DEFINITION
The performance of regional anesthesia may cause muscle injury by several mechanisms. Ischemia from an occlusive tourniquet such as during an intravenous regional anesthetic may produce muscle damage, but only after 6 hours of inflation, which is typically longer than the duration of the anesthetic. Injury by ischemia may also follow either compartment syndrome from bleeding or direct occlusion of a vessel from needle damage.1 However, the dominant mechanism by far is myotoxicity, which may be defined as the direct action of the local anesthetic agent on the muscle cell (myocyte) that initiates cellular process leading to the destruction of the cell.
 SCOPE OF THE PROBLEM
SCOPE OF THE PROBLEM
Local anesthetic solutions used in regional anesthesia are uniformly toxic to muscle tissue. Since most local anesthetic injections are in the immediate vicinity of muscle tissue, myotoxicity likely accompanies nearly all regional anesthetic blocks. Despite this predictable muscle damage, myotoxicity is only rarely a clinically important event.2,3 Several factors may explain this. Only adult myocytes are damaged by local anesthetic, so basal lamina, vasculature, neural elements, and most importantly the immature myocytes (myoblasts) remain intact. This allows complete regeneration in 3 to 4 weeks.4,5 In fact, it may be this destruction of old cells and the prompting of new growth that provides the therapeutic benefit in trigger point injection of local anesthetics for myofascial pain, possibly combined with the growth of new vessels.6 Other factors concealing the muscle injury from clinical recognition may include postsurgical immobilization, injury sites too deep to examine, or lack of scrutiny as the pain and inflammation develop several days postinjection. Also, pain due to myotoxicity may be mistakenly attributed to the surgery. An exception to the generally low impact of local anesthetic myotoxicity is extraocular muscle damage, in which there is a growing recognition of extraocular muscle dysfunction after regional anesthesia for the eye. There are no large studies formally evaluating the incidence of local anesthetic injury of the extraocular muscles. However, many cases have been reported despite the apparent low frequency of this event, consequent to the common use of these injections.7,8
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Within 5 minutes of injection of usual concentrations of any local anesthetic, muscle fibers appear hypercontracted.9,10 Within 15 minutes, lytic degeneration of the muscle cell’s sarcoplasmic reticulum (SR) and mitochondria is evident.9,11 By 24 hours, the myocyte becomes edematous and necrotic. Inflammation ensues with phagocytosis of cellular debris and appearance of eosinophils,12 and eventually fibers are regenerated from precursor cells (Fig. 15-1). Abnormal muscle fibers may be evident for months.13 These changes happen predictably in all subjects.
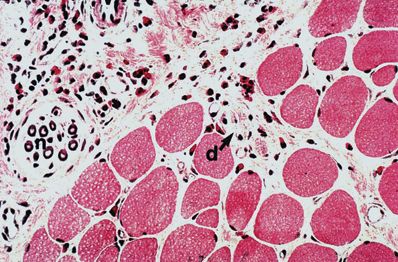
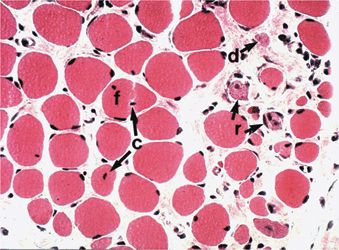
FIGURE 15-1. Histologic views (hematoxylin and eosin, ×125) of sternocleidomastoid muscle 54 days after bupivacaine injection. Myotoxic changes are evident, including eosinophilic infiltration (e), degenerating fibers (d), fiber splitting (f), fibers with central nuclei (c), and regenerating fibers (r). Nerves (n) and vessels (v) are not affected. (From Hogan Q, Dotson R, Erickson S, et al. Local anesthetic myotoxicity: a case and review. Anesthesiology 1994;80:942–947.)
The molecular mechanism of local anesthetic myotoxicity has not been fully elucidated, due to the diverse effects of local anesthetics on cellular homeostasis (Fig. 15-2). Local anesthetics irreversibly injure mature myocytes in culture, which eliminates denervation secondary to blockade of the action potential or neuromuscular junction as an etiologic possibility.14 Tetrodotoxin, a local anesthetic without direct effects on intramuscular Ca2+, is not myotoxic.15 Therefore, inhibition of the sarcolemmal Na+ channel also does not play a role.16 Local anesthetics fail to elicit contractures, the earliest phase of local anesthetic myotoxicity, if SR accumulation of Ca2+ is prevented, which indicates that toxicity is not caused by direct action on the myofibrils.17 Replication of local anesthetic myonecrosis with caffeine alone16 points to pathologic efflux of intracellular Ca2+ from the SR of mature multinucleated myocytes as a key element in local anesthetic myotoxicity.
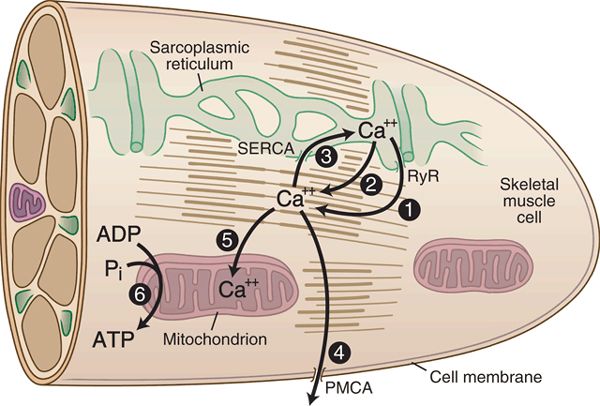
FIGURE 15-2. Cellular sites for local anesthetic myotoxicity. Upon exposure to local anesthetics, Ca2+ is released from the SR stores either through an action on the ryanodine receptor (also known as the Ca2+ release channel) (1) or directly on the SR membrane (2), causing elevated cytoplasmic Ca2+ levels. Additionally, local anesthetics may suppress the action of mechanisms that clear Ca2+ from the cytoplasm, both at the sarcoplasmic-endoplasmic Ca2+ ATP-ase (SERCA) (3) and the plasma membrane Ca2+ ATP-ase (PMCA) (4). Decrease in the mitochondrial inner membrane potential from local anesthetic exposure also interferes with Ca2+ uptake by the mitochondria (5) and reduces cellular energy production (6).
Mature myocytes maintain a concentrated store of Ca2+ sequestered in the internal membrane system of the SR. Release of Ca2+ from SR via the Ca2+ release channels (also known as ryanodine receptors) triggers contraction of striated muscle. Local anesthetics cause a pathologic efflux of Ca2+ from the SR18 essentially like malignant hyperthermia in miniature, producing contracture and cell destruction via activation of intracellular enzyme systems. There is some evidence that local anesthetics produce generalized permeability of the SR membrane.17 Alternatively, other studies have shown a direct action of the anesthetic molecule on the Ca2+ release channel.19 This action is complex and depends on drug concentration, pH, and Ca2+ loading status of the SR.20–22
Komai and Lokuta23 demonstrated increased Ca2+ release channel activity in the presence of bupivacaine, whereas tetracaine caused inhibition. It is possible, therefore, that a general feature of local anesthetics, including tetracaine, procaine, benzocaine, and dibucaine, is a nonspecific action on sarcoplasmic membranes that causes Ca2+ release, whereas others such as bupivacaine,23 lidocaine,19 and prilocaine19 have an additional direct action on the ryanodine receptor that accounts for their greater myotoxicity. Inhibition by local anesthetics of the Ca2+-dependent ATPase of the SR, which transports Ca2+ back into intracellular stores following release, may be a further contributing factor,24–27 and there is evidence that the plasma membrane pump that expels Ca2+ from the cell is also inhibited.28 Interestingly, local anesthetic injury of primary sensory neurons may result from processes similar to that which damages muscle cells, specifically the triggering of pathologic discharge of Ca2+ from intracellular stores.29
Local anesthetics substantially affect mitochondrial bioenergetics. In the case of skeletal muscle, bupivacaine has been shown to dissipate the potential across the mitochondrial inner membrane, resulting in disrupted oxidative phosphorylation.30,31 Because of the important role mitochondria play in regulating Ca2+ homeostasis, this mechanism may cause or interact with the features described above. Furthermore, events initiated in the mitochondria may trigger apoptosis, while depletion of Ca2+ stores may initiate SR stress, in which proteins that have been assembled but remain unfolded accumulate in the SR, which in turn may also mediate apoptosis. Thus, local anesthetics disrupt a broad set of homeostatic mechanisms, which individually or together lead to the destruction of the myocyte.32
 RISK FACTORS
RISK FACTORS
While all local anesthetics tested produce myonecrosis, bupivacaine produces the most intense effect and procaine the least.33 Recent studies confirm that ropivacaine also produces myotoxicity,34 although less than bupivacaine at equipotent concentrations.35,36 The myotoxicity that follows levobupivacaine administration has not been compared to other agents, but it would be expected to be high, since S-enantiomers show particular efficacy at releasing stored Ca2+ and disrupting cellular Ca2+ signaling.31,37 The sensitivity of muscles is not uniform. Muscles composed of fibers that have high oxidative metabolism capacity are particularly sensitive, whereas those dependent on glycolytic metabolism are resistant to bupivacaine toxicity.30 The sensitivity to local anesthetic myotoxicity in rats is greater in young animals than in adults,38 although there has been no examination of this distinction in humans.
The extent of damage is dose related39,40 and is worse with serial administration.41,42 Infusions lasting only 6-hour duration for femoral nerve blockade in pigs result in amplified damage compared to single injection, with the development of calcific deposits and scar formation.36 Similarly, slow release of bupivacaine from microparticles shows that even subanesthetic concentrations produce myotoxicity if the duration of exposure is long.43 Steroid44 and epinephrine45 in the injection amplify the muscle injury produced by local anesthetics. Injection outside a muscle, such as into the subcutaneous fat, produces damage to adjacent muscle,9,46 but intramuscular injection results in maximal injury (Box 15-1). According to some reports,47,48 hyaluronidase increases the myotoxicity of local anesthetics. However, these studies investigated effects on rat tibialis and rabbit orbicularis oculi muscles. In contrast, clinical reports indicate that the use of hyaluronidase for retrobulbar and peribulbar anesthesia may actually decrease the incidence of myotoxicity.49,50
BOX 15-1 Factors Potentiating Local Anesthetic Myotoxicity
 Bupivacaine > ropivacaine, lidocaine, prilocaine > tetracaine, procaine
Bupivacaine > ropivacaine, lidocaine, prilocaine > tetracaine, procaine
 Dose related
Dose related
 Direct intramuscular injection
Direct intramuscular injection
 Serial or continuous administration, steroid, epinephrine
Serial or continuous administration, steroid, epinephrine
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


