EVALUATION/DIAGNOSIS/TREATMENT
Lumbar punctures (LPs; spinal taps) can provide invaluable information, but only if the local culture allows them to be done and the laboratory can analyze the specimen accurately. In children ≤5 years old, consider using a short small-gauge hypodermic needle; a butterfly needle works well in infants. For children >5 years old, use a standard LP needle, if one is available. Otherwise, use a longer small-gauge hypodermic needle.
Lumbar puncture needles may need to be reused. While this is potentially dangerous, it may be safer to reuse these needles than to reuse hypodermic needles. The obturator allows large material to be cleaned out of the needle’s core. After cleaning, steam autoclave the needles. If that is not possible, high-level disinfection by boiling has been recommended.1 To do this:
Put the needles into a pan and cover them with 3 cm of potable water. Put the lid on the pan and bring the water to a boil. Once it begins to boil, keep it boiling for 15 minutes.
While holding the lid on the pan, pour out the water without letting the needles fall out. This is not as easy as it sounds; practice this before you boil the needles.
Leave the nearly dry needles in the covered pan until they are needed. Never put them anywhere else.
In resource-poor settings, finding the midline with an ultrasound for an LP may hold the key to success, because there may be no one available to help with a failed LP. In adults, use a curvilinear low-frequency (5 to 2 MHz) transducer to image non-palpable bony landmarks, even if they are deeper than 9 cm. In children, use a linear high-frequency transducer. With a musculoskeletal “preset,” adjust the depth (adults) to 10 to 12 cm and place the probe near the presumed midline with the marker cephalad. Slowly slide the transducer left or right until the facet joints are seen—they look like humps on the ultrasound screen. Then slide the probe caudad until the sacrum is seen as a horizontal hyperechoic line. Use this landmark to identify the L3 to L4 and L4 to L5 interspaces, marking their location on the skin. Then, rotate the transducer 90 degrees to locate the anatomic midline. Slide the probe cephalad and caudad, identifying and marking the spinous processes. Connect the skin markings from the midline and the interspace levels; they will cross at the ideal sites for needle entry. Follow standard LP technique.2
Patients with psychogenic neurological disorders may present with bizarre motor findings and neurological tests that indicate a functional (psychogenic) disorder. They usually will have normal muscle tone, no atrophy or fasciculations, and no ataxia, although they may try to simulate it. The following are a number of quick, easy tests that suggest a patient may be malingering—or not. In resource-poor settings, you will have to rely on these simple tests; in other settings, they can save clinicians time and effort.
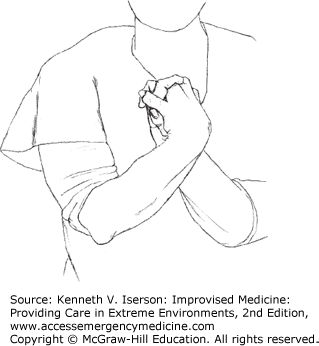
The Hoover Crossed-Leg Test examines leg strength. A supine person who tries to lift his straight leg against gravity or resistance will push down (for leverage) with the other heel. To do the test, the examiner asks the patient to lift the affected leg while keeping one hand under the patient’s other heel. No or little pressure on the examiner’s hand while the person says he is trying to lift his leg signifies malingering—as long as both legs are not affected. A more effective modification is for the examiner to put a thumb over both ankles while doing the test (Fig. 29-1). This provides additional resistance to lifting the leg and also provides information on the relative strength exerted by each leg.
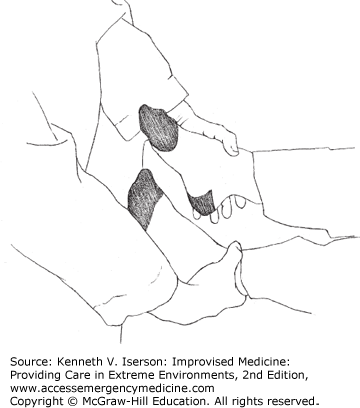
The Crossed-Hand Test (Fig. 29-2) is used to discover whether a complaint of unilateral hand numbness is valid. Have the patient cross his arms at the forearm level, turn the palms facing each other, and interlock the fingers. Then, if the patient is limber enough, he can bring his hands up in front of his face. With the patient watching, test the sensation in the fingers. Visual miscues due to the hand position will cause malingerers to pause before responding to sensation when the examiner touches each finger; they often identify the sensation incorrectly.
