Fig. 14.1
Liver metastasis on CT scan. The circle represents the location of liver metastases as seen on CT scan
CT and MRI scans conventionally delineate the anatomical features of a tumour (i.e. shape, size and density). The introduction of PET/CT scans permits for the analysis of not just morphological features but takes into account the metabolic function of the lesion as well (Fig. 14.2). In such circumstances, the use of a radiotracer (i.e. 18F-fluorodeoxyglucose) in a PET/CT scan further enhances the resolution of pathological lesions. The specific indications of radiological scans would be further illustrated in the following subsections.
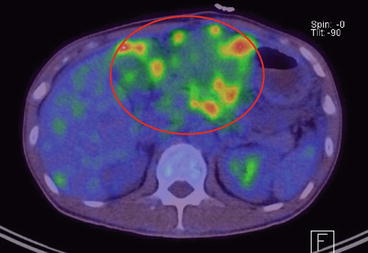

Fig. 14.2
Liver metastasis on PET/CT scan. The circled region indicates the location of liver metastases as seen on PET/CT scan
14.3 Primary Colon and Rectal Cancer
Upon diagnosis of stage IV colorectal cancer, it is important to determine if the metastatic disease is potentially resectable or definitely non-resectable. If the metastatic disease is potentially resectable, then a curative resection can be considered if the primary pathology can be removed as well (Anwar et al. 2011).
In these patients, it is imperative to discuss amongst surgical, medical and radiation oncologists whether upfront surgery to remove all apparent disease or to adopt a chemotherapy +/− radiotherapy first approach before re-evaluating the suitability for surgery is preferred.
In patients with unresectable disease, chemotherapy has always been deemed the mainstay of treatment except in emergency situations (Kaufman et al. 2007). However, there remain proponents of surgical resection of the primary pathology (Bacon and Martin 1964; Lockhart-Mummery 1959; Cady et al. 1970; Cook et al. 2005). There appears to be evidence supporting a higher median survival and also reduced future complications from the primary malignancy in the group that underwent surgery for the primary pathology. But a recent Cochrane review (Cirocchi et al. 2012) has established that there is insufficient evidence to advocate surgical resection for asymptomatic patients with stage IV colorectal cancer and the surgery itself does not lead to a reduction in the risk of future complications from occurring.
In addition, surgery itself is not without its own set of perioperative complications (Clements et al. 2009). And the majority of these studies are largely retrospective in nature and lend itself to enormous amount of selection bias. It would not be surprising if the group of patients who underwent surgery for the primary pathology was generally fitter, has lower metastatic load and the primary cancer is easily resected without much morbidity.
14.4 Dealing with Crisis
However, if a patient with metastatic colorectal cancer presents acutely with bowel perforation, obstruction or severe bleeding, these situations may warrant urgent interventions to the primary pathology regardless of the presence or extent of metastatic disease. When the crisis is resolved, the management principles of such patients would be similar to those who present electively.
14.4.1 Perforation
The rate of perforation of colorectal cancer is reported to be around 5–10 % (Ronnekleiv-Kelly and Kennedy 2011). Perforation is itself an indicator of poor prognosis as it is associated with postoperative morbidity and mortality and shorter disease-free survival (RodriGuez-Gonzalez et al. 2013). Perforation can arise from the primary tumour itself or a complication of colonic obstruction.
Conservative measures are often unsuccessful and these patients often require exploratory laparotomy to identify the site of perforation. If technically feasible and the patient fit, a resection of the perforation and the primary pathology should be performed. Otherwise, a proximal stoma to divert the faecal stream can be adopted if the patient is unfit or the tumour is too locally advanced to be operated in the emergency setting. There is also the role of comfort measures in a select group of patients after accounting for their premorbid conditions, fitness for surgery and the extent of metastatic disease.
14.4.2 Obstruction
Large bowel obstruction occurs in 10–30 % of patients with symptomatic metastatic colon and rectal cancer (Clements et al. 2009). These patients often exhibit clinical symptoms such as abdominal pain, distension, constipation and vomiting. Radiological findings of proximal bowel dilatation (Figs. 14.3 and 14.4) are commonly seen as the primary pathology is often located in the left colon or rectum (Ruo et al. 2003). Classically, surgical resection of the obstructed segment has been emphasised and the National Comprehensive Cancer Network (NCCN) guidelines also recommend surgery for patients with metastatic colon and rectal cancer presenting with obstruction. But since the 1980s, Dohmoto has proposed using endoscopic colonic stenting to bypass the obstruction in selected cases (Figs. 14.5 and 14.6) (Dohmoto 1991).
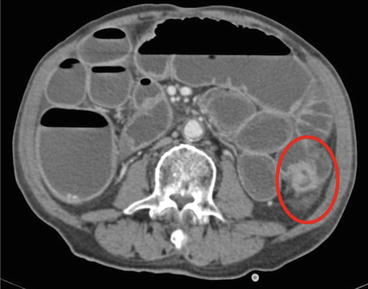
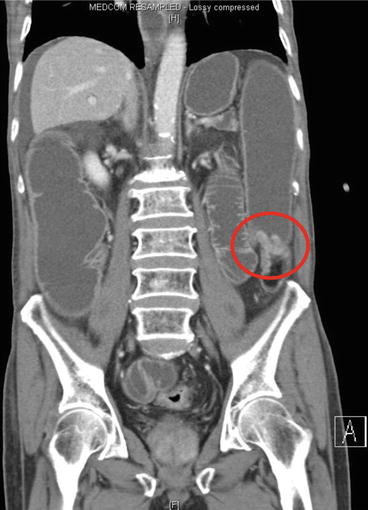
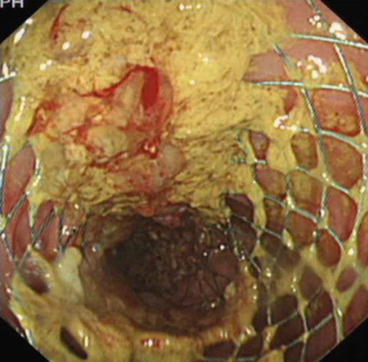
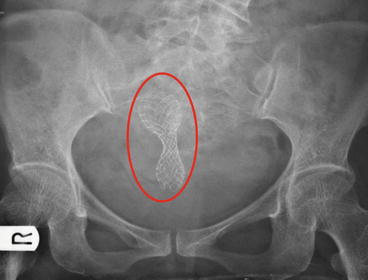

Fig. 14.3
Transverse view of an obstructing lesion in descending colon causing intestinal obstruction. The circled region shows the location of the obstructing colon lesion

Fig. 14.4
Coronal view of the same obstructing lesion in the descending colon. The circled region shows the corresponding lesion as that seen in Fig. 14.3 but on coronal view

Fig. 14.5
Deployment of an endoscopic colonic stent

Fig. 14.6
A plain X-ray of the pelvis to confirm position of the stent following endoscopic stenting. The circled region shows the location of the stent
For right-sided lesions, surgical resection with primary anastomosis is still recommended (Baron 2010). Stenting is not recommended as it is technically more challenging and is associated with higher rates of perforation (Baron 2010). Reasons postulated include the longer length of colonoscope inserted into the colon to reach the right-sided pathology which then renders the manipulation of the colonoscope harder. And the further build-up of pressure from the insufflation during the colonoscopy in the presence of a competent ileocaecal valve would only further increase the risk of caecal perforation.
In left-side-obstructing colorectal cancers, endoscopic stenting has been increasing advocated in recent years. Some of its advantages include the ability to relieve the obstruction, decompress the proximal colon and enables earlier commencement of chemotherapy (Poultsides et al. 2009). Patients also enjoy a shorter hospital stay and reduction in the rates of stoma creation (Vemulapalli et al. 2010). Although reports demonstrated no change in the median survival (McCullough and Engledow 2010), endoscopic colonic stenting is associated with fewer complications when compared to those who underwent upfront surgery (McCullough and Engledow 2010).
As more than 90 % of stents inserted remained patent for more than 6 months following placement (Camúñez et al. 2000), it is a promising option as patients who undergo non-resectional bypass procedures only have a median survival of approximately 7 months (Anwar et al. 2011). However, the availability of the technical capability and resources to perform endoscopic stenting are the major limitations. Another more dire complication is the possibility of perforating the segment of tumour during the procedure which then mandates emergency surgery.
14.4.3 Bleeding
Considerable bleeding from the colorectal cancers often presents with haematochezia with signs and symptoms of anaemia. Blood transfusion to minimise the impact of anaemia is the first step in the management of these patients.
In the presence of metastatic disease, it is imperative to consider the haematological complications of chemotherapeutic agents and also the possibility of further bleeding during the treatment. In the presence of acute emergencies where the patients are bleeding massively, a CT angiography can be performed to localise the bleeding followed by invasive mesenteric embolisation to cease the bleeding temporarily (Martí et al. 2012; Chang et al. 2011; Strate and Naumann 2010). This enables ongoing resuscitation and more definitive plans to address the primary pathology.
While numerous modalities have been described as means to stop the bleeding (Table 14.1), these are often temporary. The mainstays of treatment remain surgical resection and radiotherapy to the malignancy.
Injection | Mechanical | Thermal |
|---|---|---|
Epinephrine | Clips | Electrocautery: monopolar, bipolar |
Ethanol | Detachable loop ligators | Heater probe |
Fibrin | Band ligators | Argon plasma coagulation |
N-butyl-2-cyanoacrylate | Laser photocoagulation | |
Radiofrequency ablation |
14.5 The “Asymptomatic” One
In the majority of patients with metastatic colon and rectal cancer that does not present as a surgical emergency, chemotherapy is key to prolong the overall survival in these patients. As these patients often die from systemic disease due to the massive tumour burden, the aim of treatment should be aimed towards achieving stable disease and to delay tumour progression rather than to primarily induce tumour response. Thus, significant delay in the commencement of chemotherapy could be detrimental (Michel et al. 2004; Muratore et al. 2007; Sarela et al. 2001; Scoggins et al. 1999).
Chemotherapy has not been shown to increase the incidence of complications such as bleeding, perforation or obstruction. These complications following chemotherapy are reported to only occur in less than 10 % of the patients (Nitzkorski et al. 2012). But if the patients are deemed likely to develop any crisis during chemotherapy, surgical resection or other treatment modalities to address these potential crises should be considered so as to allow uninterrupted systemic chemotherapy (Michel et al. 2004; Muratore et al. 2007; Sarela et al. 2001; Scoggins et al. 1999).
Since the 1970s, chemotherapeutic options for colon and rectal cancer are based on 5-flurouracil (5-FU) and leucovorin given as an infusion. Reports of median survival ranged between 10 and 12 months (Glimelius and Cavalli-Bjorkman 2012). Ten years on, the introduction of “doublets”, 5-FU/leucovorin plus either irinotecan (FOLFIRI) or oxaliplatin (FOLFOX) extends survival by a further 4 months. However, the FOLFIRI and FOLFOX regimes are associated with complications such as diarrhoea and neurotoxicities, respectively. But unlike the other chemotherapeutic agents, the occurrences of haematological toxicities are relatively uncommon (Fornaro et al. 2010). The onset of neurotoxicity has been the focus for many with the use of oxaliplatin-based chemotherapeutic agents. It was reported in the OPTIMOX trial that the “stop-and-go” approach to administering FOLFOX whereby high dose of FOLFOX was given before maintenance infusion of 5-FU/leucovorin was started and FOLFOX reintroduced when disease progressed reduced occurrence of such toxicities (Tournigand et al. 2006).
Since the mid-1990s, the introduction of the oral chemotherapeutic agent capecitabine (fluoropyrimidine) radically changed the treatment of colon and rectal cancer. Its presence made chemotherapy more convenient and pushed survival towards 18–20 months. Combination “doublets” of capecitabine and irinotecan (CAPIRI) or oxaliplatin (XELOX) were noted to be non-inferior to FOLFIRI and FOLFOX, except in CAPIRI where the severe gastrointestinal toxicities experienced prematurely suspended the trial (Kohne et al. 2008). The triplet form, capecitabine/irinotecan/oxaliplatin (XELOXIRI), also resulted in grade 3–4 diarrhoea. The use of 5-FU/leucovorin plus irinotecan and oxaliplatin (FOLFOXIRI) has resulted in higher radical resection of metastasis and increase in overall survival (Masi et al. 2008). FOLFOXIRI increasingly has become the choice drug in palliative chemotherapy.
The introduction of targeted chemotherapy provided an additional avenue to push survival past the 24-month mark. Tissue samples from the primary tumour and its metastatic site can be obtained via aspiration cytology or tissue block histology. Specific genetic testing (i.e. K-ras mutation) is done routinely on these tissue samples to detect if the drug can bring about specific desired response such as tumour apoptosis. Such targeted therapies seem to be breakthroughs, but we await longer-term data to demonstrate its true efficacy.
14.5.1 Anti-vascular Endothelial Growth Factor (Anti-VEGF)
14.5.1.1 Bevacizumab
Studies show that the addition of Bevacizumab to chemotherapeutic agents allows for longer overall survival (Hurwitz et al. 2004). In the BOND-2 trial (Saltz et al. 2007), longer survival was noted when bevacizumab was added to irinotecan and cetuximab. However, the use of this targeted agent is not without its perils. The high cost involved and occurrence of hypertension question the cost-effectiveness of bevacizumab.
14.5.2 Anti-epidermal Growth Factor Receptor (Anti-EGFR)
14.5.2.1 Cetuximab
The BOND-1 trial (Cunningham et al. 2004) highlighted the use of cetuximab in patients who are resistant to irinotecan-based chemotherapeutic agents. The addition of cetuximab showed that it was possible to overcome this resistance and this therapy was associated with significantly longer progression-free survival. Further analysis with this new antitumour effect showed that having a K-RAS mutation actually hindered the effects of the drug (Van Cutsem et al. 2007; Karapetis et al. 2008). Cetuximab is now used as first line with irinotecan-based agents (Van Cutsem et al. 2009, 2011).
14.5.2.2 Panitumumab
Panitumumab is a fully human monoclonal antibody which is believed to elicit fewer drug-related allergic reactions. We are currently waiting for the results of the ASPECCT trial to determine which is more superior, cetuximab or panitumumab. Through the use of FOLFOX, the addition of panitumumab has also shown to increase progression-free survival.
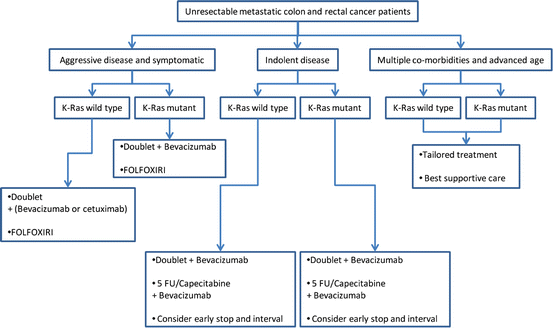

Flowchart 14.1 First-line treatment option for different unresectable metastatic colon and rectal cancer patients based on clinical and molecular factors (Ruo et al. 2003)
14.6 Management of Organ-Specific Metastasis
14.6.1 Hepatic Metastases
The liver is the most common site of colorectal cancer metastases. Unfortunately, less than 25 % of these metastases are potentially resectable upon presentation (Scheele et al. 1995; Bismuth et al. 1996). The 5-year survival amongst patients who have resectable disease is expectedly far better than those who do not. The patients with resectable disease have a median 5-year overall survival of 40–58 % following hepatic resection (Fong et al. 1999; Scheele et al. 1990).
In the modern era, treatments of hepatic colorectal metastases have evolved and include biologic agents, regional hepatic ablative technology and trans-arterial modalities (Saied et al. 2013). These advances have changed the approach in the management of hepatic metastases, such that previously deemed unresectable tumours could become resectable. The ongoing aim is to reduce the number of unresectable lesions and thereby increase the overall survival amongst patients with liver metastases.
14.6.1.1 Detection of Liver Metastases
The importance of obtaining preoperative imaging cannot be understated. Not only is it performed to ascertain the volume, number and location of the metastatic deposits, it is also important to determine the amount of remnant functional liver following liver surgery (Frankel and D’Angelica 2014). There is, however, no one best diagnostic modality to achieve this currently, and the modality adopted is often dependent on facilities and expertise available at individual institution.
The most routinely used imaging modality is the CT scan. This utilises varying contrast enhancement in the arterial and portal venous phases to detect the hepatic lesions. Colorectal cancer metastases to the liver are hypodense compared to the surrounding tissue during the portal venous phase. They also demonstrate rim enhancement which washes out in the delayed phase (Sahani et al. 2004). The disadvantage of the CT is that subcentimetre lesions cannot be accurately characterised.
MRI is another modality that can be used to identify and characterise any hepatic lesions (Braga et al. 2004; Patel et al. 2010). On the MRI scans, colorectal liver metastases appear hypointense on T1 images and hyperintense on T2 and diffusion-weighted series (Frankel and D’Angelica 2014). MRI appears to be superior in the detection of colorectal liver metastases compared to CT. One study showed the sensitivity and specificity of MRI to be 81.1 and 97.2 %, compared to 74.8 and 95.6 %, respectively, in CT scans (Floriani et al. 2010). Another study also demonstrated that MRI had a higher sensitivity than CT in detecting subcentimetre lesions (Niekel et al. 2010). However, MRI does require a significant amount of patient compliance, as the patient needs to be able to tolerate lying still in a closed, confined and noisy environment for an extended period of time.
PET/CT scans have been found to be highly sensitive in the detection of hepatic metastases. It also confers the advantage of also detecting metabolically active metastatic lesions outside the liver. This is especially important in patients who are being considered for hepatic resection for metastases as it would reduce nontherapeutic laparotomy rates considerably. Limited availability and high cost remain its biggest limitations (Frankel and D’Angelica 2014).
14.6.1.2 Role of Hepatic Metastasectomy
Liver resection is a major surgical procedure, and appropriate preoperative considerations must be made prior to surgery. The patient needs to be relatively fit to undergo hepatic resection apart from the resection of the primary. Both hepatic and colonic resection can be performed simultaneously in the same setting, or a liver-first or colon-first approach can also be adopted. When a hepatic resection is considered, apart from the absence of extrahepatic unresectable metastatic deposits, adequate remnant liver function must also be ensured to prevent postoperative liver failure. This is typically achieved through assessment of indocyanine green excretion levels while incorporating serum laboratory values and aggregate scores such as the Model for End-Stage Liver Disease (MELD) score (Schroeder et al. 2006; Uesaka et al. 1996; Imamura et al. 2005). Certain radiological findings are also instrumental to determine the feasibility of liver resection.
The consensus statement on the oncologic and technical criteria for resectability offers guidelines concerning which patients are amenable to resection (Adams et al. 2013). These criteria are summarised in Tables 14.2 and 14.3, which are adapted from the consensus statement article.
Table 14.2
Oncologic criteria for resection
Oncologic criteria for resectability |
|---|
1. Pretreatment radiological staging is required to assess for metastatic disease |
2. If patients have extrahepatic disease, these should be amenable to surgical resection before hepatic metastases resection is considered |
3. Patients should be able to have disease manageable by adjuvant therapies and should be expected to achieve long-term disease control |
4. Patients on preoperative chemotherapy who are not able to achieve control of metastatic disease should have surgical resection deferred |
Table 14.3
Technical criteria for resection
Technical criteria for resectability |
|---|
1. A margin negative resection must be achievable |
2. Two contiguous segments of the liver must be preserved |
3. Adequate vascular inflow, outflow and biliary drainage must be achieved |
4. Post-resection liver volume should be at least 20 % in normal liver and 30 % in pretreated liver with chemotherapy |
5. Liver function post-resection must be preserved |
In addition, Fong et al. have identified a number of factors that could predict for poorer overall survival following hepatic resection for colorectal metastatic deposits. These include a positive hepatic resection margin, presence of extrahepatic disease, number and size of tumours, high preoperative CEA, lymph node-positive primary and short disease-free interval (Fong et al. 1999). Patients with a poor score were found to have a median survival of only 22 months compared to 74 months in better-scoring individuals.
14.6.1.3 Surgical Points
The goal of surgical therapy is to achieve negative resection margins. While formal hepatic resections such as hemihepatectomies or tri-sectionectomies were previously performed, newer studies have suggested that limited nonanatomical hepatic resection can achieve similar oncological benefits while preserving maximal hepatic volume and function as long as negative margins are attained (Zorzi et al. 2006; Gold et al. 2008; Kokudo et al. 2001).
14.6.1.4 Regional Hepatic Therapies
Apart from surgery, regional hepatic therapies can also be considered in patients who have initially unresectable disease. These treatment modalities can broadly be classified into either ablative, arterial or non-arterial modalities (Saied et al. 2013).
Ablative methods, such as radiofrequency ablation (RFA) which is already commonly used in primary hepatic tumours, achieve tumour necrosis by inciting high temperatures. RFA is suitable for lesions up to about 5 cm, owing to the size of current probes. RFA for hepatic metastatic lesions has been associated with a recurrence rate of about 9–20 % (Gillams and Lees 2000; De Baere et al. 2000). RFA in combination with systemic chemotherapy has also been proven in the EORTC 40004 randomised controlled trial to have superior progression-free survival rates at 3 years of 27.6 % compared to 10 % in the systemic chemotherapy-only group (Ruers et al. 2012).
Radiation-based therapies can be administered intra-arterial or extra-arterial. Intra-arterial strategies include the use of yttrium 90. This agent is administered into the hepatic artery and emits beta rays. Pre-therapy planning is critical. The presence of hepatopulmonary shunting and reflux into the gastrointestinal arcades needs to be excluded as the yttrium 90 which enters these circulations can have severe consequences (Saied et al. 2013). Yttrium 90 has been associated with longer median survival rates of 70, 46 and 46 % at 6, 12 and 18 months, respectively (Stubbs and Wickremesekera 2004).
Non-arterial therapy is an evolving treatment modality which has recently entered the treatment armamentarium. A phase II trial using intensity-modulated radiation therapy with image-guided radiation therapy has been attempted. The trial reported a local tumour control at 1 year of 54 % and progression-free survival and overall survival of 14 and 78 %, respectively (Engels et al. 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








