CHAPTER 45

NONTRAUMATIC VASCULAR EMERGENCIES
Nontraumatic vascular emergencies span the entirety of the body as would be expected given the life-essential nature of the vascular system. The diverse presentations reflect the specifics of each affected tissue bed. However, common themes unite the management of these conditions. First, thorough physical examination is essential as many of the vascular beds can be interrogated directly by examination or its extension, Doppler ultrasound. Second, imaging modalities, including color duplex ultrasound, computed tomographic angiography, and angiography, complement the evaluation of a vascular patient, helping to determine the optimal treatment. Finally, technologic advances in transcatheter intervention now present the surgeon with attractive alternatives to traditional open surgery. Some endovascular interventions complement rather than replace surgery, so multidisciplinary vascular skill sets are becoming essential. Given the rapid evolution of vascular surgery, continuing medical education is essential in caring for these conditions.
In this chapter, the breadth of both arterial and venous conditions most commonly presenting in the emergency room is addressed. For the arterial system, rupture and ischemia in multiple anatomic territories and acute aortic dissection are discussed. For the venous system, the management of venous thrombosis in multiple organ systems is reviewed.
ARTERIAL EMERGENCIES AND ARTERIAL RUPTURE
Abdominal Aortic Rupture. Approximately, 15,000 deaths per year due to ruptured abdominal aortic aneurysms (rAAAs) occur in the United States, making it the 13th leading cause of death.1 These deaths represent a small fraction of the estimated 1.7 million patients with abdominal aortic aneurysm (AAA) in the United States, with 190,000 new cases discovered each year.1
To date, the cause of AAA in the majority of patients is unknown. Although atherosclerosis, hypertension, smoking (8:1), and male gender (4:1) have been found to be associated with AAA, no specific mechanism accounts for the development of AAA.2 In a minority of patients, AAA is known to be related to underlying connective tissue disorders, such as Marfan’s syndrome or Ehlers-Danlos syndrome, aortitis due to syphilis or other aggressive microorganisms, or degeneration of wall integrity with aortic dissection and cystic medial necrosis.
The classical presentation of rAAA is hypotension, abdominal or back pain, and a pulsatile abdominal mass. However, rAAA can present insidiously even while the rupture is contained by the retroperitoneum or if only a small leak has developed. Inevitably, acute decompensation with loss of tamponade occurs within 24 hours leading to hemorrhagic shock. The rupture frequently occurs through the left posterolateral aortic wall into the retroperitoneum (Fig. 45.1). Rupture into the gastrointestinal tract or inferior vena cava (IVC) (aortocaval fistula) have been reported. Patients with aortocaval fistula may also have an audible abdominal bruit and venous hypertension resulting in swollen cyanotic legs, lower gastrointestinal bleeding, and hematuria. Rarely, contained rAAA presents with radicular symptoms due to nerve root compression and bowel obstruction due to compression. Additionally, fever and leukocytosis in the presence of rAAA symptoms merit consideration of infected AAA, as these patients are at high risk for rupture.3
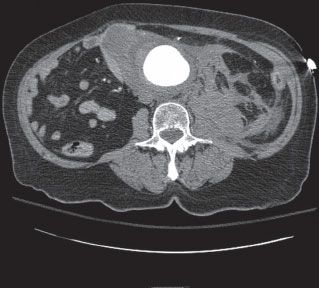
FIGURE 45.1. Abdominal aortic aneurysm ruptured with left posterolateral retroperitoneal hematoma.
The classic presentation of rAAA remains an indication for proceeding directly to the operating room (OR). However, for patients who present with stable hemodynamics, the diagnostic algorithm has evolved over the last decade. With the increased availability of spiral computed tomography (CT), patients can be screened for rAAA within minutes. The anatomical information provided by a CT angiogram (CTA) helps determine suitability for an endovascular aortic repair (EVAR).
Controversy exists with regard to the optimal technique for repair of rAAA. However, multiple studies have been published demonstrating decreased mortality for EVAR compared to open repair. In a recent meta-analysis, a 38% reduction in mortality was reported in patients treated with EVAR versus open repair for rAAA.4 However, the substantial institutional, material, and manpower commitments necessary to execute an emergent EVAR for rAAA must be emphasized.
Mehta published the Albany Vascular Group’s standardized protocol for endovascular aneurysm repair of rAAA (Algorithm 45.1).5 Hemodynamically unstable patients (systolic blood pressure <80 mm Hg) proceed directly to the OR. Permissive hypotension is utilized in these patients to limit further hemorrhage. Stable patients undergo emergent CTA to evaluate for EVAR anatomic suitability and then proceed to the OR. While in the OR, a femoral artery cutdown is completed to allow placement of a long 12–14 French arterial sheath, which is advanced over a guidewire into the juxtarenal aortic position, allowing both performance of an angiogram via the sheath and support of an aortic occlusion balloon. If a patient remains hemodynamically unstable, an aortic occlusion balloon is advanced over the wire into the supraceliac position. Inflation of the occlusion balloon replaces the traditional supraceliac aortic clamp, allowing the anesthesia team to resuscitate the patient with free rupture from the aorta thus controlled. An angiogram is performed to evaluate the neck of the aneurysm to determine if an EVAR is feasible. A detailed discussion of the technique of EVAR is beyond the scope of this chapter, but in short, the technique involves placement of a covered stent graft inside the aorta to cover the rupture and exclude the aneurysm. Current commercially available endografts require a 10–15 mm proximal neck and a 20 mm iliac landing zone to complete an EVAR successfully.
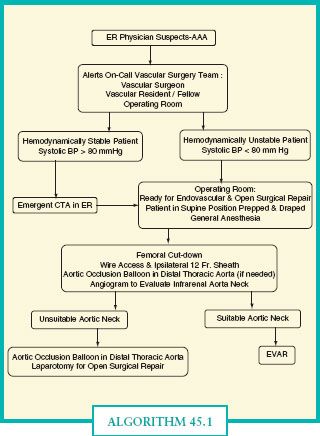
ALGORITHM 45.1.Albany vascular group ruptured AAA protocol.(Reproduced from Mehta M, et al. Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysm: outcomes of a prospecxtive analysis. J Vasc Surg. 2006;44:1–8, with permission.)
If the patient’s anatomy is not compatible with EVAR, an open repair is necessary. First, an abdominal incision is made, either midline or wide transverse for a transperitoneal approach or a left flank lazy S incision for a retroperitoneal approach. If the retroperitoneal hematoma extends to the pararenal aorta or root of the mesentery, the supraceliac aorta is exposed by dividing the left triangular ligament of the liver and opening the lesser omentum. If the hematoma is more contained, then direct exposure of the infrarenal aorta can be accomplished, taking care to avoid injury to the crossing left renal vein. Also, remember that the left renal vein is posterior to the abdominal aorta in 5% of patients. Once the supraceliac aorta is exposed, the occlusion balloon can be exchanged for a supraceliac clamp, if necessary. The distal extent of the aneurysm is determined with dissection of the distal aorta. Depending upon the status of the iliac arteries and aortic bifurcation, a bifurcated or tube graft replacement of the aorta is performed. Based on the clinical status of the patient, systemic heparinization may be used. The aorta is controlled proximally and distally with clamps and the aneurysm sac is opened longitudinally. The thrombus is removed from the aneurysm sac and lumbar arteries are suture ligated from within the aorta. If heavy back bleeding is present from the inferior mesenteric artery, its orifice is also suture ligated. A synthetic Dacron graft is sewn into place with permanent synthetic monofilament sutures. The sac should be reapproximated over the graft and the retroperitoneum is closed if possible. If not, a tongue of omentum is rotated into the retroperitoneum to cover the aortic repair in order to prevent aortoenteric fistula formation. Not infrequently, abdominal compartment syndrome develops in rAAA due to the fluid shifts associated with aggressive resuscitation, bowel edema, and accumulation of large intraperitoneal or retroperitoneal hematomas.6 Thus, consideration of delayed fascia closure or temporary mesh closure may be necessary.
Thoracic Aortic Rupture. Similar to abdominal aortic rupture, ruptured thoracic aortic aneurysms (rTAAs) comprise the majority of the nontraumatic thoracic aortic ruptures. The literature documents an annual incidence of rTAA of 5 per 100,000.7 The majority of rTAAs are localized to the ascending aorta and arch, with only 30% in the descending aorta.7 These patients have a mortality rate as high as 97%, with the majority of patients dying before reaching the hospital.7 In a recent meta-analysis, a male predominance of 70% was reported with an average age of 70 ± 5.6 years old.8
Thoracic aortic aneurysms (TAAs) most commonly result from age-related atherosclerotic medial degenerative disease (70%-93%) or aortic dissection (4%-30%).9 Connective tissue disorders such as Marfan’s syndrome (1.6%-10.9%) or Ehlers-Danlos syndrome (1.1%-4.2%), aortitis due to syphilis or granulomatous disease, aortitis such as Takayasu’s disease (0.9%-2.1%), and trauma (0.1%-1.8%) have been reported as causes for development of TAA.9
Ruptured TAA frequently presents with a mixture of aneurysm and rupture symptoms. Pain is commonly cited: ascending aortic involvement presenting with anterior chest pain, aortic arch involvement leading to neck pain, descending aortic involvement presenting with back pain localized between the scapulae, and diaphragmatic level aortic involvement causing midback and epigastric pain. Interestingly, TAA tend to be more symptomatic (48%) and more likely to rupture than AAA.9 Ascending aortic aneurysms can cause superior vena cava (SVC) obstruction, aortic valvular insufficiency, and heart failure. Arch aneurysms can cause hoarseness due to recurrent laryngeal nerve compression. Descending thoracic aneurysms can compress adjacent structures, causing dyspnea or stridor with compression of the trachea or a major bronchus, or dysphagia with compression of the esophagus. Thrombus from TAA can embolize to the anterior spinal artery, causing paraparesis or paraplegia, and also to distal tissue beds, resulting in a variety of symptoms dependent on the specific organ or limb affected. Rupture of the ascending aorta may result in pericardial tamponade, dissection of the aortic valve leading to aortic insufficiency, or dissection of the coronary arteries leading to a myocardial infarction. Rupture of a TAA into an adjacent structure can result in hemoptysis, hematemesis, gastrointestinal bleeding, and progressive dyspnea from parenchymal lung compression from intrapleural hemorrhage.
To date, surgical repair in the hands of cardiothoracic surgeons (or vascular surgeons at some centers) continues to be used in the management of ascending aorta and aortic arch rupture. The details of such repairs are beyond the scope of this chapter. In general, repair of ascending aortic rupture involves replacement of the aorta with a Dacron supracoronary tube graft from the sinotubular junction to the origin of the innominate artery under cardiopulmonary bypass with possible aortic valvuloplasty or replacement. For aortic arch aneurysms, deep hypothermic circulatory arrest with cerebral perfusion is used with replacement of the arch with a Dacron graft extending to the descending thoracic aorta. The supra-aortic trunk vessels are either reimplanted or sewn as a patch to the graft. If a concomitant descending thoracic aneurysm is present, an elephant trunk procedure may be completed, in which the graft is allowed to telescope into the descending aorta beyond the arch repair’s distal anastomotic suture line.
With the advent of thoracic endovascular aortic repair (TEVAR), the paradigm for repair of ruptured descending TAA has evolved, although the rarity of rTAA limits the availability of class I evidence. TEVAR technology, in short, involves intravascular deployment of a covered stent, which covers the rupture and excludes blood flow from the aneurysm sac, (Fig. 45.2). TEVAR requires proximal and distal landing zones of 20 mm and a commercially available device large enough to exclude blood flow from the aneurysm at these seal zones. Other adjunctive procedures, such as common carotid to common carotid bypass, common carotid to subclavian bypass, or visceral debranching procedures may also be necessary. A recent meta-analysis for ruptured descending TAA demonstrated a lower 30-day mortality in patients treated with TEVAR compared to open repair, 18.9% versus 33.3%.8 Additionally, myocardial infarction was less common, 3.5% versus 11.1%, and a trend toward improved stroke and permanent paraplegia rates was demonstrated.10 Other retrospective series support the benefits of this paradigm shift in management.10,11
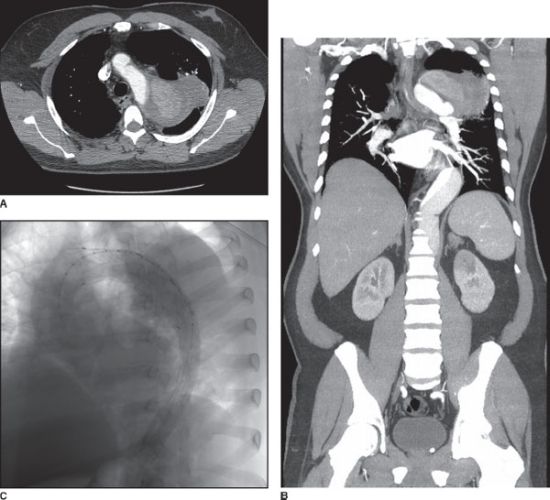
FIGURE 45.2. Ruptured thoracic aneurysm from chronic aortic dissection. A,B: Transverse and coronal section. C: After repair with TEVAR with left subclavian artery fenestration and stenting.
If the patient’s anatomy is unfavorable for endovascular repair, surgical repair of the ruptured descending TAA is necessary. A detailed discussion of such surgical technique is beyond the scope of this chapter. Simplified, the surgical technique involves a left posterolateral thoracotomy or thoracoabdominal incision and clamping the aortic arch either distal to the left subclavian artery or between the left common carotid and left subclavian artery. At the surgeon’s judgment, atrio-femoral bypass may be utilized to provide retrograde perfusion to the spinal cord, viscera, and kidneys. Finally, a segmental aortic clamp and sew technique is utilized to minimize segmental spinal ischemia, reimplanting large intercostals arteries as deemed necessary. If mesenteric and renal arteries are compromised by the aortic repair, they should be reimplanted either separately or via a Carrell patch including the compromised arteries.
Iliac Artery Rupture. Similar to rAAA, iliac artery rupture presents in the setting of preexisting iliac artery aneurysms (IAAs). Due to their location in the pelvis, IAAs frequently are not discovered until they are larger than the currently recommended repair size of 3.5 cm. The average mortality for emergent repair of ruptured arteries is 28% versus 5% for elective repair, emphasizing the importance of early detection.12
Isolated IAAs are rare with a prevalence of only 0.4%-1.9% of all aneurysms.12 IAAs are more commonly found with AAAs, being prevalent in 10%-20% of cases of AAA.12 The primary iliac artery segments involved are: common iliac artery (CIA) (70%), internal iliac artery (IIA) (20%), multiple segments (10%), and external iliac artery (rare).12 Also, one-third of patients with an IAA will have an aneurysm in the contralateral iliac artery. Etiologies for these aneurysms are unknown, but thought to be similar to the etiologies observed for AAA, with medial degeneration from atherosclerosis being the most common cause.
Ruptured iliac arteries frequently present with sudden abdominal, groin, or thigh pain with a pulsatile mass in the affected groin. Prior to rupture, 50% of IAA will be symptomatic due to compression or erosion into surrounding structures: compression of ureters leading to pyelonephritis and sepsis, compression of the rectum leading to pain with defecation, and compression of pelvic nerves leading to paraesthesias of the lower extremities. Rarely, IAA present with thromboembolic symptoms or cardiac overload due to erosion into an iliac vein.
Open operative repair of IAA remains the gold standard. In short, CIA aneurysms are repaired with interposition graft and IIA aneurysms are repaired by ligation with or without interposition graft placement.12,13 With the advent of endovascular interventions, multiple series now document endovascular treatment of IAA. IAA in the setting of AAA is now treated by exclusion with endograft iliac limbs as part of EVAR.12,13 Additionally, embolization of the IIA, consisting of placement of coils in the main IIA trunk if nonaneurysmal and additional coiling of the anterior and posterior divisions of the IIA if aneurysmal, may be a necessary adjunct to prevent a type II endoleak in these cases. In the setting of isolated CIA aneurysm, an endograft iliac limb is deployed if the proximal CIA landing zone is ≥ 20 mm; otherwise, a bifurcated EVAR is completed with iliac limb coverage of the IIA.12,13 If an isolated IIA aneurysm is present, the IIA is embolized as described above with or without coverage with an iliac endograft limb.12,13
Peripheral Artery Rupture. Nontraumatic rupture of peripheral arteries, consisting of arteries distal to and including the subclavian artery and distal to and including the femoral artery, is rare. This is in part due to the rarity of bland aneurysms of these arteries and their tendency to present with thromboembolic symptoms rather than rupture.
In contrast, mycotic aneurysms of peripheral arteries usually rupture. The root cause is infection with bacteria or fungi that lodge in vessels and cause transmural necrosis, leading to aneurysm formation. The clinical presentation of these aneurysms ranges from overwhelming sepsis to more insidious symptoms of fever, malaise, chills, night sweats, and pain. Often on examination, the skin demonstrates erythema, tenderness, and a palpable pulsatile mass.
The high rate of rupture of mycotic peripheral aneurysms justifies intervention in all cases, as antibiotics are inadequate to treat the compromised arterial wall. Debridement of necrotic tissue is essential in all cases. For subclavian and axillary arteries, a discussion of surgical approaches to repairing these arteries is beyond the scope of this chapter, as exposure of these arteries depends on location of involved segment, ranging from median sternotomy or left posterolateral thoracotomy to simple supraclavicular or axillary incisions. In short, if injury to the subclavian vein and brachial plexus can be avoided, treatment involves excision and reconstruction of artery with vein interposition graft.14 If adjacent structures are likely to be injured, arterial ligation with incision and drainage of the aneurysm has been reported. Forearm and hand mycotic aneurysms usually require only excision and ligation.15 Finally, femoral and popliteal artery mycotic aneurysms require aneurysm excision and arterial reconstruction with saphenous or femoral vein conduit.14 Adjuncts such as a sartorius flap may be needed to help cover the arterial reconstruction, allowing then use of aggressive local wound care including negative pressure dressings.
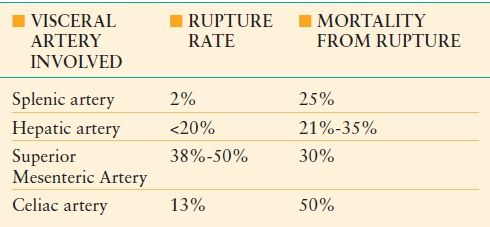
Visceral Artery Rupture. Ruptured visceral arteries are rare yet lethal entities, with 20%-70% presenting as emergencies and 8.5%-75% of patients dying from their ruptured visceral artery.16 In most cases, rupture is the result of unrecognized preexisting aneurysms, which themselves are rare with a 0.01%-0.2% incidence during autopsy.17 More than 3,000 visceral artery aneurysms have been reported in the literature, with variable rupture rates depending on the tissue bed involved (Table 45-1). In decreasing order of prevalence in the general population, the most commonly involved visceral arteries include the splenic, hepatic, superior mesenteric, and celiac arteries.18,19 In reported series, there is a male predominance for visceral artery aneurysms, except for splenic artery aneurysms where there is a 4:1 female predominance. Reported rupture rates are summarized in Table 45-1.20,21
TABLE 45.1
COMPILATION OF REPORTED VISCERAL ARTERY ANEURYSM RUPTURE RATES AND ASSOCIATED MORTALITY
In most series, atherosclerosis is cited as the most common etiology for ruptured visceral arteries.16–19,22 Other etiologies include fibrodysplasia, mycotic aneurysms, blunt trauma, connective tissues disorders such as Marfan’s syndrome and Ehlers-Danlos syndrome, vasculitides such as giant cell arteritis and polyarteritis nodosa, and gastrointestinal conditions such as pancreatitis and chronic peptic ulcer disease.16–19,22 Pregnancy merits special mention as the hormonal-mediated changes in vessel wall structure and increased splanchnic and splenic arterial flow result in an increased incidence of rupture with a maternal and fetal mortality rate of 64%-75% and 72.5%-95%, respectively.23
Clinical presentation of visceral artery rupture is dependent on the pattern of rupture, with free peritoneal rupture acutely presenting as hemorrhagic shock and retroperitoneal rupture possibly presenting delayed symptoms due to initial tamponade. Most commonly cited presentations are hypotension, abdominal and back pain, and gastrointestinal bleeding, including hemobilia in cases of hepatic artery rupture.16,18,19 Splenic aneurysm rupture not infrequently presents with minimal symptoms followed by acute decompensation.
Traditional treatment of visceral artery rupture has been open surgical approach consisting of ligation and bypass or possible reconstruction of the vessel as tolerated by the patient. For splenic artery aneurysm rupture, splenic artery ligation as part of splenectomy has historically been the treatment of choice. However, with the recognition of the importance of the spleen in immune function, more attempts at splenic salvage, consisting of splenic artery aneurysmectomy or exclusion, have occurred. Of special note, because of the exceedingly high maternal and fetal mortality rates from ruptured splenic artery aneurysms, elective surgical management of splenic aneurysms in females of childbearing age should be recommended when the splenic artery aneurysm is ≥2 cm.23 In fact, some experts recommend surgical intervention on all splenic artery aneurysms during pregnancy regardless of size, ideally after the first trimester.23 Hepatic arterial rupture can be managed with aneurysmectomy or aneurysmal exclusion as long as gastroduodenal and gastroepiploic collaterals are patent. Superior mesenteric artery (SMA) rupture frequently requires aneurysmorrhaphy and simple ligation, assuming adequate collateral blood flow through the inferior pancreaticoduodenal and middle colic arteries. Given the importance of the SMA to intestinal perfusion, attempts at repair of the SMA in the hemodynamically stable patient with aneurysmorrhaphy or interposition graft have been reported. Celiac artery rupture has been treated with aneurysmectomy and primary reanastomosis or interposition graft.
With the advent of endovascular technology, multiple case series have been published on endovascular embolization with coils and glues and exclusion of visceral artery aneurysms with covered stent grafts. However, the numbers of endovascular interventions for ruptured visceral arteries are limited in these series and no long-term follow-up on these interventions is available. Common complications from endovascular interventions include inadequate embolization, end tissue infarction from embolization, abscess formation from infarcted tissue, and embolization of glue or coils from initial delivery site.16,19,23–26
Arterial Ischemia
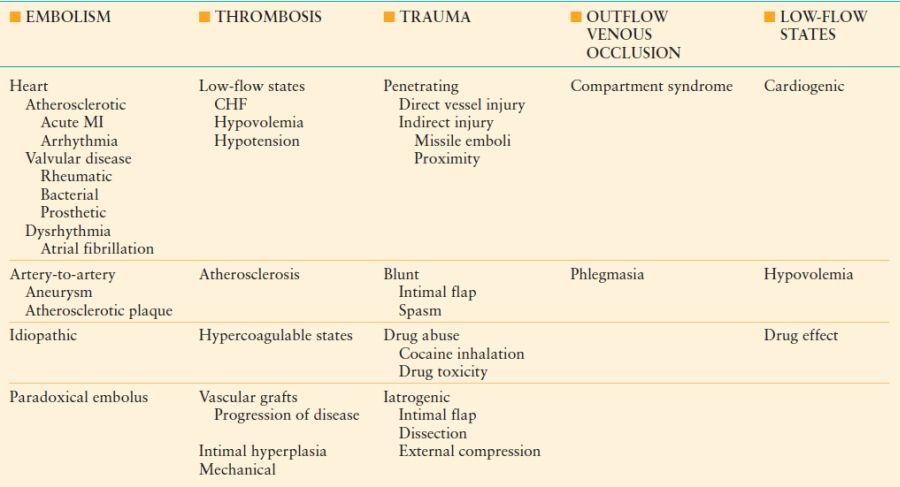
The bulk of emergency vascular interventions can be attributed to arterial ischemia. This seemingly diverse group of disease processes shares a common mechanism—compromised arterial blood flow whether due to embolism, thrombosis, dissection or poor cardiac function (Table 45-2). Symptoms and long-term outcomes are partially dependent on the ischemic tolerance of the affected tissue bed, for example, 6 hours for lower extremities and 5 minutes for the brain. Even after successful revascularization, risk of reperfusion injury and development of a compartment syndrome or systemic inflammatory response syndrome plagues these patients. This section will review arterial ischemia according to the limb or organ affected.
TABLE 45.2
ETIOLOGIES OF ARTERIAL ISCHEMIA
Lower Limb Ischemia. The incidence of acute leg ischemia (ALI) has been reported to be 9–14 per 100,000 with a peak incidence of 180 per 100,000 in patients older than 90 years.27,28 The most common causes of lower limb ischemia are thrombosis and embolism. Most emboli to the lower extremities originate from the heart (80%-90%), and 60%-70% of these patients have an underlying cardiac condition.27,29 These cardiogenic emboli lodge at branch points due to changes in laminar blood flow caused by vessel divisions and sequential reduction in diameter of branch arteries with each division. In decreasing order of relative frequency: the femoral bifurcation, the aortic bifurcation, the popliteal trifurcation. Aggressive medical management of atrial fibrillation with anticoagulation and rheumatic heart disease with antibiotics has shifted the incidence toward thrombosis.
The Trans-Atlantic Inter-Society Consensus (TASC II) Working Group in 2007 redefined acute limb ischemia as “any sudden decrease in limb perfusion causing a potential threat to limb viability.”30 As no reliable biochemical or radiologic indices of limb viability exists, a high index of clinical suspicion is required for rapid diagnosis and management. The clinical severity and presentation of ALI depends not only on the etiology, but on the location, proportion of luminal obstruction, and the capability of existing collateral circulation to transport blood around the obstruction. ALI due to emboli is more likely to present with sudden, severe limb-threatening ischemia. In contrast, patients with chronic arterial occlusive disease may also develop acute thrombosis, but their chronically diseased vascular beds develop collateral circulation, partially mitigating ischemia.
Acute limb ischemia is essentially a clinical diagnosis with a range of symptoms classically described as the 6 P’s: pain, paresthesia, paralysis, pallor, pulselessness, and poikilothermia. Pain in the toes and feet is usually the first presenting symptom of ALI. As ischemia continues, paraesthesia develops as large sensory nerves transmitting pain, temperature, and light touch become malperfused. Finally, in the most severe ischemic conditions, paralysis ensues with loss of toe flexion and extension. This is followed by an absence of foot dorsiflexion and plantarflexion due to ischemic myopathy of the calf muscles. As the muscles infarct, the leg swells and becomes more tender, and eventually the foot loses passive movement. After 6–8 hours, muscles become rigid and contracted and the limb is unsalvageable. Amputation is necessary at this point to prevent renal failure from rhabdomyolysis.
The clinical findings of pallor include a white, waxy extremity with absent capillary refill. With thrombosis, as the collateral circulation dilates to restore flow, there may be gradual improvement in capillary refill. If there is still refill after blanching, the limb is still salvageable. However, with fixed mottling or cyanosis the capillaries have thrombosed or ruptured and limb recovery is unlikely. Pulselessness is the sine qua non of acute limb ischemia. Palpable pulses in the contralateral extremity suggest embolism as the cause. With thrombosis, there are usually signs of chronic limb ischemia such as thickened toe nails, loss of hair, reduced or absent contralateral pulses. Finally, poikilothermia, or perishing cold, is the finding of decreasing skin temperature from the proximal unaffected limb to the distal ischemic limb.
With the advent of endovascular therapy, the range of management options for acute limb ischemia has expanded. These options include open surgical revascularization, endovascular revascularization, or anticoagulation with observation. First, a determination of ischemia severity is completed using the clinical classification of acute limb ischemia listed in Table 45-3.31 In this initial step, the degree of motor and sensory dysfunction is determined and the presence of arterial and venous flow verified with a handheld continuous Doppler unit. If no contraindication exists, the patient is started on systemic anticoagulation with heparin to prevent further clot propagation. Patients with level I ischemia should be treated with heparinization and close observation for symptom deterioration, especially older sedentary patients with substantial comorbidities who may become completely asymptomatic with aggressive management of associated comorbidities. In the younger, more active patient with level I ischemia, a more aggressive approach of proceeding directly to endovascular intervention, using catheter-directed thrombolysis or thrombectomy, or surgery is more prudent since the earlier the thrombus is removed, the better the outcome. This same strategy is employed for level IIA and IIB ischemia, but revascularization must be done expeditiously and four compartments calf fasciotomy much more likely to be needed to treat compartment syndrome. Most patients with level III ischemia should receive primary amputation only with treatment of comorbidities due to the potential consequences of reperfusion injury.
TABLE 45.3
RUTHERFORD’S CLASSIFICATION OF ACUTE LIMB ISCHEMIA
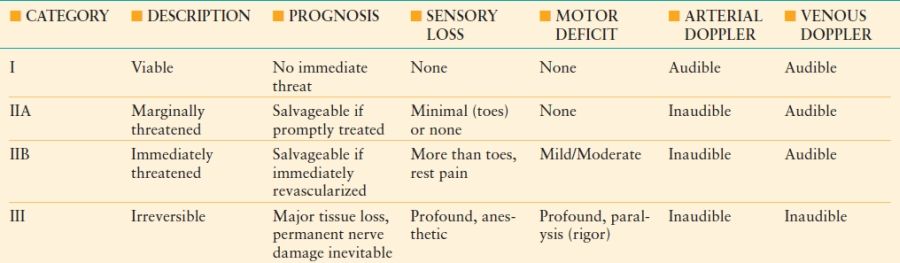
Modified from Rutherford RB, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