“Splitting the tuning fork” identifies patients with psychogenic sensory disturbance. These patients typically have a sharply demarcated loss of sensation to the midface. Use a tuning fork to test vibratory sensation on both sides of the forehead. These patients often complain that they lack vibratory sensation when tested on the affected side of the forehead, but will have intact vibratory sensation on the unaffected side. Of course, that is not possible, because the vibration is transmitted through the frontal bone to both sides.3
Use “optokinetic testing” for individuals who claim to be blind but are not. This test produces nystagmus in those who can see. To induce optokinetic nystagmus (vertical, horizontal, or rotational), have the patient look at moving visual stimuli, usually on a special rotating drum. If patients have nystagmus, it means they can see the drum. Improvising optokinetic testing equipment is easy: Make an optokinetic drum by attaching a paper with alternating black and white stripes around an empty soda can. Alternatively, use a black sheet of paper with multiple strips of white tape (~2 cm wide) attached so that there are alternating stripes of black and white. Make a photocopy of this and wrap it around an empty soda can. Extend a straight piece of metal, such as a hanger, through the top and bottom parts of the can as a handle to rotate the drum. Another method of eliciting optokinetic nystagmus is to pass a vertically striped cloth (such as a striped tie) horizontally across the patient’s visual field at about 5 to 10 cm/second. A measuring tape with large markings also works well. This reflex can be elicited starting at about 4 to 6 months of age and confirms cortical vision.4
Tunnel vision may be another manifestation of psychogenic visual disturbance. Patients may complain that they can see only a tiny part of the visual field in the center of vision, not unlike looking through a soda straw. To test whether such a complaint is legitimate, do the following: Make a small hole (about the diameter of a pencil) in a piece of paper. Have the patient close one eye and hold the paper with the hole positioned over his or her open eye. Move your face in front of the paper such that the patient can see only your nose, and then move back 3 to 6 feet from the patient and ask him or her what they see. If he or she says that they can see only your nose, they are feigning, because the degree of visual arc subtended has increased. In other words, they should see your whole face (at the very least). (Geoffrey Ahern, MD, PhD, University of Arizona, Tucson. Personal written communication, September 1, 2008.)
If the patient complains of being blind, but you do not think he or she is, leave some money on the floor and see how the patient reacts when he or she enters the room. (Joseph Miller, MD, PhD, University of Arizona, Tucson. Personal written communication, September 8, 2008.)
Monocular diplopia that does not correct using a pinhole device is almost always a functional problem.5 And eyelids that flutter when a person is in “coma” means that they are completely awake. If you do not have time to wait for their spontaneous awakening, break two or three ammonia capsules and hold them under the patient’s nose.
Use the sedative-hypnotic interview in Chapter 38 to easily assess suspected complex psychogenic complaints.
An early sign of a peripheral neuropathy is the inability to feel vibration. If a low-pitched tuning fork is not available, use a pager or a cell phone (set on vibratory mode, or use a “women’s vibrator” app) to see if a patient feels vibrations over bony prominences.6
If concerned about polyneuropathy in a patient, test the Achilles tendon reflex. Patients with normal Achilles reflexes rarely have a clinically significant polyneuropathy.7
A large number of patients, especially when populations are under stress, will come in with the complaint of headache, often a “migraine.” Historical and physical findings usually can differentiate their headaches from more serious problems.
