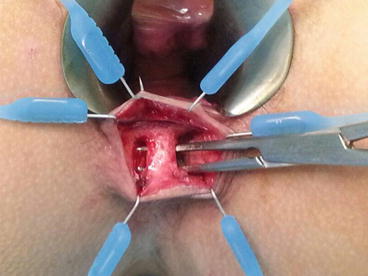
Fig. 12.1
Endoanal ultrasonographic picture showing the course of the fistula with respect to the sphincter musculature
Endoanal ultrasound (EAUS), also known as anal endosonography, has been shown to be a cheap and accurate diagnostic modality that enables preoperative visualisation of the fistula tract (Nevler et al. 2013). One of its main advantages is the ability to delineate the anatomy of the fistula tract distinctly and to correlate its path with respect to the integrity and relation of the internal and external sphincters. The use of hydrogen peroxide coupled with the advent of 3-dimensional images also increase the accuracy of the test and help to better identify the location of the internal opening and determine the presence of any secondary fistula tracts (Kim and Park 2009). When used properly, the radiological findings correspond closely with the surgical findings, and the images provide a blueprint to plan the optimal surgical intervention.
As in all sonographic modalities, the main disadvantage lies in its heavy dependence on the competency of the operator. In addition, EAUS has a limited focal range whereby pathology beyond the anal sphincters complex may not be as clearly visualised.
12.2.2 Magnetic Resonance Imaging
The superior ability to discern soft tissue planes has resulted in magnetic resonance imaging (MRI) being used in various specialties. With a reported concordance rate of over 90 % with operative findings, MRI has slowly become a recognised modality in the preoperative assessment of recurrent and complex anal fistulas (Holzer et al. 2000). It is also able to evaluate the deeper surgical planes, underlying pathologies and complex characteristics of the anal fistulas more accurately than EAUS (Fig. 12.2). The availability of the MRI, or the lack of it, and its significant cost are its main limitations.
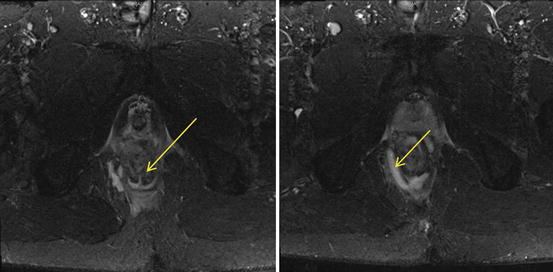

Fig. 12.2
MRI images of the pelvis. Image on the left shows an intersphincteric fistula tract at the 6 o’clock position (arrowed), and image on the right shows a right ischiorectal abscess (arrowed), which is communicating with the fistula tract
12.3 Surgical Management for Anal Fistula: A Journey Through Time and Innovation
The principles of surgical management in anal fistulas are the eradication of sepsis, prevention of recurrence and preservation of continence. While numerous evolving techniques have sprung up over the years in the hope of replacing lay-open fistulotomy as the standard of care in the management of all anal fistulas, none has succeeded thus far. We hereby evaluate the various techniques that have been advocated for anal fistula over the past years.
12.3.1 Fistulotomy
Lay-open fistulotomy was first described by John Arderne, an English surgeon, in the fourteenth century. As the name implies, this technique essentially involves laying open the fistula tract after establishing the path of the fistula from the external opening to the internal opening. This would facilitate drainage of the perianal sepsis and enables the wound to heal by secondary intention. A recent study demonstrated a 3-year recurrence rate of as low as 7 % with this technique (Benjelloun et al. 2013). The key component of this technique involves curetting the fistula tract to eradicate any infected and unhealthy tissue. A variation of the technique involves marsupialisation of the edges to prevent the skin from closing over the wound before healing from the wound bed, thereby reducing recurrence rates. This approach has been shown to be associated with faster wound healing and shorter duration of wound discharge (Ho et al. 1998).
Despite its high success rates, the risk of subsequent faecal incontinence increases if this technique is performed in fistulas with considerable involvement of the external anal sphincter or in patients with predisposing impaired anal continence. It has been reported that mild incontinence occurred in 9 % while severe incontinence is documented in up to 4 % of the patients (Stremitzer et al. 2011). This has a detrimental impact on the patients’ quality of life. Thus, lay-open fistulotomy has gradually fallen out of favour in patients with high anal fistula if considerable amount of anal sphincter muscles would be divided (Mylonakis et al. 2001; Cavanaugh et al. 2002).
12.3.2 Seton
First described by Hippocrates, seton placement within the fistula tract, either loosely or tightly, has been around for a long time. It is often used either as a bridging procedure to fistulotomy or a single modality in high complex anal fistula to avoid upfront division of excessive amount of sphincter muscle (McCourtney and Finlay 1995; Memon et al. 2011). There are two ways to insert a seton – draining (loose) and cutting (tight). The primary aim of inserting a seton within the fistula tract is to enable drainage of the sepsis.
Although a draining seton effectively relieves the perianal sepsis, it is often not definitive. A subsequent procedure such as fistulotomy is typically performed when the seton has migrated more superficially. A recent meta-analysis actually highlighted a fistula recurrence rate of 3–5 %, depending on whether the anal sphincters were divided with placement of seton. The group that had their anal sphincters divided had slightly better outcomes. But the faecal incontinence rate was also significantly higher in the group that had their anal sphincter divided compared with those who only had seton without division of sphincters (25.2 % vs. 5.2 %) (Vial et al. 2010).
Conversely, when a cutting seton is used, one of the aims is to exteriorise a deep tract by inducing fibrosis superficial to the seton. This would gradually make the tract more superficial as the seton is tightened sequentially in intervals after its insertion. Apart from significant pain and discomfort arising from the rigidity of the seton coupled with the frequent tightening procedures, the reported incontinence rates are actually considerable in spite of its purported sphincter-sparing properties. The faecal incontinence rate of as high as 12 %, with almost 60 % of patients complaining of minor persistent loss of continence to flatus and fluid, has been reported (Ritchie et al. 2009). This complication is likely secondary to the disruption of the anal sphincters as the tightening process of the seton actually cuts through the sphincters gradually, and the ensuing fibrosis and scarring of the muscle fibres resulted in suboptimal function of the sphincters.
12.3.3 Mucosal Advancement Flap
This technique was first described for the repair of rectovaginal fistulas in 1902 which later extended to the treatment of complex anal fistulas. The two primary aims of this technique are to disrupt any communication of the tract with the anal canal and to completely remove the diseased and inflamed tissue from the anal mucosal surface (Elting 1912). A meta-analysis review of the success rate for anal fistula following mucosal advancement flap procedures revealed a recurrence rate from 7.0 to 51.7 %, while the wound healing time was reported to be 24.5 (±5.5) days. The incontinence rate was documented to be from 3.7 to 13.4 %. But as this technique is typically reserved for mainly the recurrent and/or complex high anal fistulas, one would expect their success rates to be less than first-line options such as fistulotomy.
One of the limitations precluding its widespread adoption is the technical difficulties in mastering this procedure. A thorough understanding of the anal anatomy and the underlying pathology is critical to maximise the success and minimise its complication rates (Golub et al. 1997; Leng and Jin 2012). To pursue a higher success rate for this technique, some surgeons have combined the mucosal advancement flap with the administration of platelet-rich plasma which is an autologous form of tissue glue. A 16.0 % recurrence rate after a median follow-up period of 27 months was reported which did not appear to improve the procedural outcome significantly (Göttgens et al. 2014).
12.3.4 Utilising Technology for Anal Fistula Treatment
The problems of the aforementioned techniques are apparent. Anal fistulas involving minimal sphincter muscles can be easily dealt with by the fistulotomy procedure. But none of the “traditional” methods have been deemed safe and effective in the treatment of high, complex and recurrent anal fistulas. With the continual advancement in science and technology, newer and innovative techniques have been introduced with the aim to improve the success rates following the treatment of this difficult condition while preserving the anal sphincter function.
12.3.4.1 Fibrin Glue
The rationale of fibrin glue in anal fistula is the application of an adhesive substance into the fistula tract with the aim to facilitate its closure by laying down a fibrin network. If proven to be successful, this is an attractive, fast and non-invasive method for treating anal fistulas. In the initial years following its introduction in the 1990s, the overall closure rate reported could be as high as 85–100 % after single or multiple treatments (Sentovich 2001). However, longer-term follow-up studies revealed suboptimal results with one study reporting a healing rate of 39.1 % at 12 weeks post-operatively. Another randomised study highlighted a 27-month post-operative healing rate of only 21 % (Singer et al. 2005; Chung et al. 2009).
It is postulated that the high failure rates could be attributed to its inability to eradicate the infected and inflamed tissue and its failure to tackle complex fistulas with secondary extensions. Coupled with the high failure and recurrence rates and the cost of the fibrin glue, this technique has largely fallen out of favour (Swinscoe et al. 2005).
12.3.4.2 Fistula Plug
As we enter the new millennium, a bioprosthetic plug (Fig. 12.3) was introduced to address the liquid nature of the fibrin glue which was one of the postulated reasons for its poor outcome. These plugs are placed within the fistula tract, typically after perianal sepsis has subsided with the aid of a draining seton. The fistula plug is then sutured in place at the internal opening with the excess plug material being removed if it extrudes from the external opening (Fig. 12.4).
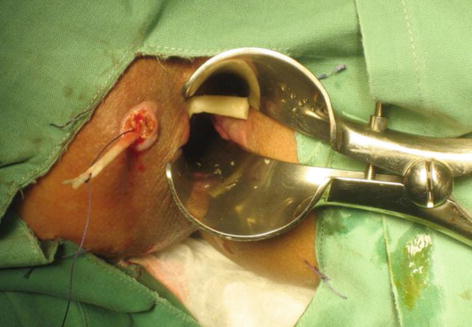

Fig. 12.3
Anal fistula plug

Fig. 12.4
Placement of the fistula plug within the fistula tract
Initial interest was overwhelming following a prospectively conducted study which involved a cohort of 25 patients, where 15 patients were treated with plug and 10 with glue. A 6-month fistula closure success rate of 87 % was reported with plug treatment, compared to 40 % in those who had glue application (Johnson et al. 2006). Just like the fibrin glue, varying institutions and longer-term follow-up failed to replicate the success shared by the initial group. A retrospective review in an Australian centre reported a failure rate of 86.7 % at 59 weeks (Tan et al. 2013). A meta-analysis including three retrospective and two randomised studies also demonstrated that the incidence of recurrence for the anal fistula plug was 62.1 % compared to 47 % for conventional surgical approach (Pu et al. 2012).
A multicentre study of 126 patients over 5 years attempted to evaluate the risk factors that could predispose to failure of the fistula plug. While they concurred with the very low success rates, they found that anteriorly located fistula tends to have a lower chance of closure (12 %, hazard ratio = 2.98, confidence interval [CI] 1.01–8.78) (Blom et al. 2014). Other factors that have been reported as risk factors for failures included the extrusion of the fistula plug and its inability to deal with fistulas with secondary extensions.
12.3.4.3 Video-Assisted Anal Fistula Treatment
Video-assisted anal fistula treatment (VAAFT) is one of the latest treatment modalities advocated for anal fistula. This technique which is first described by Meinero and Mori, combines the use of a small rigid endoscope inserted through the external opening to follow the tract until the internal opening (Fig. 12.5). This is then followed by electrocautery to fulgurate the fistula tract as the endoscope was slowly withdrawn. The internal opening is then closed either with a local mucosal flap or by stapler technique. One of the purported advantages of the scope includes its ability to exclude secondary fistula tracts or chronic deep abscesses.
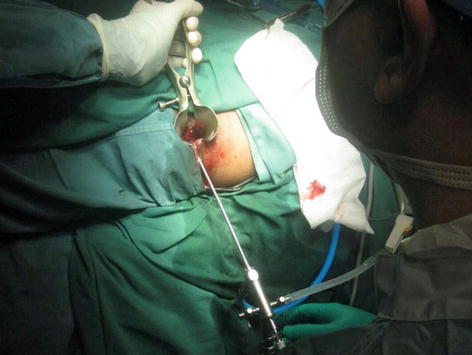

Fig. 12.5
Rigid fistuloscope being inserted into the fistula
This method theoretically discontinues the fistula tract with the anal mucosa and preserves the sphincter continuity by preventing further disruption to the sphincter integrity in high and complex anal fistulas. Meinero’s study of 136 patients revealed an 87.1 % healing rate 1 year following the operation (Meinero and Mori 2011). The same study group later included incontinence scoring over a 24-month period for a total of 203 patients and found that none had a change in their continence ability (Meinero et al. 2014).
The promising results of this new technique have made it very attractive, but just like the fibrin glue and fistula plug techniques, this technique needs to be validated by other surgeons from numerous institutions to determine if similar outcomes can be achieved. The cost of the disposables required for this procedure is also a considerable limitation.
12.3.4.4 Fistula Tract Laser Closure
The persistence of the fistula epithelium was deemed to be one of the main reasons for failure in fistula treatment. In recent years, the incorporation of laser technology to ablate the fistula tract has been attempted in the form of fistula tract laser closure (FiLaC).
A laser-emitting diode is threaded through the fistula tract, and the emission of a radial laser from the diode results in contraction of the tract around the laser fibre to induce fibrosis around the fistula tract in an attempt to obliterate it. This procedure is recommended as a follow-up procedure after perianal sepsis is alleviated with draining setons. It can also be combined with another procedure, like a mucosal advancement flap, to seal the internal opening.
12.3.5 Back to Basics?
Despite the prominence and keen interest in adopting technology and innovation in treating anal fistula, their outcomes have yet to reach levels comparable to conventional treatment modalities. Perhaps, success in fistula surgery may not necessarily lie with continual advancement in technology and innovation.
12.3.5.1 Ligation of Intersphincteric Fistula Tract
A small group of surgeons opted to refocus on the basics of perianal anatomy and the pathological process of anal fistula rather than to continue to apply technology and innovation in this field. Rojanasakul and group described the ligation of intersphincteric fistula tract (LIFT) procedure which puts the emphasis back on the clear delineation of the anatomy and pathology of the anal fistula. It was postulated that the eradication of the infected crypt gland in the intersphincteric space is vital to the LIFT procedure (Fig. 12.6).