduced rates of nosocomial infection by 48%.172 • Materials used for fixtures, furniture, and other surfaces should be smooth and easy to clean; surfaces made of porous materials foster bacterial colonization.173 • An adequate number of sinks must be available for convenient handwashing by ICU personnel. Ideally, a sink should be located at the entrance of each cubicle or patient room to encourage handwashing by all entering personnel who will have contact with the patient or the immediate environment.174,175 Separate sinks should be used for cleaning and reprocessing contaminated equipment. Sinks and sink drains are normally contaminated by pseudomonads,176 although their role in the epidemiology of nosocomial infection is as yet unclear. However, sinks should be designed to minimize aerosol formation and splashback. • All ICUs should be equipped with one or more class A isolation rooms, which include an anteroom for gowning and handwashing and the necessary modifications (negative pressure, roofline exhaust) to permit it to be used for patients with tuberculosis or other airborne infections such as chickenpox, measles, disseminated HSV infection, or a highly contagious emerging pathogen such as the severe acute respiratory syndrome (SARS) human coronavirus. If an ICU treats bone marrow transplant patients or other patients with prolonged severe granulocytopenia, positive-pressure isolation rooms using high-efficiency particle-arrest (HEPA) filters should be available. Isolation rooms for patients with infections transmitted by the respiratory route or to protect profoundly granulocytopenic patients must be kept closed to maintain control over the direction of airflow. • A centralized, filtered air-handling system that provides at least six room exchanges per hour is essential.170,177 Ideally, each patient’s room should have the capacity of being set at positive or negative pressure with respect to the rest of the unit; if it cannot be, the room should be maintained permanently at positive pressure. A variety of microorganisms including bacteria, mycobacteria, fungi, and parasites can be isolated from hospital water and have been implicated in endemic and epidemic nosocomial infections.178 Many of these outbreaks were caused by bacteria typically thought of as “water” organisms such as P. aeruginosa,176 Stenotrophomonas maltophilia,179 and A. baumanii16,180,181; however, the most important and epidemiologically linked hospital water pathogen is the Legionella group.182 Nosocomial legionellosis was first described in 1979,183 and it is estimated that up to 50% of cases of legionellosis are acquired in the health care setting,184 with a mortality rate that approaches 30%.185 Contamination of hospital potable water remains underappreciated despite studies showing that Legionella species can be recovered from 12% to 70% of hospital water systems,186 and a number of studies in which nosocomial cases were identified only when specific diagnostic and surveillance methods were employed.187,188 Characteristics of hospital water systems that are associated with Legionella contamination include piping systems with dead-ends that facilitate stagnation, large-volume water heaters that result in inefficient heating of hospital water, sediment buildup, water heater temperatures less than 60° C and tap water temperatures less than 50° C, maintaining water pH greater than 8 and receiving municipal water untreated with monochloramine.189–191 Despite the ubiquity of water systems colonized with Legionella species and studies demonstrating a correlation between the level of colonization and risk of infection, the CDC does not recommend routine surveillance of hospital water systems,184 although this stance is controversial.186 Researchers from Pittsburgh, Pennsylvania, and the Allegheny County Health Department have recommended a more proactive stepwise approach that involves initial surveillance of hospital water for Legionella contamination, regardless of the presence or absence of institutional nosocomial legionellosis, followed by continued surveillance based on the level of water contamination found or the presence of institutional legionellosis.186 Legionella species are resistant to chlorine and heat, making it challenging to eradicate them from contaminated hospital water systems.191 Attempts to hyperchlorinate hospital water have been partially successful if chlorine levels are continuously maintained between 2 and 6 parts per million at all times but produce rapidly accelerated corrosion of water pipes and are expensive.192 Thermal eradication is feasible, using a “heat-and-flush” method to raise water tank temperatures to greater than 70° C and distal water sites to greater than 60° C for short periods of time.193 Although effective, superheating is labor intensive and there is the constant fear that patients or health care personnel may sustain scald injuries if they wash or shower with tap water during a flushing period. The use of technologies such as instantaneous steam heat for incoming water193 and ultraviolet light194 are technically feasible with newer hospital water systems but may be incompatible with older hospital water systems. Perhaps the most attractive, effective, safe, and cost-efficient method for Legionella eradication may be the use of continuous copper-silver ionization systems to sterilize hospital water systems. These systems have been well studied over the past decade and have proved to be highly effective for reliably eradicating Legionella contamination of hospital water and, most importantly, for eliminating nosocomial legionellosis in institutions when other interventions have failed.195 In our own institution (Maki DG), two clusters of nosocomial legionellosis prompted a retrospective review that identified 10 cases over an 11-year period. Surveillance of the hospital water system found that 75% of all samples contained low levels of L. pneumophila, which were shown to be clonally related to the 10 cases of nosocomial legionellosis. Installation of a continuous copper-silver ionization system led to complete eradication of Legionella from water samples, and no further cases of nosocomial legionellosis have been identified at our institution since 1995, among 255,000 patients hospitalized. Reliable sterilization, disinfection, and antisepsis embrace virtually all measures aimed at prevention of nosocomial infection. Critical objects, which are introduced directly into the bloodstream or into other normally sterile areas of the body, such as surgical instruments, cardiac catheters, and implanted devices, must be reliably sterile and sterilized with steam, gas, hydrogen peroxide gas, or chemical sterilization. Semicritical items, which come into contact with intact mucous membranes, such as fiberoptic endoscopes, endotracheal tubes, or ventilator circuit tubing, can be decontaminated between patients by pasteurization or the use of high-level chemical disinfection with glutaraldehyde, peracetic acid, hydrogen peroxide, ethyl alcohol, or hypochlorite. Noncritical items, which normally come into contact only with intact skin, such as blood pressure cuffs or electrocardiograph electrodes, require hygienic cleansing or low-level disinfection with an iodophor, hypochlorite, quaternary ammonium or phenolic disinfectants, or alcohol.196,197 The lone exception to this classification scheme is devices that pose a risk of transmitting prion-related diseases, including Creutzfeldt-Jakob disease (CJD) and variant CJD (vCJD).198,199 Prions are not readily inactivated by conventional disinfection and sterilization procedures.196,199 As a result, devices that pose a risk for transmission of prion-related diseases should undergo special sterilization procedures after cleaning that involve sodium hydroxide followed by low-temperature autoclaving (121° C) or high-temperature autoclaving (132° C for 1 hour or 134° C for ≥18 minutes).197,199 Despite concerns that procedures involving semicritical items such as endoscopes and bronchoscopes may pose a risk for transmission of prion-related infections, there has not been a single report of CJD or vCJD associated with these devices. As a result, current guidelines recommend that only critical items and semicritical items that have come in contact with neurologic tissue (e.g., brain [including dura mater], spinal cord, eye, and pituitary tissue) should undergo special prion inactivation sterilization procedures.197,199,200 Numerous epidemics of gram-negative infection have been described in association with respiratory therapy equipment,97,98 diagnostic equipment such as bronchoscopes and endoscopes,98,102–104 and solutions used for cutaneous antisepsis.201,202 Most of these outbreaks were traced to improper procedures or malfunction of automated systems used for the disinfection and sterilization of medical devices, although a number of epidemics in years past arose as a result of extrinsic contamination of solutions used for cutaneous antisepsis.201,202 For these reasons, the importance of strict adherence to recommended policies and procedures for cleaning and reprocessing medical equipment used in the ICU cannot be overemphasized. Endoscopes and bronchoscopes are essential diagnostic and therapeutic instruments in the ICU. Although most postendoscopy nosocomial infections are caused by inoculation of colonizing mucosal flora into normally sterile, vulnerable anatomic sites during the procedure, numerous epidemics have been traced to contaminated endoscopes.98,102–104 Following use for bronchoscopy, endoscopes are typically contaminated with 6×104 colony-forming units per milliliter (CFUs/mL).203 All endoscopes are considered semicritical medical devices by the Spaulding classification and therefore require high-level disinfection following use.200 In order to ensure their safe use, flexible endoscopes should be reprocessed with the following procedures: (1) physical cleaning to reduce microbial bioburden and remove organic debris; (2) high-level disinfection—glutaraldehyde and automated chemical sterilizing systems that use peracetic acid are most commonly used in the United States—with adequate contact time between the disinfectant and device surface; (3) following disinfection, rinsing with sterile or filtered tap water to remove disinfectant residue; (4) flushing of all channels with 70% to 90% ethyl or isopropyl alcohol; and (5) drying with forced air.200 Devices used with endoscopes that violate mucosal barriers, such as biopsy forceps, need to be reprocessed as critical medical items with full sterilization.200 Other devices used in the delivery of respiratory care are also considered semicritical under the Spaulding classification and therefore should be reprocessed in a manner similar to endoscopes prior to reuse.135 Iodophors (e.g., 10% povidone-iodine), until recently, have been the most common agents used for cutaneous disinfection in North America. However, a large, prospective, randomized trial of cutaneous antiseptics used for drawing blood cultures showed that chlorhexidine was superior to 10% povidone-iodine and was associated with a more than twofold reduced rate of contaminated blood cultures (odds ratio [OR] = 0.40, 95% CI 0.21 to 0.75, p = 0.004).204 Moreover, a meta-analysis examining the impact of different cutaneous antiseptic agents found that chlorhexidine was superior to povidone-iodine for both the prevention of intravascular catheter colonization and catheter-related bloodstream infection.205 On the basis of these and other recent studies,206,207 chlorhexidine-containing solutions are the preferred cutaneous antiseptics for insertion of intravascular devices in the ICU.132 Whatever agent is used, it is essential that it be applied with vigorous scrubbing for a minimum of 1 minute to allow adequate time for germicidal activity. The major reservoir of nosocomial infection in the ICU is infected or colonized patients, and the major mode of spread of most nosocomial bacterial pathogens, many viruses, and even Candida from patient to patient is by transient carriage on the hands of medical personnel (see Fig. 50.3). Studies of hand carriage of nosocomial pathogens by ICU personnel, using a simple rinse technique to quantify the transient flora,208 have shown that, on average, approximately 60,000 CFUs (or 4.6 logs) are recovered from the hands of ICU personnel randomly sampled (Table 50.4). Nearly half of persons cultured at any point in time will be found to be carrying gram-negative bacilli, and 10% will be carrying S. aureus.
Nosocomial Infection in the Intensive Care Unit
![]() APPROACH TO A NOSOCOMIAL EPIDEMIC
APPROACH TO A NOSOCOMIAL EPIDEMIC
![]() Additional online-only material indicated by icon.
Additional online-only material indicated by icon.
Incidence and Profile
Definitions
Incidence
Profile and Secular Trends
Morbidity and Economic IMPACT
General Infection Control Measures
Hospital Infection Control Programs
Pathogenesis and Epidemiology
Pathogenesis
Reservoirs and Transmission
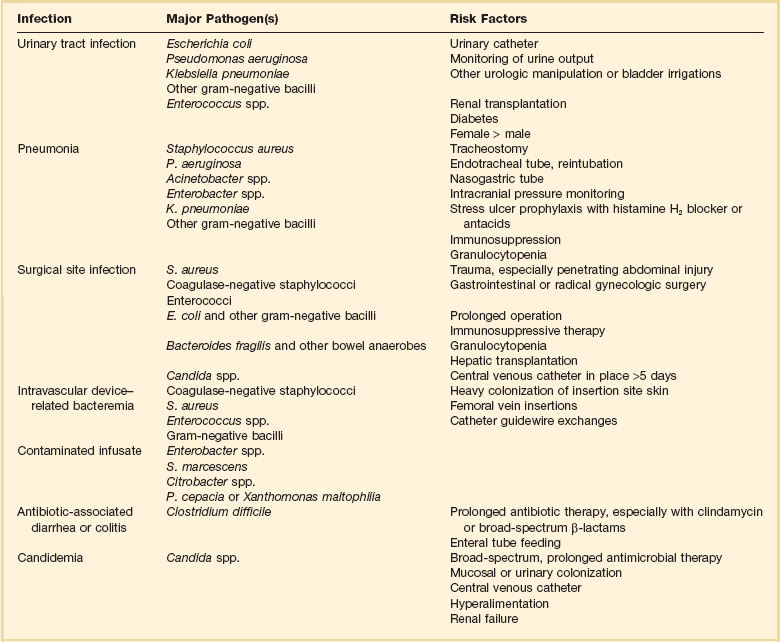
Risk Factors

Role of the Microbiology Laboratory
Architectural and Environmental Issues
Reliable Sterilization Procedures, Chemical Disinfectants, and Antiseptics
Hand Hygiene
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nosocomial Infection in the Intensive Care Unit