For patients with non-migraine headaches, 20 mg IV metoclopramide plus 25 mg IV diphenhydramine provides better immediate (<1 hour) pain relief, decreases the likelihood of needing “rescue medications,” and provides more sustained pain relief at 24 hours than does 30 mg IV ketorolac.8 In some cases, peripheral nerve blocks also may be useful. The most commonly used blocks include the greater occipital, lesser occipital, supratrochlear, supraorbital, and auriculotemporal. If used, the block should produce cutaneous anesthesia and, hopefully, relieve an acute headache attack or terminate a headache cycle. Limit the adult local anesthetic dose per treatment session to <300 mg lidocaine or <175 mg bupivacaine. However, with the exception of using a greater occipital nerve block (often with corticosteroids) for cluster headaches, there is little consistent evidence about its effectiveness.9
If a migraine is diagnosed, and if medications are limited, try using a medication from Table 29-1. Even when other medications are available, 1 gram magnesium sulfate IV in 100 mL 0.9% normal saline given over 20 minutes has been shown to provide better relief than a combination of dexamethasone and metoclopramide.10
| Medication | Dose |
|---|---|
| I. Abortive Agents | |
| A. Oral or Sublingual | |
| Acetaminophen | 325-1300 mg |
| Acetaminophen/butalbital/caffeine | 325-650 mg acetaminophen |
| Aspirin | 325-1950 mg |
| Aspirin/butalbital/caffeine | 325-650 mg aspirin |
| Dexamethasone | 4-8 mg |
| Ergotamine tartrate (sublingual) | 2 mg; then 1 q30min prn. Max 6 tabs/24 hr and 10 mg/week |
| Ergotamine tartrate/caffeine | 2 tabs, then 1 q30min × 4, prn. Max 6 tabs/attack |
| Ergotamine tartrate/caffeine (suppository) | 0.5-2 mg ergotamine; may repeat once after 1 hour |
| Ibuprofen | 400-800 mg |
| Indomethacin | 25-50 mg |
| Isometheptene/acetaminophen/dichloralphenazone | 325-975 mg acetaminophen |
| Ketorolac | 10 mg |
| Naproxen | 500-1000 mg |
| Sumatriptan | 50-100 mg; may repeat in 2 hours. Max 200 mg/24 hr |
| B. Parenteral | |
| Butorphanol | 2 mg IM or intranasally |
| Chlorpromazine | 7.5-37.5 mg IV (slowly) or 25-50 mg IM |
| Dexamethasone | 12-20 mg IV or IM, may follow with prednisone taper: 60 mg × 1 day; 40 mg × 1 day; 20 mg × 1 day |
| Dihydroergotamine | 0.75-1 mg IV or SQ, or IV over 2-3 min (premedicate with 10 mg metoclopramide or prochlorperazine); may repeat qh to max 3 mg/24 hr and 6 mg/week 1 spray (0.5 mg) into each nostril; may repeat q15min. Max 3 mg/24 hr |
| Droperidol | 2.5 mg IV or IM |
| Ergotamine/caffeine | 1 rectal suppository; may repeat in 1 hour |
| Hydrocortisone | 100-250 mg IV over 10 min; may follow with prednisone taper: 60 mg × 1 day; 40 mg × 1 day; 20 mg × 1 day |
| Ketamine | 0.1-0.2 mg/kg IV |
| Ketorolac | 30 mg IV or 60 mg IM |
| Magnesium sulfate | 1-2 g IV over 10 to 30 min |
| Metoclopramide | 10-20 mg IV |
| Oxygen | 100% by mask at 8-10 L/min for 30 min |
| Prochlorperazine | 5-10 mg IV |
| Sumatriptan | 6 mg SQ; 5-20 mg intranasally; may repeat after 2 hours.Max 40 mg/24 hr |
| Zolmitriptan | 5 mg intranasally; may repeat after 2 hours |
| II. Prophylactic Agents | |
| Amitriptyline | 30-150 mg qhs |
| Candesartan | 8-32 mg qd |
| Divalproex sodium | 250-500 mg bid; extended-release 500 mg-1g qd |
| Lisinopril | 5-40 mg qd |
| Metoprolol | 50-100 mg bid; extended-release 100-200 mg qd |
| Nadolol | 20-40 mg qd |
| Naproxen | 250 mg bid or tid |
| Nortriptyline | 10-150 mg qhs |
| Propranolol | 160-240 mg/day divided bid, tid, or qid; extended-release 160-2400 mg qd |
| Timolol | 10-15 mg bid or 20 mg qd |
| Topiramate | 50 mg bid |
| Verapamil | 80 mg tid or qid; extended-release 240 mg qd |
Abort cluster headaches, often considered the most painful type of headache, with oxygen by face mask, if it is available. Various medications, including corticosteroids, can suppress attacks during cluster periods. Two “street drugs,” the ergot derivative lysergic acid diethylamide (LSD) and the related drug, psilocybin, have been the only medications to induce remission of a cluster period. In “sub-hallucinogenic doses,” LSD aborted the cluster period after one dose; psilocybin generally took three doses.15
If a patient with a transient ischemic attack (TIA) presents, how many resources must you devote to this patient? In other words, what is the chance of this patient developing a stroke in the next 7 days? Use a scoring system, such as the one below, to help determine the answer.
The 9-point ABCD3 Scale uses a score calculated on the basis of the patient’s Age, Blood pressure (BP), Clinical features, Duration of symptoms, the presence of Diabetes, and Dual TIA symptoms (a TIA prompting medical attention plus at least one other TIA in the preceding 7 days). Assign points according to the list in Table 29-2, and then add them to get the ABCD3 score. Table 29-2 can also be used to calculate the shortened ABCD and ABCD2 scores, as described below.
| Age | ≥60 years = 1 point <60 years = 0 points |
| Blood pressure | Systolic >140 mm Hg or diastolic >90 mm Hg = 1 point |
| Clinical features | Unilateral weakness = 2 points Speech disturbance without weakness = 1 point Other symptoms = 0 points |
| Duration of symptoms | ≥60 min = 2 points 10-59 min = 1 point <10 min = 0 points |
| Diabetes mellitus | 1 point |
| Dual TIA | 2 points |
The 6-point ABCD score (which does not include Diabetes or Dual TIA) has been validated. In one validation study of 274 consecutively enrolled patients with TIA (based on World Health Organization [WHO] standards), no patient with an ABCD score ≤3 had a stroke within 30 days. Of the patients suffering a stroke within 7 days, 20% had an ABCD score of 4, 40% scored 5, and 40% scored 6.16
Neither the 7-point ABCD2 score (without Dual TIA) nor the 9-point ABCD3 score has been thoroughly validated. Neither can identify all patients who will have a new stroke within two weeks or 30 days. However, an ABCD2 score <2 can identify patients at very low risk; an ABCD3 score <4 identifies even more patients at low risk. Those with an ABCD3 score ≥4 or an ABCD2 score ≥2 suggest a higher likelihood of stroke within 14 days.17,18
Nearly one-third of patients presenting with a possible stroke have a different diagnosis. With limited imaging and consultation, rapid clinical differentiation becomes especially important. It saves resources and can guide therapy and diagnostic measures in the correct direction.
The likelihood that the patient had an acute stroke increases dramatically if there is a definite history of focal neurological symptoms (odds ratio = 7.21), if you know the exact time that symptoms began (odds ratio = 2.59), or in the presence of any cardiovascular abnormality (systolic BP >150 mm Hg, atrial fibrillation, valvular heart disease, or absent peripheral pulses; odds ratio = 2.54).19 On presentation, a stroke is more likely if the patient has facial paresis, arm drift, or abnormal speech (odds ratio = 5.5). If none of these symptoms are present, it is unlikely that the patient had a stroke (odds ratio = 0.39).20
TRAUMA
Is the patient unconscious? Hysteria, psychological disease, drugs, or alcohol may all make patients seem unconscious when they are not. The following are simple, rapid methods to test for the level of consciousness (LOC) in these patients:
Sternal rub: Use your knuckles and progressively increase pressure as you rub.
Ammonia capsules: Break three or four and cup them in your hand. Place your cupped hand firmly over the patient’s mouth and nose.
Supraorbital nerve pressure: Similar to a sternal rub; be careful that your finger does not slip and injure the eye.
Jaw pull: Place your second and third fingers behind the ascending ramus of the mandible and pull the patient’s jaw forward.
Nose-hair tickle: Use cotton strands (e.g., from a cotton swab) and gently stimulate the hair inside the nares.
Hand–face drop: When the patient’s hand is held over his or her face and dropped, the patient in psychogenic coma typically avoids letting the hand hit their face by making subtle movements to the side.3
Corneal reflex: Use saline drops to get a response, rather than a wisp of cotton.
Rectal examination: Part of the normal full examination for unconscious patients, this procedure may arouse the hysteric. When done in unconscious patients who are about to be chemically paralyzed for intubation, the absence of tone means they are paralyzed from a spinal injury.
Foley catheter: (especially males) This stimulation will usually awaken any male who can be awakened by external stimuli.
The simplest way to transmit information about the level of head injury is either to use a coma score that the receiver understands or to describe the patient’s response to the score’s elements. Neurological consultants will want to know this critical information, especially when you ask for their advice or want to transfer head-injured patients to them. Do not assume that everyone uses the same scoring system. The best way to deliver or receive information about a patient is to ask for the specific brief neurological findings that the clinician found when determining any of the coma scores described next.
The most widely understood coma score is the Glasgow Coma Score (GCS; also known as the Glasgow Coma Scale), although its 13 levels are confusing, unreliable, unnecessarily complex, and were never designed to be used for acute care. If used, the 6-point motor component of the GCS provides the same results as the entire scale. If the entire GCS is used, note that patients ≥70 years old with a GCS score of 14 have a greater mortality than do those <70 years old with a GCS of 13.21
Two similar alternatives now commonly used in prehospital care are the AVPU scale and the ACDU scale (Table 29-3). These, as well as the Simplified Motor Scale (SMS), provide essentially the same prognostic information as the GCS, but are easier to use and describe to other clinicians. Steve Green, MD, has suggested that we change SMS to TROLL (Test Responsiveness: Obeys, Localizes, or Less), because the name is both memorable and a mnemonic for the test elements.
| AVPU Scale | ACDU Scale | SMS (TROLL) |
|---|---|---|
A = Alert V = Responds to verbal stimuli P = Responds to painful stimuli U = Unresponsive | A = Alert C = Confused D = Drowsy U = Unresponsive | Test responsiveness: Obeys commands Localizes pain Withdrawal from pain or Less responsive |
Do not rely on the ophthalmoscopic examination to show evidence of acute rises in intracranial pressure. These changes appear only in chronic conditions, including retinal hemorrhages in infants with head injuries from child abuse.
In the midst of trauma resuscitation turmoil, be careful not to let easily salvageable head-injured patients die because (a) you confused a bad scalp laceration with an open skull fracture or (b) you did not control significant scalp bleeding. Control scalp hemorrhage by simply wrapping a tourniquet-type bandage around the head above the eye line. (Remember that all vessels to the scalp travel up from the neck.) When the bleeding slows, quickly use scalp clips, staples, or big sutures to stop the bleeding; then remove the tourniquet. Do a better closure later, if necessary.
Knowing which patients will not survive is the key to maximizing the use of limited resources to treat hordes of patients with multiple severe injuries. As the US military recognizes, “the prognosis of brain injuries is good in patients who respond to simple commands, are not deeply unconscious, and do not deteriorate. The prognosis is grave in patients who are rendered immediately comatose (particularly those sustaining penetrating injury) and remain unconscious for a long period of time.”23
Similarly, Dr. Husum and colleagues, in their book War Surgery, write about patients who are “beyond salvation,” saying that “bilateral dilated pupils that do not improve after a few hours in a comatose [head-injured] patient are a sign of major brain injury which normally will not respond to treatment. Operation in such cases is wasted.”24 In a similar vein, the US military writes that “a GCS <5 indicates a dismal prognosis despite aggressive comprehensive treatment, and the casualty should be considered expectant [comfort care provided, but not treatment].”25
In austere situations where computed tomographic (CT) scans are not readily available, it may be an extremely weighty and costly decision to send patients for a CT scan. The question is: How much chance of making an error is reasonable in your situation? In these environments, the best option may be to use the Canadian Rules (adult and child), because these will detect more patients who actually need neurosurgical intervention. Those at high risk (adult or child) have one of the listed findings:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree