Key Concepts
 The minimum alveolar concentration (MAC) progressively decreases during pregnancy—at term, by as much as 40%—for all general anesthetic agents; MAC returns to normal by the third day after delivery.
The minimum alveolar concentration (MAC) progressively decreases during pregnancy—at term, by as much as 40%—for all general anesthetic agents; MAC returns to normal by the third day after delivery.
 Pregnant patients display enhanced sensitivity to local anesthetics during regional anesthesia and analgesia, and neural blockade occurs at reduced concentrations of local anesthetics; dose requirements may be reduced as much as 30%.
Pregnant patients display enhanced sensitivity to local anesthetics during regional anesthesia and analgesia, and neural blockade occurs at reduced concentrations of local anesthetics; dose requirements may be reduced as much as 30%.
 Obstruction of the inferior vena cava by the enlarging uterus distends the epidural venous plexus and increases the risk of intravascular injection during epidural anesthesia.
Obstruction of the inferior vena cava by the enlarging uterus distends the epidural venous plexus and increases the risk of intravascular injection during epidural anesthesia.
 Approximately 5% of women at term develop the supine hypotension syndrome, which is characterized by hypotension associated with pallor, sweating, or nausea and vomiting. The incidence of maternal hypotension syndrome may be higher in women receiving neuraxial analgesia.
Approximately 5% of women at term develop the supine hypotension syndrome, which is characterized by hypotension associated with pallor, sweating, or nausea and vomiting. The incidence of maternal hypotension syndrome may be higher in women receiving neuraxial analgesia.
 The reduction in gastric motility and gastroesophageal sphincter tone place the parturient at high risk for regurgitation and pulmonary aspiration.
The reduction in gastric motility and gastroesophageal sphincter tone place the parturient at high risk for regurgitation and pulmonary aspiration.
 Ephedrine, which has considerable β-adrenergic activity, has traditionally been considered the vasopressor of choice for hypotension during pregnancy. However, clinical studies suggest that α-adrenergic agonists such as phenylephrine and metaraminol are just as effective in treating hypotension in pregnant patients and are associated with less fetal acidosis than ephedrine.
Ephedrine, which has considerable β-adrenergic activity, has traditionally been considered the vasopressor of choice for hypotension during pregnancy. However, clinical studies suggest that α-adrenergic agonists such as phenylephrine and metaraminol are just as effective in treating hypotension in pregnant patients and are associated with less fetal acidosis than ephedrine.
 Volatile inhalational anesthetics decrease blood pressure and, potentially, uteroplacental blood flow. In concentrations of less than 1 MAC, however, their effects are generally minor, consisting of dose-dependent uterine relaxation and minor reductions in uterine blood flow.
Volatile inhalational anesthetics decrease blood pressure and, potentially, uteroplacental blood flow. In concentrations of less than 1 MAC, however, their effects are generally minor, consisting of dose-dependent uterine relaxation and minor reductions in uterine blood flow.
 The greatest strain on the parturient’s heart occurs immediately after delivery, when intense uterine contraction and involution suddenly relieve inferior vena caval obstruction and increase cardiac output as much as 80% above late third trimester values.
The greatest strain on the parturient’s heart occurs immediately after delivery, when intense uterine contraction and involution suddenly relieve inferior vena caval obstruction and increase cardiac output as much as 80% above late third trimester values.
 Current techniques employing very dilute combinations of a local anesthetic (eg, bupivacaine, 0.125% or less) and an opioid (eg, fentanyl, 5 mcg/mL or less) for epidural or combined spinal-epidural (CSE) analgesia do not appear to prolong the first stage of labor or increase the likelihood of an operative delivery.
Current techniques employing very dilute combinations of a local anesthetic (eg, bupivacaine, 0.125% or less) and an opioid (eg, fentanyl, 5 mcg/mL or less) for epidural or combined spinal-epidural (CSE) analgesia do not appear to prolong the first stage of labor or increase the likelihood of an operative delivery.
Maternal & Fetal Physiology & Anesthesia: Introduction
Physiological Changes during Pregnancy
Pregnancy affects most organ systems (Table 40-1). Many of these physiological changes appear to be adaptive and useful to the mother in tolerating the stresses of pregnancy, labor, and delivery. Other changes lack obvious benefits but nonetheless require special consideration in caring for the parturient.
| Parameter | Change |
|---|---|
| Neurological | |
| MAC | −40% |
| Respiratory | |
| Oxygen consumption | +20 to 50% |
| Airway resistance | −35% |
| FRC | −20% |
| Minute ventilation | +50% |
| Tidal volume | +40% |
| Respiratory rate | +15% |
| Pao2 | +10% |
| Paco2 | −15% |
| HCO3 | −15% |
| Cardiovascular | |
| Blood volume | +35% |
| Plasma volume | +55% |
| Cardiac output | +40% |
| Stroke volume | +30% |
| Heart rate | +20% |
| Systolic blood pressure | −5% |
| Diastolic blood pressure | −15% |
| Peripheral resistance | −15% |
| Pulmonary resistance | −30% |
| Hematologic | |
| Hemoglobin | −20% |
| Platelets | −10% |
| Clotting factors2 | +30 to 250% |
| Renal | |
| GFR | +50% |
 The minimum alveolar concentration (MAC) progressively decreases during pregnancy—at term, by as much as 40%—for all general anesthetic agents; MAC returns to normal by the third day after delivery. Changes in maternal hormonal and endogenous opioid levels have been implicated. Progesterone, which is sedating when given in pharmacological doses, increases up to 20 times normal at term and is at least partly responsible for this observation. A surge in β-endorphin levels during labor and delivery also likely plays a major role.
The minimum alveolar concentration (MAC) progressively decreases during pregnancy—at term, by as much as 40%—for all general anesthetic agents; MAC returns to normal by the third day after delivery. Changes in maternal hormonal and endogenous opioid levels have been implicated. Progesterone, which is sedating when given in pharmacological doses, increases up to 20 times normal at term and is at least partly responsible for this observation. A surge in β-endorphin levels during labor and delivery also likely plays a major role.
 Pregnant patients also display enhanced sensitivity to local anesthetics during regional anesthesia and analgesia, and neural blockade occurs at reduced concentrations of local anesthetics. The term minimum local analgesic concentration (MLAC) is used in obstetric anesthesia to compare the relative potencies of local anesthetics and the effects of additives; MLAC is defined as the local analgesic concentration leading to satisfactory analgesia in 50% of patients (EC50). Local anesthetic dose requirements during epidural anesthesia may be reduced as much as 30%, a phenomenon that appears to be hormonally mediated but may also be related to engorgement of the epidural venous plexus.
Pregnant patients also display enhanced sensitivity to local anesthetics during regional anesthesia and analgesia, and neural blockade occurs at reduced concentrations of local anesthetics. The term minimum local analgesic concentration (MLAC) is used in obstetric anesthesia to compare the relative potencies of local anesthetics and the effects of additives; MLAC is defined as the local analgesic concentration leading to satisfactory analgesia in 50% of patients (EC50). Local anesthetic dose requirements during epidural anesthesia may be reduced as much as 30%, a phenomenon that appears to be hormonally mediated but may also be related to engorgement of the epidural venous plexus.  Obstruction of the inferior vena cava by the enlarging uterus distends the epidural venous plexus and increases epidural blood volume. The latter has three major effects: (1) decreased spinal cerebrospinal fluid volume, (2) decreased potential volume of the epidural space, and (3) increased epidural (space) pressure. The first two effects enhance the cephalad spread of local anesthetic solutions during spinal and epidural anesthesia, respectively, whereas the last may complicate identification of the epidural space (see Chapter 45). Bearing down during labor further accentuates all these effects. Positive (rather than the usual negative) epidural pressures have been recorded in parturients. Engorgement of the epidural veins also increases the likelihood of placing an epidural needle or catheter in a vein, resulting in an unintentional intravascular injection. It is unclear whether pregnancy lowers the seizure threshold for local anesthetics.
Obstruction of the inferior vena cava by the enlarging uterus distends the epidural venous plexus and increases epidural blood volume. The latter has three major effects: (1) decreased spinal cerebrospinal fluid volume, (2) decreased potential volume of the epidural space, and (3) increased epidural (space) pressure. The first two effects enhance the cephalad spread of local anesthetic solutions during spinal and epidural anesthesia, respectively, whereas the last may complicate identification of the epidural space (see Chapter 45). Bearing down during labor further accentuates all these effects. Positive (rather than the usual negative) epidural pressures have been recorded in parturients. Engorgement of the epidural veins also increases the likelihood of placing an epidural needle or catheter in a vein, resulting in an unintentional intravascular injection. It is unclear whether pregnancy lowers the seizure threshold for local anesthetics.
Oxygen consumption and minute ventilation progressively increase during pregnancy. Tidal volume and, to a lesser extent, respiratory rate and inspiratory reserve volume also increase. By term, both oxygen consumption and minute ventilation have increased up to 50%. Paco2 decreases to 28-32 mm Hg; significant respiratory alkalosis is prevented by a compensatory decrease in plasma bicarbonate concentration. Hyperventilation may also increase Pao2 slightly. Elevated levels of 2,3-diphosphoglycerate offset the effect of hyperventilation on hemoglobin’s affinity for oxygen (see Chapter 23). The P50 for hemoglobin increases from 27 to 30 mm Hg; the combination of the latter with an increase in cardiac output (see section on Cardiovascular Effects below) enhances oxygen delivery to tissues.
The maternal respiratory pattern changes as the uterus enlarges. In the third trimester, elevation of the diaphragm is compensated by an increase in the anteroposterior diameter of the chest; diaphragmatic motion, however, is not restricted. Thoracic breathing is favored over abdominal breathing. Both vital capacity and closing capacity are minimally affected, but functional residual capacity (FRC) decreases up to 20% at term; FRC returns to normal within 48 h of delivery. This decrease is principally due to a reduction in expiratory reserve volume as a result of larger than normal tidal volumes. Flow-volume loops are unaffected, and airway resistance decreases. Physiological dead space decreases but intrapulmonary shunting increases toward term. A chest film may show prominent vascular markings due to increased pulmonary blood volume and an elevated diaphragm. Pulmonary vasodilation prevents pulmonary pressures from rising.
The combination of decreased FRC and increased oxygen consumption promotes rapid oxygen desaturation during periods of apnea. Preoxygenation (denitrogenation) prior to induction of general anesthesia is therefore mandatory to avoid hypoxemia in pregnant patients. Closing volume exceeds FRC in some pregnant women when they are supine at term. Under these conditions, atelectasis and hypoxemia readily occur. The decrease in FRC coupled with the increase in minute ventilation accelerates the uptake of all inhalational anesthetics. The reduction in dead space narrows the arterial end-tidal CO2 gradient.
Capillary engorgement of the respiratory mucosa during pregnancy predisposes the upper airways to trauma, bleeding, and obstruction. Gentle laryngoscopy and smaller endotracheal tubes (6-6.5 mm) should be employed during general anesthesia.
Cardiac output and blood volume increase to meet accelerated maternal and fetal metabolic demands. An increase (55%) in plasma volume in excess of an increase in red cell mass (45%) produces dilutional anemia and reduces blood viscosity. Hemoglobin concentration, however, usually remains greater than 11 g/dL. Moreover, in terms of tissue oxygen delivery, the reduction in hemoglobin concentration is offset by the increase in cardiac output and the rightward shift of the hemoglobin dissociation curve (see the section on Respiratory Effects). A decrease in systemic vascular resistance by the second trimester decreases both diastolic and, to a lesser degree, systolic blood pressure. The response to adrenergic agents and vasoconstrictors is blunted.
At term, blood volume has increased by 1000-1500 mL in most women, allowing them to easily tolerate the blood loss associated with delivery; total blood volume reaches 90 mL/kg. Average blood loss during vaginal delivery is 400-500 mL, compared with 800-1000 mL for a cesarean section. Blood volume does not return to normal until 1-2 weeks after delivery.
The increase in cardiac output (40% at term) is due to increases in both heart rate (20%) and stroke volume (30%). Cardiac chambers enlarge and myocardial hypertrophy is often noted on echocardiography. Pulmonary artery, central venous, and pulmonary artery wedge pressures remain unchanged. Most of these effects are observed in the first and, to a lesser extent, the second trimester. In the third trimester, cardiac output does not appreciably rise, except during labor. The greatest increases in cardiac output are seen during labor and immediately after delivery (see the section on Effect of Labor on Maternal Physiology). Cardiac output often does not return to normal until 2 weeks after delivery.
Decreases in cardiac output can occur in the supine position after week 20 of pregnancy. Such decreases have been shown to be secondary to impeded venous return to the heart as the enlarging uterus compresses the inferior vena cava.  Approximately 5% of women at term develop the supine hypotension syndrome (aortocaval compression), which is characterized by hypotension associated with pallor, sweating, or nausea and vomiting. The cause of this syndrome appears to be complete or near-complete occlusion of the inferior vena cava by the gravid uterus. When combined with the hypotensive effects of regional or general anesthesia, aortocaval compression can readily produce fetal asphyxia. Turning the patient on her side typically restores venous return from the lower body and corrects the hypotension in such instances. This maneuver is most readily accomplished by placing a wedge (>15°) under the right hip. The gravid uterus also compresses the aorta in most parturients when they are supine. This latter effect decreases blood flow to the lower extremities and, more importantly, to the uteroplacental circulation. Uterine contraction reduces caval compression but exacerbates aortic compression.
Approximately 5% of women at term develop the supine hypotension syndrome (aortocaval compression), which is characterized by hypotension associated with pallor, sweating, or nausea and vomiting. The cause of this syndrome appears to be complete or near-complete occlusion of the inferior vena cava by the gravid uterus. When combined with the hypotensive effects of regional or general anesthesia, aortocaval compression can readily produce fetal asphyxia. Turning the patient on her side typically restores venous return from the lower body and corrects the hypotension in such instances. This maneuver is most readily accomplished by placing a wedge (>15°) under the right hip. The gravid uterus also compresses the aorta in most parturients when they are supine. This latter effect decreases blood flow to the lower extremities and, more importantly, to the uteroplacental circulation. Uterine contraction reduces caval compression but exacerbates aortic compression.
Chronic partial caval obstruction in the third trimester predisposes to venous stasis, phlebitis, and edema in the lower extremities. Moreover, compression of the inferior vena cava below the diaphragm distends and increases blood flow through the paravertebral venous plexus (including the epidural veins), and to a minor degree, the abdominal wall.
Lastly, elevation of the diaphragm shifts the heart’s position in the chest, resulting in the appearance of an enlarged heart on a plain chest film and in left axis deviation and T wave changes on the electrocardiogram. Physical examination often reveals a systolic ejection flow murmur (grade I or II) and exaggerated splitting of the first heart sound (S1); a third heart sound (S3) may be audible. A few patients develop small, asymptomatic pericardial effusion.
Renal plasma flow and the glomerular filtration rate increase during pregnancy, and as a result serum creatinine and blood urea nitrogen may decrease to 0.5-0.6 mg/dL and 8-9 mg/dL, respectively. A decreased renal tubular threshold for glucose and amino acids is common and often results in mild glycosuria (1-10 g/d) or proteinuria (<300 mg/d), or both. Plasma osmolality decreases by 8-10 mOsm/kg.
Gastroesophageal reflux and esophagitis are common during pregnancy. Gastric motility is reduced, and upward and anterior displacement of the stomach by the uterus promotes incompetence of the gastroesophageal sphincter.  These factors place the parturient at high risk for regurgitation and pulmonary aspiration. However, neither gastric acidity nor gastric volume changes significantly during pregnancy. Opioids and anticholinergics reduce lower esophageal sphincter pressure, may facilitate gastroesophageal reflux, and delay gastric emptying.
These factors place the parturient at high risk for regurgitation and pulmonary aspiration. However, neither gastric acidity nor gastric volume changes significantly during pregnancy. Opioids and anticholinergics reduce lower esophageal sphincter pressure, may facilitate gastroesophageal reflux, and delay gastric emptying.
Overall hepatic function and blood flow are unchanged; minor elevations in serum transaminases and lactic dehydrogenase levels may be observed in the third trimester. Mild elevations in serum alkaline phosphatase are due to its secretion by the placenta. A mild decrease in serum albumin is due to an expanded plasma volume, and as a result, colloid oncotic pressure is reduced. A 25-30% decrease in serum pseudocholinesterase activity is also present at term but rarely produces significant prolongation of succinylcholine’s action. The breakdown of ester-type local anesthetics is not appreciably altered. Pseudocholinesterase activity may not return to normal until up to 6 weeks postpartum. High progesterone levels appear to inhibit the release of cholecystokinin, resulting in incomplete emptying of the gallbladder. The latter, together with altered bile acid composition, can predispose to the formation of cholesterol gallstones during pregnancy.
Pregnancy is associated with a hypercoagulable state that may be beneficial in limiting blood loss at delivery. Fibrinogen and concentrations of factors VII, VIII, IX, X, and XII all increase; only factor XI levels may decrease. Accelerated fibrinolysis can be observed late in the third trimester. In addition to the dilutional anemia (see the section on Cardiovascular Effects), leukocytosis (up to 21,000/μL) and a 10% decrease in platelet count may be encountered during the third trimester. Because of fetal utilization, iron and folate deficiency anemias readily develop if supplements of these nutrients are not taken.
Complex metabolic and hormonal changes occur during pregnancy. Altered carbohydrate, fat, and protein metabolism favors fetal growth and development. These changes resemble starvation, because blood glucose and amino acid levels are low whereas free fatty acids, ketones, and triglyceride levels are high. Nonetheless, pregnancy is a diabetogenic state; insulin levels steadily rise during pregnancy. Secretion of human placental lactogen, also called human chorionic somatomammotropin, by the placenta is probably responsible for the relative insulin resistance associated with pregnancy. Pancreatic beta cell hyperplasia occurs in response to an increased demand for insulin secretion.
Secretion of human chorionic gonadotropin and elevated levels of estrogens promote hypertrophy of the thyroid gland and increase thyroid-binding globulin; although T4 and T3 levels are elevated, free T4, free T3, and thyrotropin (thyroid-stimulating hormone) remain normal. Serum calcium levels decrease, but ionized calcium concentration remains normal.
Elevated levels of relaxin throughout pregnancy help prepare for delivery by softening the cervix, inhibiting uterine contractions, and relaxing the pubic symphysis and pelvic joints. Ligamentous laxity of the spine increases the risk of back injury. The latter may contribute to the relatively high incidence of back pain during pregnancy.
Uteroplacental Circulation
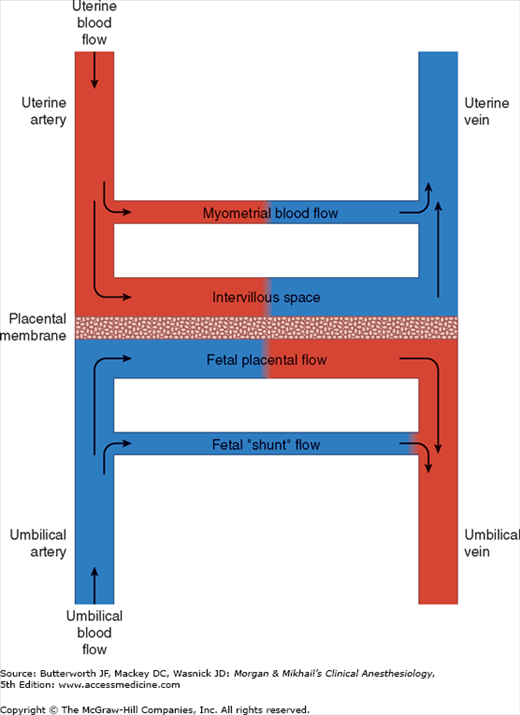
A normal uteroplacental circulation (Figure 40-1) is critical in the development and maintenance of a healthy fetus. Uteroplacental insufficiency is an important cause of intrauterine fetal growth retardation, and when severe, can result in fetal demise. The integrity of this circulation is, in turn, dependent on both adequate uterine blood flow and normal placental function.
At term, uterine blood flow represents about 10% of the cardiac output, or 600-700 mL/min (compared with 50 mL/min in the nonpregnant uterus). Eighty percent of uterine blood flow normally supplies the placenta; the remainder goes to the myometrium. Pregnancy maximally dilates the uterine vasculature, so that autoregulation is absent, but the uterine vasculature remains sensitive to α-adrenergic agonists. Uterine blood flow is not usually significantly affected by respiratory gas tensions, but extreme hypocapnia (Paco2 <20 mm Hg) can reduce uterine blood flow and causes fetal hypoxemia and acidosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree