INFLAMMATORY CONDITIONS OF THE GASTROINTESTINAL TRACT
INFLAMMATORY EMERGENCIES OF THE ESOPHAGUS
Acute, inflammatory conditions of the esophagus requiring urgent treatment are associated with significant morbidity, and can result in the rapid progression of mediastinitis, sepsis and death.1 Thoughtful and expeditious diagnostic testing and prompt operative intervention (when required) are critical in optimization of patient outcome. In this section, we review three severe inflammatory conditions of the esophagus: esophagitis, perforation, and caustic ingestion. Other esophageal emergencies related to obstruction, bleeding, trauma, and foreign bodies will be covered in the respective chapters devoted to these conditions.
Esophagitis
Acute inflammation of the esophagus can occur due to a variety of causes (Table 35.1). A careful History and Physical Examination can frequently reveal the probable etiology. Symptoms may include heartburn, chest pain, dysphagia, or odynophagia. Chest radiograph is frequently negative, but may reveal effusion or signs of aspiration. Barium esophagography may demonstrate irregular mucosal contour, ulceration, or stricture. Computed tomography (CT) can reveal esophageal thickening or distension. Esophagogastroduodenoscopy (EGD) is the most accurate diagnostic modality, providing both visual inspection and the opportunity for biopsy and pathologic confirmation of the underlying inflammatory process.
Gastroesophageal Reflux Disease. The most common cause of esophageal inflammation is gastroesophageal reflux disease (GERD). It is the most common benign condition affecting the esophagus encountered in the emergency room,2 and accounts for up to 75% of patients with esophageal pathology.3 Over 60 million Americans suffer from heartburn and indigestion (overall prevalence: 10%–20%).4 The prevalence of GERD has been steadily increasing over the last two decades and may contribute to the rapid rise in the incidence of other complications of GERD (Barrett’s esophagus, adenocarcinoma) along with the large societal costs associated with the treatment of reflux disease.3
Reflux esophagitis arises from repeated exposure of the distal esophagus to both acidic and bilious gastric contents secondary to an incompetent lower esophageal sphincter, hiatal hernia, or impaired gastric emptying. Symptoms are frequently episodic, and can be acute in nature. At endoscopy, the degree of mucosal damage can be assessed and graded based upon severity. The most widely-recognized grading systems are that of Savary and Miller5 and the Los Angeles Classification6 (Table 35.2). The degree of esophagitis can range from focal mucosal erythema to severe, circumferential hemorrhagic esophagitis. The optimal management for severe, reflux esophagitis is the institution of proton-pump inhibitor (PPI) therapy, which will resolve the inflammatory mucosal changes and patient symptoms in the majority of cases.7 Other antacid therapy (calcium carbonate, H2-blockers, carafate) can be added to PPI therapy, as necessary. Acute surgical intervention is rarely required beyond endoscopy to secure the diagnosis. In patients with refractory end-organ damage and persistent symptoms despite maximal medical therapy, a definitive antireflux procedure (e.g., Nissen fundoplication) may be beneficial.8
TABLE 35.2
ESOPHAGITIS GRADING SYSTEMS

Candida Esophagitis. Infectious causes of acute esophagitis are rare, and most commonly encountered in the immunocompromised host (e.g., HIV or posttransplantation setting), in cases of prolonged antibiotic use or in association with an underlying structural esophageal abnormality (e.g., stricture).9 The most commonly encountered infectious pathogen within the esophagus is Candida albicans. The most common symptoms of Candida esophagitis are dysphagia and odynophagia. The diagnosis should be suspected if oral thrush is evident on physical examination, although the absence of thrush does not negate the possibility of esophageal candidiasis. The diagnosis is confirmed with EGD, which reveals characteristic white mucosal plaques and pseudomembranes (Fig. 35.1). For mild cases, the institution of fluconazole or ketoconazole, in addition to oral nystatin swish and swallow, is effective in eradication of the infection in conjunction with the withdrawal of any predisposing antibiotic or corticosteroid therapy. C. esophagitis can become severe, with circumferential, pseudomembranous exudative infiltrates. The infectious process can penetrate the esophageal wall, leading to the development of candidal sepsis or massive hemorrhage.10 Patients manifesting signs of systemic illness are frequently treated with amphotericin B or caspofungin.
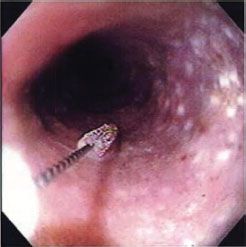
FIGURE 35.1. C. esophagitis. Severe inflammation of the esophagus is accompanied by the development of white plaques and pseudomembranes.(From Postma GN, Belafsky PC, Aviv JE. Atlas of Transnasal Esophagoscopy. Philadelphia, PA: Lippincott Williams & Wilkins Figure 4.6.)
Viral Esophagitis. The most commonly encountered causes of viral esophagitis are cytomegalovirus (CMV) and herpes simplex virus (HSV). CMV is more common in the setting of AIDS, but each of these infections can occur in immunocompetent patients as well.11 The most prominent presenting complaint is odynophagia. EGD reveals multiple shallow ulcerations or vesicles, in the case of HSV esophagitis. The degree of esophagitis can be severe, and patients can present with inability to swallow or gastrointestinal (GI) hemorrhage. Endoscopic biopsy confirms the diagnosis. Cytologic specimens reveal cytopathic effect restricted to the squamous epithelium and intranuclear eosinophilic inclusion bodies (Cowdry type A bodies). Severe HSV esophagitis generally responds to 2–4 weeks of acyclovir therapy.
CMV esophagitis is unusual in the normal host. CMV esophagitis can be associated with deep, longitudinal ulcers. The cytologic damage extends through the mucosa to involve underlying mesenchymal cells. Gancyclovir given over 2–4 weeks is the treatment of choice for CMV esophagitis. Long-term prophylaxis may be required in the transplant setting.
Bacterial Esophagitis. Bacterial etiologies of acute esophagitis are extremely rare. Mycobacterium tuberculosis has been associated with the development of esophagitis and ulceration. This disease is believed to be more commonly the result of direct extension from infected nodal tissue.12 Primary tuberculous infection can also present as an exophytic mass, or pseudotumor.13 Patients typically present with dysphagia. EGD with biopsy typically will reveal the diagnosis. Standard antimycobacterial regimens are the treatment (e.g., isoniazid, rifampin, ethambutol).14
Radiation Esophagitis. Patients who have undergone prior radiation for intrathoracic malignancy (e.g., lung or esophageal cancer, lymphoma) can develop inflammatory changes within the esophagus that are proportional to the dose administered.15 The esophageal mucosa becomes thickened and friable. Swallowing can become painful (odynophagia). Dysphagia can result from esophageal muscular fibrosis and stricture formation. Treatment is typically symptomatic (viscous lidocaine) until symptoms improve. Dilation or stenting of strictures may ultimately become necessary for recurrent, refractory dysphagia.16
Pill Esophagitis. The esophagus can become acutely inflamed from direct contact with ingested medications. Symptoms typically include chest pain and dysphagia within 4–6 hours of medication consumption. Common causes include antibiotics (esp. tetracycline), quinidine, potassium, and antiinflammatory medications. Treatment requires washing down the medication with water or physical removal of the pill by endoscopy in cases of underlying esophageal motor disorder or obstruction.17
Eosinophilic Esophagitis. Eosinophilic esophagitis is a unique disorder of the esophagus more commonly found in men. Patients present with dysphagia, heartburn, and recurrent food impaction. Symptoms typically will not respond to conservative measures or standard antisecretory therapy. EGD reveals multiple concentric rings in the proximal-mid esophagus. Mucosal biopsies reveal the presence of >20 eosinophils per high power field.18 The etiology of this condition is not well understood, but is believed to represent an immunologic derangement in cytokine and T-cell function.19 Symptomatic treatment with systemic or topical corticosteroids is recommended.
Iatrogenic Causes. Significant esophageal erosions can occur following long-term placement of nasogastric or feeding tubes. These erosions are typically an incidental finding of no clinical significance. Rarely, erosions can become severe resulting in esophagitis or bleeding. Treatment is removal of the offending foreign body to allow mucosal healing.20 Esophageal inflammation can also occur following esophageal instrumentation (EGD), PEG tube placement, or following endoscopic esophageal interventions (photodynamic therapy, endoscopic mucosal resection).21. In select cases, patients may demonstrate an elevated white blood cell count and chest discomfort. Conservative management with intravenous antibiotics and hydration typically will lead to resolution of symptoms. Barium esophagography can be utilized to exclude full thickness perforation.
Uncommon Inflammatory Disorders. The esophagus can demonstrate involvement with primary dermatologic conditions, such as epidermolysis bullosa, pemphigus, erythema multiforme, or Behcet’s disease. Sarcoidosis may present with nodularity and ulceration of the esophagus. Crohn’s disease can occur anywhere from the mouth to the anus, but esophageal involvement is noted in only 1%–3% of cases.22 Similar to ileocolic disease, Crohn’s disease is characterized by aphthous ulceration, esophagitis, and long fibrotic strictures. In patients undergoing bone marrow transplantation, graft-versus-host disease may present with dysphagia, ulceration, and fibrosis. Protozoan infections (e.g., cryptosporidiosis and pneumocystis) in the setting of AIDS are rare, but have also been reported.17
Esophageal perforation is a life-threatening problem associated with high mortality rate (9%–36%).23,24 Perforation can occur spontaneously in the setting of forceful retching/vomiting (Boerhaave’s syndrome). More commonly perforation results from penetrating trauma or following esophageal instrumentation (EGD, dilation). Typical presenting symptoms include acute chest pain, shortness of breath and fever. Additional symptoms include dysphagia, odynophagia, epigastric pain, or productive cough. The clinical presentation may be fulminant, with signs of toxicity and shock. Chest x-ray reveals pneumomediastinum and subcutaneous emphysema, mediastinal air-fluid levels, and pleural effusions. Esophagography confirms and localizes the site of leak. CT is valuable in further definition of the site and extent of the leak, as well as the detection of undrained fluid collections, which is of particular value to plan the most appropriate surgical approach.25
The hallmarks of therapy include expeditious diagnosis, prompt institution of broad-spectrum antibiotics and early, aggressive surgical intervention directed at drainage, débridement, and control of the perforation site to prevent or treat sepsis.26 Several surgical approaches can be employed in the management of spontaneous or iatrogenic esophageal perforation including débridement and primary closure, mediastinal drainage, placement of an esophageal T-tube, esophageal exclusion with proximal diversion, and esophagectomy. The selection of approach is influenced by the underlying cause and duration of the perforation, the degree of tissue injury and inflammation as well as the clinical condition of the patient. As expertise is gained in video-assisted esophageal surgery, minimally invasive surgical approaches to esophageal perforation and leak have been developed, and have become the preferred approach in many situations.27 Given the lack of level 1 evidence to establish clear standards, a lack of consensus exists regarding various aspects of management of this complex and life-threatening problem.
The decision to operate is typically based upon the extent of mediastinal contamination and systemic sepsis rather than cause of perforation. The extent of perforation may be classified as contained or noncontained. A noncontained perforation is defined by free extravasation of contrast in the mediastinal or peritoneal cavities (Fig. 35.2). A contained perforation is defined as minimal extravasation of contrast at the perforation site, or the presence of pneumomediastinum or pneumoperitoneum without apparent extravasation of contrast (Fig. 35.3). The interval between the time of perforation and initiation of treatment is also considered in the management decision. The surgical tenets in management of esophageal perforation include: (1) identification and localization of the esophageal perforation, (2) débridement of all infected and necrotic tissue, (3) control of the leak (primary closure or T-tube placement), and (4) wide drainage of the mediastinum.28
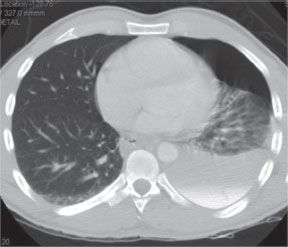
FIGURE 35.2. Esophageal perforation. Uncontained perforation with free flow of contrast material into the left pleural space.(Figure 1 from Abbas G, Schuchert MJ, Pettiford BL, et al. Contemporaneous management of esophageal perforation.Surgery. 2009;146(4): 749–756.)
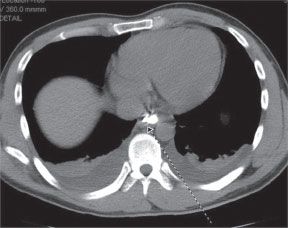
FIGURE 35.3. Esophageal perforation. Contained perforation of the distal esophagus.(Figure 2 from Abbas G, Schuchert MJ, Pettiford BL, et al. Contemporaneous management of esophageal perforation. Surgery. 2009;146(4): 749–756.)
Nonoperative management can be considered in patients with contained perforation and no clinical signs of sepsis. Patients are managed with no oral intake (NPO) for 24–72 hours, intravenous hydration, and antibiotics. Covered esophageal stents can be placed in select patients to occlude the perforation. The extent of contamination and signs of sepsis are the most important clinical variables in the outcome of esophageal perforation. The patient with a contained leak without signs of sepsis may generally be safely managed with a nonoperative approach.29 Surgical intervention can subsequently be performed if clinical signs of sepsis arise.
In cases of noncontained perforation, esophageal repair represents the preferred surgical approach, when feasible. Primary repair is typically undertaken in patients with limited mediastinal contamination and necrosis, regardless of the time interval from perforation event to surgery.30 During repair, it is critical to identify the full extent of injury. Devitalized esophageal and mediastinal tissues are first debrided. The full extent of mucosal injury is exposed, and the esophagus is closed in layers. Mobilization of a flap of vascularized tissue (e.g., intercostal muscle, pleura, pericardial fat, omentum) is recommended to buttress the repair. Multiple closed suction drains and chest tubes are inserted to achieve wide drainage of the mediastinum and pleura. Repair over a drain (e.g., T-tube) is a useful option when formal repair is not feasible. Repair can be performed by either minimally-invasive or open technique. The side chosen for thoracotomy or VATS is selected on the basis of known extravasation within the pleural space, taking into consideration extent of contamination and CT or endoscopic findings. Laparotomy or laparoscopy can be performed for the repair of gastroesophageal junction perforation into the peritoneal cavity. Gastrostomy and jejunostomy tubes are placed as adjuncts for drainage and enteral feeding access, respectively. Esophagectomy may be required in the setting of perforation and cancer or severe esophageal stricture. Esophageal exclusion is rarely performed, but can represent a life-saving option to the unstable patient with severe contamination.
Transcervical drainage is efficacious in the control of proximal esophageal perforation, usually secondary to trauma (gunshot wound, difficult intubation). This approach allows adequate exposure to control and repair perforations extending into the upper mediastinum down to the level of the carina. Transcervical drainage is also useful in the treatment of descending cervical mediastinitis, which may be odontogenic, peritonsillar, cervicofacial, or esophageal in origin.31 Aided by gravity, as well as negative intrathoracic pressure, organisms and pus rapidly accumulate in the deep cervical and mediastinal fascial planes, with mortality rates as high as 30%–40%.32 Esophageal leak after esophagectomy can be managed in most instances by opening the neck incision and packing the wound. Cervical exploration via this wound allows access to the upper mediastinum, facilitating assessment of the leak, the condition of the gastric tube, and esophagogastric anastomosis. It permits adequate exposure for débridement and drain placement.
Mid-distal esophageal perforation typically requires transthoracic drainage. Some authors advocate the routine use of thoracotomy to provide maximal exposure for thorough débridement, repair, and drainage. Increasingly, thoracoscopy is supplanting thoracotomy when conditions are appropriate.33 The initial step is retraction of the lung and evacuation of fibrinous debris and purulent exudate. Intraoperative endoscopy is performed to assist in identification of the site of perforation. The suspected region can be submerged under irrigation during endoscopic insufflation to pinpoint the precise location of perforation. Once identified, the devitalized margins of the perforation are debrided, and the decision whether to attempt a primary closure, depending on the degree of surrounding tissue injury as well as the clinical condition of the patient, is made. If the defect is small (<1 cm), and surrounded by viable tissue, a primary closure can be performed with interrupted sutures. In the case of larger injury or perforation, surrounded by severely inflamed tissue, wide drainage is performed with placement of a T-tube to control the leak. Wide defects may allow direct placement of a T-tube through the perforation site. Jackson-Pratt drains as well as a 28–32 French chest tube are positioned strategically to provide wide drainage of the mediastinum and chest.
Special circumstances encountered during esophageal perforation provide opportunities for creative surgical approaches to this serious problem. Perforation involving an esophageal diverticulum can be managed by diverticulectomy (when possible) and drainage.34 An important part of the surgical approach is to relieve distal obstruction with myotomy of either the cricopharyngeus (Zenker’s diverticulum) or lower esophageal sphincter (epiphrenic diverticulum). Perforation of the distal esophagus after esophageal dilatation for achalasia occurs with a frequency of up to 15%. The location of the perforation is typically the left posterior esophagus. Full-thickness perforation tends to begin within a centimeter of the squamocolumnar junction, and extends proximally from a few millimeters to as much as 10 cm. These perforations can usually be repaired utilizing a thoracoscopic35 or trans-abdominal36 laparoscopic technique with suture closure of the perforation, contralateral Heller myotomy, and Toupet posterior fundoplication. A contralateral myotomy is performed extending 5 cm along the length of the esophagus and extending 1 cm onto the surface of the stomach, with care taken to spare the vagus nerves. A posterior fundoplication is then performed in the manner of Toupet, suturing the edges of the myotomy to the edges of the plicated stomach over a length of 4 cm.37 This technique has the advantage of covering the closed esophageal perforation with a gastric serosal patch, at the same time treating the underlying motility disorder. A closed suction drain is placed into the mediastinum prior to closure.
Caustic Injury of the Esophagus
Caustic ingestion can result in significant injury to the esophagus and stomach. This condition is most commonly encountered with accidental ingestion in children, adults with underlying psychiatric conditions, or those with suicidal ideation. Caustic ingestion can involve both acidic and alkaline substances which produce severe esophagitis and gastritis. Full thickness esophageal and gastric injuries are not uncommon in this setting. Inciting agents include strong alkali products such as sodium or potassium hydroxide (lye), commonly utilized in drain/pipe cleaners. Strong acid compounds include hydrochloric, sulfuric, and phosphoric acid that are primary constituents of many household cleaners. Bleach ingestion (sodium hypochlorite) is commonly encountered in this setting but is rarely associated with significant esophageal injury.38 The degree of damage depends upon the specific corrosive properties of the ingested agent, the amount, concentration, physical form (solid, liquid), and the duration of contact with the esophageal and gastric mucosa.
Acid burns result in coagulative necrosis. Given their reduced viscosity, these solutions typically pass quickly to the stomach resulting in a greater degree of gastric injury, and a lesser degree of esophageal damage. Outcome following acid injury has been reported to be worse than following alkaline injuries, with an associated higher rate of transmural injury and perforation.39 In contrast to acid ingestion, alkaline injuries typically result in more extensive damage to both the esophagus and stomach. The degree of mucosal damage can be deep and penetrating (liquefaction necrosis); esophageal injury may be severe in this setting. The stomach itself may be partially preserved by neutralization by gastric acid. The affected mucosa is devitalized by direct contact with the alkaline agent. Severe concomitant inflammation ensues in viable tissues with vascular thrombosis, mucosal ulceration, and sloughing. Depending upon injury severity, progressive thinning of the esophageal wall may ultimately result in perforation and mediastinitis several days after the initial injury.
Patients will typically present with a known or suspected history of toxic substance ingestion reported by family members or EMS personnel. Symptoms commonly include severe nausea, vomiting, and hematemesis. The patient may complain of chest pain and severe abdominal pain. Inability to swallow and drooling may also result. The presence of fever, tachycardia, or hypotension portends a severe injury and a poor prognosis. Patients may have severe lip and oropharyngeal burns, associated with dysphonia and stridor. These findings should heighten suspicion of laryngeal and airway involvement, and early definitive control of the airway should be accomplished.40 If there is evidence of significant laryngeal edema or stridor, tracheostomy should be performed urgently.
Initial therapeutic measures include prompt assessment and control of the airway, volume resuscitation, and institution of intravenous antibiotics. No attempt should be made to induce vomiting, as this may worsen the caustic injury. Nasogastric tubes should not be placed blindly as this may induce vomiting and may create a full thickness esophageal perforation. Radiographic evaluation with chest and abdominal x-rays is performed. Upper endoscopy is performed within the first 24 hours following ingestion. This permits assessment of the extent of esophageal and gastric damage and is critical in guiding therapy. The development of clinical manifestations of mediastinitis, peritonitis, and sepsis are indications for emergent surgery.
At endoscopy, mucosal injury can be categorized based upon the degree of injury. Grade 1 is associated with esophagitis and hyperemia. Grade 2 is denoted by esophageal mucosal ulcerations. Grade 3 is associated with necrosis extending into the wall of the esophagus, and may be focal or circumferential.41 Superficial (grade 1) injuries typically resolve without chronic sequelae. Grade 2 injuries associated with significant or penetrating ulcers are associated with a significant risk of stricture formation. Grade 3 injuries typically result in mediastinitis and sepsis. Esophagectomy is commonly required in Grade 3 injuries, with delayed reconstruction following recovery from the initial presentation. The stomach is frequently unsuitable for reconstruction, and delayed re-construction with a colonic conduit is often required. Late sequelae of caustic injuries include the development of strictures and squamous cell carcinoma within the injured segment. Long-term endoscopic surveillance is recommended.42
INFLAMMATORY DISEASES OF THE STOMACH AND SMALL INTESTINE
When planning elective operations surgeons have the luxury of time to properly consider and execute an operation under optimal circumstances. Under emergent conditions, however, acute care surgeons and general surgeons must make rapid decisions both inside and outside the operating room. The decision of whether and if so, which operation should be performed, will usually determine surgical success. The patient’s underlying medical condition and hemodynamic status should guide the type of operation and approach. This section focuses on inflammatory emergencies of the intestine that the acute care and general surgeons are likely to encounter in practice.
Gastroduodenal Perforation
Surgeons may encounter perforations of the stomach or duodenum secondary to trauma, neoplasm, foreign body ingestion, and iatrogenic perforations from diagnostic or therapeutic procedures. The most common cause of gastroduodenal perforation today remains ulcer disease with an incidence between 2% and 10% in patients with ulcers.43,44
Surgery is almost always indicated in the case of perforated peptic ulcer. Nonsurgical management can occasionally be considered in the carefully selected stable patient without evidence of a free leak. It is estimated that approximately half the perforations will seal spontaneously. Patients under the age of 70 without peritonitis or signs of sepsis and with the onset of symptoms of <24 hours may be candidates for a trial of nonoperative management. CT abdomen with oral contrast or gastroduodenography with water-soluble contrast should be performed. Free contrast extravasation mandates operation regardless of the patient’s symptoms or clinical status.
The treatment plan for nonoperative management should include nasogastric tube decompression, fluid resuscitation, intravenous PPI, antimicrobial therapy, and close observation of hemodynamic status with serial abdominal exams. Any clinical deterioration warrants operation. Observation longer than 12 hours without improvement worsens the outcome and is not recommended. While favorable outcomes have been demonstrated in healthy patients, results in patients with major comorbidities are unknown.–4446
Most perforated peptic ulcers occur in the first portion of the duodenum (35%–65%); 25%–45% occur in the pylorus, and 5%–25% in the stomach. The pathophysiology of peptic ulcer has shifted from acid hypersecretion to Helicobacter pylori infection. Most patients with peptic ulcer disease will have evidence of H. pylori infection of the antral mucosa. The proportion of peptic ulcer caused by NSAID use is increasing, particularly in the elderly population. This is likely a significant contributing factor to the increasing frequency of complicated peptic ulcer disease requiring surgical intervention. Cigarette smoking and excessive alcohol consumption have traditionally been considered risk factors in the development of peptic ulcer but recent studies have not definitively confirmed their significance. Smoking does appear to increase the likelihood of requiring surgery for complications of peptic ulcer.–4751
In an emergency operation the surgeon must focus on the goals of the operation. Indeed, there is inherent danger in trying to accomplish more than what is necessary to achieve a good outcome for the patient. In the case of a gastroduodenal perforation, the primary goal is closure of the perforation, providing adequate drainage, and controlling abdominal sepsis. The patient’s age, medical comorbidities, nutritional status, NSAID use, history of previous peptic ulcer disease, prior medical therapy for ulcer disease, and most importantly, hemodynamic status must be considered in plan and emergency operation for perforated peptic ulcer. Simple closure is associated with decreased morbidity and mortality in the acute setting, but with a higher rate of recurrence when compared with definitive resective procedures.52 With the currently available highly effective acid suppression medications and H. pylori treatments, a definitive acid-reducing operation in the emergent setting is rarely indicated. Simple patch closure alone should be performed in patients with hemodynamic instability and with perforation of more than 24 hours’ duration–5356 (Fig. 35.4). In more stable patients, truncal vagotomy with pyloroplasty incorporating the ulcer has shown favorable results. Postoperative medical therapy with PPIs with or without therapy to eradicate H. pylori is indicated (Table 35.3). H. pylori infection can be demonstrated by tissue biopsy, serologic studies, or urea breath test. H. pylori eradication rates are similar between quadruple and triple therapy with comparable compliance. Prescribed therapy should be tailored to the individual patient.57,58
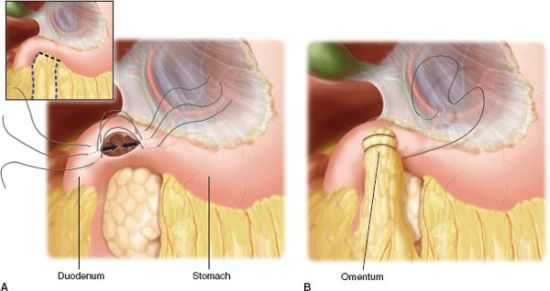
FIGURE 35.4. Omental patch closure of perforated ulcer. A:The right upper quadrant may be accessed through an upper midline incision. B: Adjacent omentum is placed over the perforation site.
TABLE 35.3
MEDICAL TREATMENT FOR ERADICATION OF H. PYLORI (10–14 DAY REGIMEN)
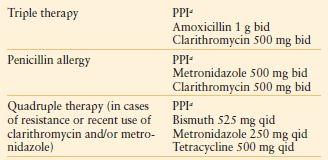
aPPI (proton pump inhibitor) choices include: lansoprazole 30 mg bid, omeprazole 20 mg bid, pantoprazole 40 mg bid, rabeprazole 20 mg bid, and esomeprazole 40 mg qd.
Formal gastric resection with reconstruction (Billroth I, Billroth II, Roux-en-Y) with or without vagotomy is rarely required today but should be considered for stable patients with recent (<12 hours) perforation and history of chronic or refractory ulcer disease.44,59 The stable patient who is deemed unreliable or noncompliant may also be better served with a definitive ulcer operation. Any ulcer clearly associated with NSAID use may be managed with simple closure and discontinuation of the offending medication.
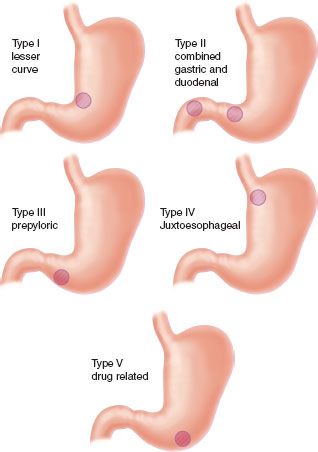
Unlike duodenal ulcers, gastric ulcers (GUs) are generally treated by excision. There are five types of GUs classified according to location and relationship to acid hypersecretion (Fig. 35.5). Type I GUs are the most common and are found at the incisura along the lesser curvature. There are two ulcers present in type II GUs—one in the body of the stomach and the other in the duodenum. Type III GUs are prepyloric. Type IV GUs are the least common and are found high on the lesser curve near the gastroesophageal junction. Type V GUs are caused by NSAIDs and respond to discontinuation of NSAID use.52,60
In elective or semielective surgery for intractable or recurrent ulcer, the operation of choice depends on the type of GU. Type II and Type III GUs are associated with acid hypersecretion and are typically treated with vagotomy and antrectomy including the GU. In an emergency operation for perforation, the surgeon must focus on the primary goals of the operation—to eliminate the defect, ensure adequate drainage, and treat abdominal sepsis. Perforated GUs tend to occur in elderly patients with multiple medical comorbidities and carry an associated mortality rate as high as 40%.61,62 Local wedge excision with or without truncal vagotomy and drainage are recommended for perforation (Figs. 35.6 and 35.7). In the stable patient with a history of chronic peptic ulcer disease or failed prior operation, distal gastrectomy is preferred with the addition of vagotomy in type II or III GUs. When excision or resection is not possible because of patient instability or prohibitive risk, eight to ten biopsies of the ulcer should be taken to evaluate for malignancy and H. pylori infection. Follow-up endoscopy should be performed in 6 weeks to ensure healing of the ulcer and eradication of H. pylori.63 The operation performed for a given patient will depend on the patient’s history, preexisting and current clinical status, as well as the type and location of the ulcer (Algorithm 35.1).
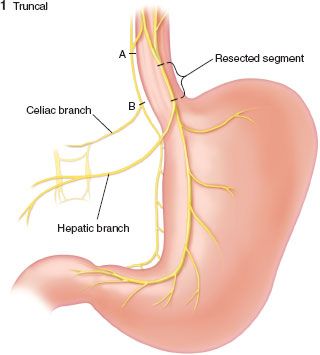
FIGURE 35.6. Truncal vagotomy.
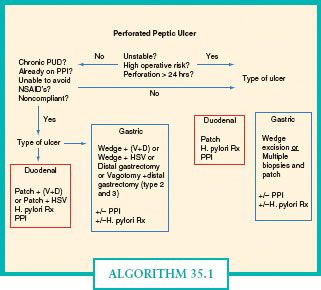
ALGORITHM 35.1. Algorithm for management of perforated peptic ulcers.(Adapted from Figure 26.44, p. 924 from Dempsey DT. Stomach. In: Brunicardi FC, ed. Schwartz’s Principles of Surgery. 9th ed. The McGraw-Hill Companies, Inc.; 2010.)
Morbidity after perforation is common, ranging from 17% to 63%.44,64 The Boey score is a useful scoring system to predict perioperative morbidity and mortality for a given patient–6567 (Table 35.4). Pneumonia is the most common complication (up to 30%) followed by wound and intraabdominal infections. Fungal infection after perforation is common and associated with high mortality (up to 21%). Perioperative shock, renal failure, cirrhosis, advanced age, immunocompromise, and delayed operative intervention are identified risk factors for major morbidity and mortality. A history of diabetes mellitus or cardiopulmonary disease is associated with mortality up to 50%. Delay of more than 24 hours dramatically increases mortality. Morbidity and mortality is significantly higher with perforated GUs than with duodenal ulcers.44,46,65,68
TABLE 35.4
BOEY SCORE
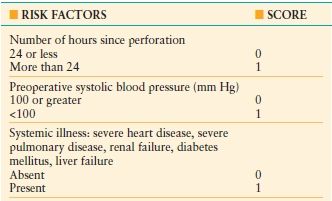
The predicted mortality is 0% with a score of 0, 10% with a score of 1, 45.5% with a score of 2, and 100% with a score of 3.(Adapted from Boey J, Choi SK, Poon A, et al. Risk stratification in perforated duodenal ulcers: A prospective validation of predictive factors.Ann Surg. 1987;205: 22.)
The minimally invasive approach to perforated gastroduodenal perforation is increasingly utilized for select patients with good results.69,70 As with many other minimally invasive operations, laparoscopic repair of perforated duodenal ulcer has been associated with decreased postoperative analgesia requirements, decreased incidence of wound infection, shorter hospital stay, and earlier return to work.71 In a recent review of 56 papers, overall conversion rate from laparoscopic to open correction of perforated peptic ulcer was 12.4% (range 0%–28.5%). The main reason for conversion was diameter of the perforation. Generally, perforations larger than 1 cm should be converted to open repair. The next two most common reasons for conversion are inability to adequately localize the ulcer and technical difficulties in placing reliable sutures due to inflammation.43 A Boey score of 2 or 3, age over 70 years, and symptoms >24 hours were associated with higher morbidity and mortality and should be considered relative contraindications to laparoscopic intervention,64,72 although several authors have demonstrated good results with laparoscopic repair in patients with prolonged peritonitis.43,73 A few papers have reported higher incidence of leakage with a laparoscopic approach, but the techniques used for repair were not necessarily comparable.43,72 Shock is a contraindication to a laparoscopic approach.
Available data on recurrence rates should be considered in the context that most data predates the routine use of PPIs and eradication of H. pylori. Ulcer recurrence is <2% with vagotomy and antrectomy, 5%–15% with vagotomy and pyloroplasty, and 5%–20% with proximal gastric vagotomy.50,52,60 Recent studies have shown patch closure of perforated duodenal ulcer followed by H. pylori eradication and a PPI to yield excellent results with low recurrence. This lends support to current practices of performing simple patch repair in the acute setting.74,75 A careful history should be obtained from patients with recurrent ulcers with attention to potential contributing risk factors such as smoking, alcohol intake, and NSAID use. In patients who underwent resective procedures without identifiable etiology for ulcer recurrence, incomplete vagotomy, retained gastric antrum, or Zollinger-Ellison’s syndrome should be considered. The diagnosis of Zollinger-Ellison’s syndrome is confirmed by fasting hypergastrinemia while off PPIs and a secretin stimulation test. An increase in serum gastrin of 200 pg/mL (or possibly 120 pg/mL) or greater suggests the presence of a gastrinoma.76
Marginal Ulcer
A perforated marginal or perianastomotic ulcer presents a unique problem in a patient whose anatomy is already surgically altered from previous gastrojejunostomy or other gastric anastomosis. With increasing number of patients undergoing bariatric surgery, short- and long-term complications are better understood.77 Elective surgery is occasionally performed for intractable ulcers, or ulcers not responding to maximal medical therapy after 3 months. Emergency surgery may be necessary in the case of perforated marginal ulcer and quite often it is the on-call general or acute care surgeon who finds himself/herself performing these challenging operations.
Roux-en-Y-gastric bypass (RYGB) remains the most commonly performed operation for obesity in the United States (Fig. 35.8). The reported incidence of marginal ulcers after RYGB varies widely, from <1% up to 16%.78,79 A large single center study reported a 1% incidence of perforated marginal ulcer with the median time of presentation 18 months after RYGB.78 A higher incidence of marginal ulcer may be associated with gastric partition without transection (commonly performed in open gastric bypass) than with transection of the stomach. Smoking, excessive alcohol intake, NSAID use, and steroids have been implicated as risk factors for the development of marginal ulcers, but results of studies have varied. The role of H. pylori is unclear although most authors recommend treatment if positive. Ulceration is more often on the jejunal side of the anastomosis, suggesting acid exposure from the stomach as an instigating factor. However, neither prophylactic acid suppression nor vagotomy has definitively been shown to reduce the rate of marginal ulcers after RYGB. Some studies suggest decreased incidence of marginal ulcer formation with retrocolic rather than antecolic gastrojejunal anastomosis.–7883
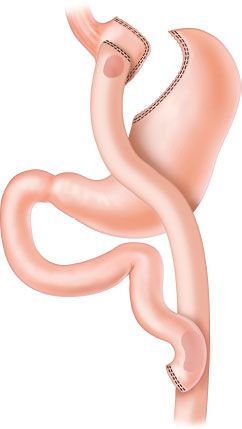
FIGURE 35.8. Roux-en-Y gastric bypass.
The goals of an emergency operation for perforated marginal ulcer are the same as in any upper GI perforation—close the defect, ensure adequate drainage, and manage sepsis. Establishment of alternate enteral access for nutritional support may be required. In the case of a prohibitively large ulcer or abscess, resection may be necessary. Creation of an anastomosis in the face of frank purulence or shock is not advisable and should be deferred until the patient has recovered physiologically and inflammation has subsided. Laparoscopic repair of perforated marginal ulcer has been described with favorable results in stable patients without abscess.
Iatrogenic injury is an increasingly common cause of gastroduodenal perforation with more liberal use of esophagoduodenoscopy for diagnostic and therapeutic purposes. ERCP (endoscopic retrograde cholangiopancreatography) carries a risk of perforation between 0.5% and 2%.84,85 Perforation has also been described as a complication following placement of inferior vena cava filters and biliary stents.86,87 Microperforation, secondary to a wire or sphinchterotomy during ERCP may be managed conservatively in many cases. However, the standard therapy for free duodenal wall rupture has traditionally been surgical repair. Advances in endoscopic techniques now allow for possible closure of a duodenal perforation endoscopically if recognized immediately and the defect is not too large.88,89 Intravenous antibiotics and close observation are mandated. If surgical repair of an iatrogenic gastric or duodenal perforation is indicated, the patient’s hemodynamic status should guide surgical management.90 Repair of the defect, peritoneal lavage, and placement of drains can be achieved laparoscopically as well as with laparotomy. The patient with frank peritonitis and in shock is better managed with laparotomy.91 A repaired gastric defect may not require extraluminal drains, but a duodenal injury should be drained widely. Proximal decompression in either case is achieved adequately with a well-positioned nasogastric tube on sump suction. We do not recommend lateral duodenostomy, but rather favor placement of closed suction drains near the site of repair to provide an outlet for potential duodenal leak in the form of a controlled fistula. Surgical enteral access for postoperative nutritional support should be obtained. In cases of gross peritonitis or septic shock antifungal coverage should be included in the perioperative antimicrobial therapy.
Crohn’s Disease of the Small Bowel
Crohn’s Disease is the most common primary surgical disease of the small intestine. It is an inflammatory process that can affect any portion of the alimentary tract from mouth to anus but most commonly affects the terminal ileum. Forty to fifty-five percent of patients with Crohn’s disease have ileocolic disease, 30% have small bowel disease, and 15%–30% have disease involving only the colon or anorectum.92,93 Grossly, the affected bowel is characterized by creeping fat and a thickened bowel wall and mesentery (Fig. 35.9A). Cobblestoning of the mucosa is characteristic but is not pathognomonic of Crohn’s disease (Fig. 35.9B). In contradistinction to ulcerative colitis, Crohn’s disease is transmural and discontinuous (segmental) with rectal sparing. Crohn’s disease is also associated with a greater degree of extraintestinal manifestations as compared to ulcerative colitis with up to 25% of patients having at least one extraintestinal manifestation of disease (Table 35.5). The incidence of Crohn’s disease is highest in North America and Northern Europe. African Americans and Caucasian Americans are affected equally though certain ethnic groups, particularly Jews, have a greater incidence. Males and females appear to be affected equally. Risk is increased in smokers and in urban populations. Peak incidence is in the third decade of life with a smaller peak in the sixth decade of life. Genetic, immunologic, and infectious etiologies have been proposed and there appears to be a familial association but the cause of Crohn’s disease remains elusive.92,93
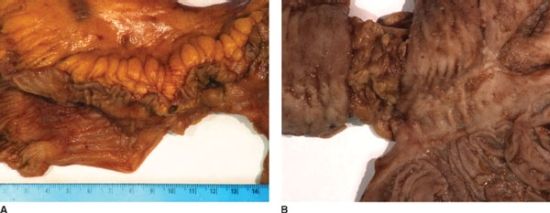
FIGURE 35.9. A,B: Crohn’s disease of the small intestine.
TABLE 35.5
EXTRAINTESTINAL MANIFESTATIONS OF CROHN’S DISEASE
There is no cure for Crohn’s disease. The goals of treatment are amelioration of symptoms and improvement in quality of life.94 Symptoms and complications of Crohn’s disease are treated medically with operation reserved for refractory cases or serious complications. Most patients (50%–70%) will require surgery at some point in their lives. Patients with Crohn’s disease are optimally managed using a multidisciplinary team approach involving gastroenterologists and surgeons who specialize in inflammatory bowel disease, as well as radiologists, nutritionists, and stoma and wound care nursing specialists. Venous thromboembolism prophylaxis should be routine in hospitalized patients as inflammatory bowel disease is a risk factor for development of deep venous thrombosis.95
The three clinical patterns of Crohn’s disease are stricturing , perforating , and inflammatory. Fibrosis and stenosis of discontinuous segments of bowel are the hallmark of the stricturing pattern. Repeated episodes eventually lead to strictures that no longer respond to medical therapies and patients ultimately require surgery. The perforating pattern is characterized by abscess or fistula formation. A combination of medical and surgical therapy is generally utilized. The inflammatory pattern is characterized by a more diffuse distribution along the alimentary tract and is treated medically with operation reserved for refractory cases.
Treatment of Inflammatory Crohn’s Disease
The first line of therapy for mild symptoms is 5-ASA (Pentasa, Asacol). For a severe acute episode, or Crohn’s flare, corticosteroids are the gold standard treatment to induce remission. Budesonide, a synthetic glucocorticoid, is often used to induce remission because of its reduced systemic absorption and more tolerable side effects when compared with prednisone. Antibiotics, typically metronidazole, may be used as adjunctive therapy. Infliximab, a monoclonal antibody against tumor necrosis factor-α (TNF-α), is used for the treatment of severe active Crohn’s disease resistant to conventional therapy. Infliximab is effective as an induction agent with up to 70% patients responding within 1–2 weeks from a single infusion. Unfortunately infliximab use is associated with an increased risk of opportunistic infections such as tuberculosis, aspergillosis, and listeriosis. It is also generally necessary to administer immunosuppressants concomitantly with infliximab to prevent transfusion reactions due to the formation of antibodies to infliximab. Following successful induction therapy the goal of treatment becomes maintenance of remission. Steroids are ineffective as maintenance therapy. The two most common agents used for maintenance of remission are azathioprine and 6-mercaptopurine. Treatment with these agents is usually initiated during induction therapy because of their delayed onset of action. Patients must be monitored closely for complications such as bone marrow suppression, hepatotoxicity, and pancreatitis.96
Treatment of Perforating Crohn’s Disease
The primary goal of treatment for abscess or fistula is control of sepsis. Image-guided drainage of an abdominal abscess with parenteral antibiotics is the mainstay of therapy (Fig. 35.10). Nutritional and metabolic support is essential. The three most important types of fistulae are enterocutaneous, enteroenteral, and enterovesical.
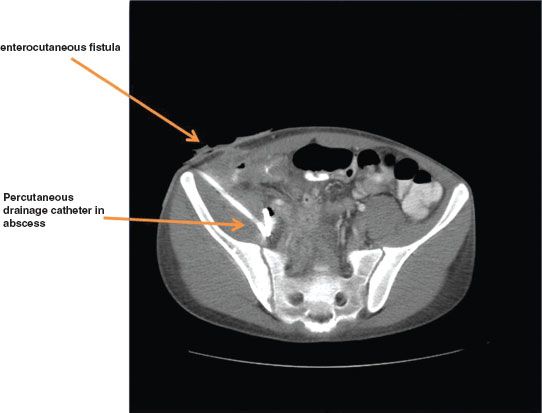
FIGURE 35.10. Abdominal abscess from Crohn’s disease.
Enterocutaneous fistulae (ECF) occur in 4% of patients with Crohn’s disease, either as a direct extension from the diseased bowel to the skin or from ongoing drainage via a percutaneous drainage site. They may also occur as a postoperative complication in patients who have recently undergone operation. Medical management should be initiated with antibiotics, nutritional support, and infliximab.97 Infliximab has been associated with >60% response rate for closure of Crohn’s related fistulae.98,99 However, more recent studies have not demonstrated a positive impact of infliximab on operative rates for bowel resection or fistula repair.100
Enteroenteral fistulae in themselves also do not necessarily mandate operation. Surgery is indicated, however, if the fistulae are associated with an acute inflammatory exacerbation refractory to medical management, abdominal mass, hemorrhage, or uncorrectable malnutrition. Enterovesical fistula is characterized by chronic or recurrent urinary tract infections (88%), pneumaturia (88%), fecaluria (38%), and hematuria (63%).92,93 CT of the abdomen and pelvis typically shows air in the bladder (Fig. 35.11). Diagnosis is confirmed by cystoscopy. Operative repair of enterovesical fistulae should be performed to prevent injury to the kidneys from recurrent urinary tract infections.
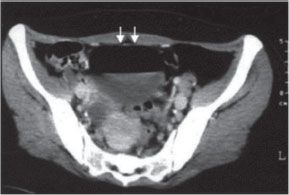
FIGURE 35.11. CT demonstrating air in the bladder.(From Chebli JM, Gaburri PD, Pinto JR. Enterovesical fistula in Crohn’s disease. Lancet.2004; 364–368.)
Surgical therapy in Crohn’s disease is aimed at treatment of complications, relief of symptoms, and optimization of quality of life. Urgent operation may be necessary for free perforation (rare), acute hemorrhage, or uncontrolled sepsis from abscesses not amenable to percutaneous drainage. Less urgent operations are indicated for enterovesical fistula or acute Crohn’s flare, obstruction, or fistula refractory to medical management.101
The preoperative workup for an elective operation for Crohn’s disease should include a contrast radiographic study (small bowel follow through) to determine the length of bowel, location of disease, identify fistulae, and evaluate strictures. CT identifies masses, fluid collections, and bowel wall thickening. Colonoscopy and video capsule endoscopy may help establish the presence and extent of disease. The patient’s nutritional status should ideally be optimized prior to surgery. Except in the case of high-grade obstruction, a standard mechanical and antimicrobial bowel prep should be performed. Consultation with the stoma care nursing team for preoperative counseling and marking for potential stoma sites is helpful to both the surgeon and to the patient. It is imperative that a history of any recent corticosteroid use be elicited to determine whether perioperative steroid replacement will be necessary. Other immunosuppressive medications may be safely discontinued preoperatively. Corticosteroid use in the perioperative period is associated with increased postoperative complications so a rapid taper postoperatively should be the goal when possible.102
In emergency operations for abdominal catastrophe with significant contamination or shock, a damage control approach may be prudent. The emergency “damage control” operation would consist of resection of the offending segment of bowel to grossly negative margins and peritoneal lavage with evacuation of any GI spillage or purulence. The surgeon must then determine whether to complete the operation with an assessment of the length of disease-free bowel, creation of an end ostomy (if applicable), and primary closure of the fascia, or to defer the second stage until hemodynamic stability has been achieved and gross peritonitis resolved. Primary anastomosis in the face of shock or gross contamination is associated with a high incidence of anastomotic leak and should be avoided if possible. Temporary closure may be achieved with either a running nonabsorbable suture closure of the skin or placement of a negative pressure vacuum dressing designated for the abdomen. The second, reconstructive operation 24–48 hours later consists of further lavage of the abdominal cavity, reestablishment of GI continuity with creation of an end ostomy or with primary anastomosis with or without protective loop ileostomy, and definitive closure of the abdomen. In the difficult case of duodenal disease, options include resection or bypass with gastrojejunostomy if resection is not feasible.
Operative management of fistulae involves identification of the inciting segment of bowel and the target structure. The abnormal connection is divided and the instigating segment of bowel resected. Resection of the target organ should only be performed if it is not amenable to simple repair. In cases of enterovesical fistula, division of the fistula is followed by primary repair of the bladder. Preoperative placement of ureteral stents may prove useful for identification of the ureters while navigating through the complex inflammatory mass. Foley catheter drainage of the bladder postoperatively is generally sufficient although an additional perivesical drain may be utilized in cases of severe inflammation or tenuous repair.
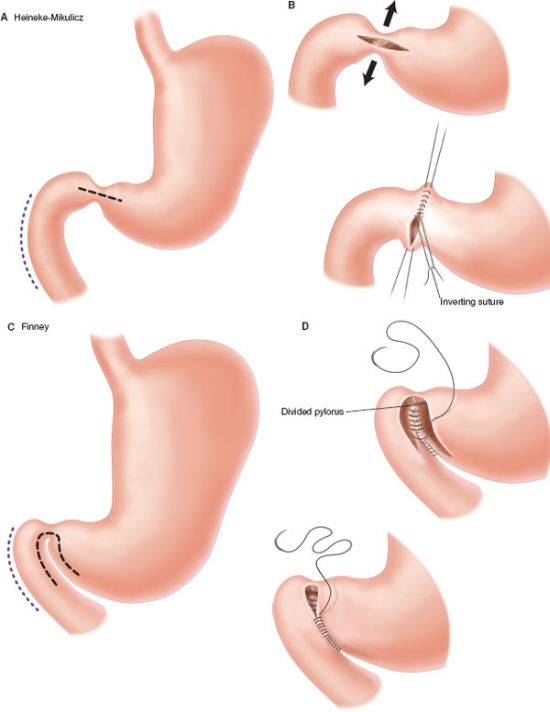
When dealing with obstruction due to stricture, viable alternatives to resection include balloon dilatation or stricturoplasty, particularly for short segment strictures (<4 cm). These alternatives may be more appropriate than resection when there is concern for short gut.–103105 A Heinecke-Mikulicz-type stricturoplasty (longitudinal enterotomy with transverse repair) is typically used for strictures <10–12 cm in length. For longer strictures up to 25 cm in length a Finney-type strictureplasty is preferred. In the case of duodenal stricture, options include endoscopic balloon dilatation, stricturoplasty, bypass with gastrojejunostomy, or resection.106 Leaving the diseased segment of bowel does however expose the patient to potential future complications such as -hemorrhage or -malignant degeneration.107
Recurrence rates after bowel resection and anastomosis have not been shown to have increased with microscopic involvement of resection margins.108 The type of anastomosis, stapled or hand sewn, also does not seem to have impact on anastomotic recurrence rate, although patients with a side-to-side anastomosis may have a lower incidence of reoperation for anastomotic recurrence than those with end-to-end anastomosis.–109111 We prefer a two-layer side-to-side hand sewn anastomosis when bowel is inflamed or edematous.
Selected patients are candidates for laparoscopic surgery for Crohn’s disease.112,113 Length of stay, morbidity, and overall cost in the first 3 postoperative months have been shown to be lower in patients who underwent a minimally invasive surgical approach. A laparoscopic approach also allows faster initiation of steroid therapy when needed and potentially reduces adhesion formation for future operations. Diagnostic laparoscopy is an excellent tool in the workup of patients in whom Crohn’s disease is suspected but cannot be confirmed otherwise.114,115 Contraindications to laparoscopic surgery in Crohn’s disease include a known “frozen” abdomen and emergency surgery in an acutely ill patient with peritonitis, bleeding, or complete bowel obstruction. General medical contraindications to laparoscopy such as severe COPD, severe heart failure, and coagulopathy must be considered in planning the operative approach. The presence of dense adhesions is the most common reason for conversion from laparoscopic to open operation. Extensive inflammation, large inflammatory mass, and the inability to safely dissect a fistula are other cited reasons for conversion.116 The number of prior abdominal operations has not been shown to be a predictor of conversion. When laparoscopy is utilized, resection may be performed externally or within the abdomen using the Ligasure device or ultrasonic scissors. A 5 cm periumbilical or suprapubic incision is used to deliver the specimen. Anastomosis is typically performed externally.
When Crohn’s disease is first diagnosed during laparoscopy or laparotomy for suspected appendicitis or bowel obstruction, it may be advisable to treat the Crohn’s disease medically rather than with resection. Intraoperative decision-making should take into account the patient’s age, clinical status, and nature of the disease (inflammation, stricture). If the surgeon elects to treat surgically he/she should adhere to the basic principles of surgical management in Crohn’s disease.
Whether elective or emergent, open or laparoscopic, certain basic principles apply to the surgical management of Crohn’s disease. (1) Only the segment causing complications should be resected and only to grossly (not microscopically) negative margins. (2) Stricturoplasty may be the better choice when dealing with obstruction. (3) Bypass should be considered a last resort because of the risk of bleeding, perforation, and future malignancy in the bypassed portion, though it may be the only viable option with duodenal disease. (4) In high-risk patients (high dose steroids, profound malnutrition) end ostomy or protective loop ileostomy for a distal anastomosis is advisable. (5) Appendectomy should be performed when possible and safe to avoid future diagnostic dilemmas.
Crohn’s Disease During Pregnancy
Active Crohn’s disease during pregnancy is associated with increased risk of fetal loss, preterm delivery, low birth weight, and developmental defects.93 Diagnosis and workup become challenging and may involve endoscopy, ultrasound, and magnetic resonance imaging in lieu of traditional studies associated with radiation exposure. Continued severe disease poses greater risk to both mother and fetus than does surgery. A frank, detailed discussion of risks, benefits, and alternatives is indicated preoperatively as is consultation with Maternal-Fetal Medicine. If surgical resection is required, ileostomy may be preferred to anastomosis to reduce the risk of postoperative complications.
Enterocutaneous Fistula
The management of ECF continues to challenge even seasoned surgeons. Fistulae are often complicated by sepsis, fluid and electrolyte abnormalities, malnutrition, and complex wound management issues. Mortality ranges from 10% to 20% depending upon coexisting conditions. ECF develop as a result of technical factors from a previous operation, factors related to healing and tissue integrity inherent to the patient, and factors related to specific disease processes.117
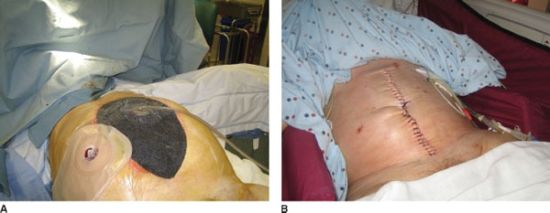
The vast majority of ECF occur as a complication of an abdominal operation. ECF may occur as a result of anastomotic leak, unrecognized enterotomy, incorporation of bowel in the fascial closure, and ischemia. Emergency abdominal procedures and contaminated operations are additional risk factors. Factors related to the patient’s ability to heal include severe malnutrition, steroid use, radiation, and chemotherapy. A serum albumin <3.0 g/dL is a well-established risk factor for the development of ECF.117 Enterocutaneous or enteroatmospheric fistulae are common among patients with an open abdomen related to multiple trauma or abdominal compartment syndrome. We recommend managing the open abdomen with vacuum negative pressure dressings specialized for the abdomen with a nonadherent plastic sheet placed over exposed bowel before the vacuum sponge is applied (Fig. 35.12A). While some studies have suggested increased risk of fistula formation with the use of negative pressure dressings, other studies have not been able to demonstrate this. When early fascial closure cannot be achieved after open abdomen we close the abdomen either with a skin-only closure with a heavy, running nonabsorbable suture (Fig. 35.12B), or with an absorbable mesh followed by skin grafting when a satisfactory bed of granulation tissue is present. The resulting ventral hernia can be repaired electively in 6–12 months.
FIGURE 35.12. A: Abdominal VAC dressing. B: Running suture for skin-only closure of the abdomen.
An ECF classically presents as a wound infection following abdominal surgery. Drainage from the wound with the appearance and odor of enteric contents is diagnostic. The underlying fascial integrity should be evaluated as ongoing leakage of enteric contents may lead to fascial dehiscence and evisceration. Conversely, ECF should be in the differential diagnosis of a patient presenting with fascial dehiscence or evisceration.
An upper GI contrast study or CT with oral contrast may demonstrate an ECF. A chronic fistula tract can be injected with oral contrast to locate the site of communication with the intestinal tract (fistulogram). In general, the more proximal the fistula, the more pronounced the electrolyte abnormalities, fluid losses, and malnutrition. Distal fistulae may essentially function as an ileostomy or colostomy and may not be associated with any fluid, electrolyte, or nutritional abnormalities. More proximal fistulae may produce management problems with volume loss and electrolyte abnormalities.
The initial primary goal of treatment of an ECF is source control and control of sepsis. Correction of fluid and electrolyte imbalances, nutritional support, and skin care are essential adjuncts. Uncontrolled sepsis is the major cause of mortality in patients with ECF. Image-guided drainage of an abdominal abscess usually precludes the need for early operation which is associated with high morbidity and mortality. Early surgical intervention may be necessary in septic patients with uncontrolled leakage of enteric contents into the abdomen or abscesses not amenable to percutaneous drainage. If the patient is not septic despite having an intra-abdominal fluid collection, an attempt at nonoperative management can be made. A trial of medical management may eliminate the need for surgery in some patients altogether; in those patients who still require operation, the additional time allows the fistula tract to mature and inflammation to subside, creating a less hostile abdomen at the time of operation. Consultations should be held with the wound and ostomy care nursing team to assist with the complex wound management that most of these patients will have. Many surgeons champion the use of specially-fashioned abdominal negative pressure vacuum dressings in the management of enteroatmospheric fistulae to promote healing. Creative skin grafting around the fistula may simplify wound care and provide patient comfort while the fistula matures or closes.
Adequate nutritional support is essential for spontaneous closure of an ECF. Patients with ECF are often profoundly catabolic and depleted in lean body mass, albumin, and prealbumin. Protein and caloric requirements may be double their baseline requirements. Enteral feeding, either by mouth or by a small-caliber nasogastric or nasoenteral tube, should be the first line of nutritional support in the absence of a contraindication.118 Providing as little as 10%–20% of nutritional needs enterally is associated with maintenance of gut mucosal integrity and immunological function. Parenteral nutrition is associated with more infectious complications but may be the only means of providing nutrition to a patient with a high output fistula, obstruction, or short gut. Parenteral nutrition may sometimes be used to supplement enteral feeding for profoundly malnourished patients or patients who cannot tolerate full enteral feeds. If fistula output increases in response to enteral feeding it may be beneficial to provide nutritional support parenterally to try to allow for spontaneous closure of the fistula.119
The overall spontaneous closure rate of an ECF is 10%–40% with conservative management. The use of octreotide, an analogue of somatostatin, as adjunctive therapy may decrease fistula output, making a high-output fistula more manageable, but has not been shown to definitively impact spontaneous closure.120,121 An ECF that persists 6–8 weeks after resolution of sepsis and correction of nutritional deficits is unlikely to close spontaneously, especially if the output is high (>500 mL/d). Up to 90% of ECF that are going to close spontaneously, close within 5 weeks after sepsis is controlled. Factors associated with failure of an ECF to close spontaneously include high output fistula, ongoing malnutrition, distal bowel obstruction, short fistula tract, abscess, and foreign body (including mesh). Malignancy, radiation, and steroids are also risk factors for nonclosure of ECF.
In planning an elective operation to repair an ECF preoperative imaging such as fistulogram and CT is obtained to identify the location of the fistula, evaluate for distal obstruction or potential foreign body, and to evaluate the abdominal wall in cases of loss of domain (large ventral defect) for possible abdominal wall reconstruction. A small but important subset of patients may benefit from earlier operation rather than delaying time to operation which exacerbates the catabolic state and leads to further debilitation and morbidity despite control of sepsis and aggressive nutritional support.
A mechanical and oral antimicrobial bowel prep should be performed when possible preoperatively. At laparotomy the bowel should be fully mobilized to allow proper identification of involved segments, ensure patency of the length of the bowel, and to perform segmental resection of the fistulae with tension-free anastomosis; fastidious technique is essential. Closure of the abdominal wall and distancing the repair from the incision should be routine. Surgical enteral access for postoperative nutritional support should be considered.
Infectious Enteritis
About 200–500 cases of typhoid fever and typhoid enteritis are reported annually in the United States. About 80% of cases occur in people with a history of recent travel to endemic areas. Typhoid enteritis is an acute systemic infection caused by ingestion of Salmonella typhi through contaminated food or water. Fever, abdominal pain, rash, and diarrhea (sometimes bloody) along with lymphadenopathy and hepatosplenomegaly are characteristic. Diagnosis is by positive blood or stool culture.92 Because of high resistance to the original first-line therapy (ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole) the recommended treatment for adults with typhoid fever and uncomplicated typhoid enteritis is now with fluoroquinolones or third generation cephalosporins. In areas of high fluoroquinolone resistance, azithromycin 1 g orally for 5 days is recommended.122,123 Hemorrhage or perforation through an ulcerated Peyer’s patch may necessitate operation. Perforation is usually solitary in the antimesenteric terminal ileum and managed with either simple closure or segmental resection. In the unusual event of multiple perforations resection with primary anastomosis or creation of end ileostomy may be required.124
Patients with AIDS or on immunosuppressive agents after organ transplantation are susceptible to GI infection by a host of pathogens. Protozoa (Cryptosporidium, Isospora, Microsporidium) are the most frequent class of pathogens causing diarrhea in patients with AIDS. The small bowel is the most common site of infection. Cryptosporidial transmission is by fecal–oral route. Public swimming pools have been implicated as a significant source of infection. Cryptosporidial enteritis generally resolves spontaneously in nonimmunocompromised hosts. In patients with AIDS, it is characterized by severe watery diarrhea (>10 L/d), cramps, nausea, and weight loss. Biliary involvement is common. Diagnosis is by isolation of oocysts in stool, bile, or tissue. Treatment is primarily HAART (highly active antiretroviral therapy) as raising the CD4 count to >100 cells/mm3 usually leads to resolution of enteritis. Supportive care with oral or parenteral hydration and antidiarrheal agents may be life-saving and continues until there is resolution of diarrhea. A trial of nitazoxanide in conjunction with HAART may be considered.125
Enteric bacterial infection with Salmonella , Shigella , and Campylobacter are more common and more virulent in immunocompromised patients than healthy individuals. Affected patients present with abdominal pain, high fever, tenesmus, and hematochezia. Stool cultures will usually diagnose Salmonella and Shigella but may be falsely negative with Campylobacter infection. Treatment is parenteral antibiotics.
Mycobacterial infection in the immunocompromised patient may be due to either M. tuberculosis or the atypical Mycobacterium avium complex (MAC). Infection is characterized by fever, night sweats, abdominal pain, diarrhea, and weight loss. Routes of entry to the intestinal tract include the oral route (infected sputum or contaminated food or milk), hematogenous spread from pulmonary or military tuberculosis, or by contiguous spread. Intestinal tuberculosis involves the ileocecal region in 85%–90% of cases. Up to half of affected patients will have a palpable right lower quadrant mass and ascites is often present. Gross findings include ulcerations or tubercles covering the bowel surface and mesenteric lymphadenopathy. Strictures and fistula formation may be present. The diagnosis of mycobacterial infection is made by identification of the organism in tissue, either by acid-fast stain, by culture of the excised tissue, or by PCR. The treatment is similar for immunocompromised and immunocompetent hosts with multi-drug antimicrobial therapy. Most fistulae will respond to medical management.126,127 Obstruction is the most common indication for surgery. Stricturoplasty may be an option for obstruction related to stricture as may be colonoscopic balloon dilatation.
The MAC organisms (M. avium and M. intracellulare) are ubiquitous in the environment and contracted by inhalation or ingestion.128 Risk of infection is increased with CD4 count <50 cells/mm3. Diagnosis is made with positive blood culture. MAC infection is characterized by massive thickening of the proximal small bowel. Perforation or other surgical emergencies are rare. Multi-drug therapy with clarithromycin or azithromycin and ethambutol is standard therapy. MAC infection in patients infected with HIV increases the risk of death independent of CD4 count.125
CMV is the most common viral cause of diarrhea in immunocompromised patients. Patients present with abdominal pain, fever, and weight loss. Diagnosis is made by demonstration of viral inclusions histologically or by serology. The treatment is usually ganciclovir or foscarnet.129 Perforation of the distal small bowel or colon or massive hemorrhage may rarely necessitate urgent operation.
GI histoplasmosis occurs in association with disseminated histoplasmosis, a disease primarily of immunocompromised patients. Diagnosis is made by fungal smear and culture of infected tissue or blood. Treatment is most often -amphotericin B.130
Small Bowel Diverticular Disease
Small bowel diverticulosis is a relatively rare entity and <4% of all small bowel diverticula will become symptomatic in a person’s lifetime. Because the clinical presentation may be dramatic with bleeding, perforation, or acute inflammation, the acute care surgeon should be prepared to manage complications that may arise from these diverticula. There are two types of small bowel diverticula. False diverticula are acquired defects with herniation of mucosa and submucosa along the mesenteric side of the bowel where vessels penetrate the bowel wall. True diverticula are congenital anomalies arising in the antimesenteric side of the distal ileum (Meckel’s diverticula).131,132
Duodenal Diverticula
A duodenal diverticulum typically presents during or after the fifth decade of life as a solitary outpouching along the mesenteric side of the duodenal wall. Duodenal diverticula are the most common small bowel diverticula, 45%–79% of all small bowel diverticula. Less than 1% require treatment.133,134 Diagnosis is by EGD, ERCP, contrast radiography, or CT with oral contrast. They are reported to be found in 10%–20% of patients undergoing ERCP.135
The majority of duodenal diverticula (61%–75%) are located in the periampullary area, or within 2 cm of the ampulla of Vater. The inherent defects in the muscularis propria caused by the penetration of the pancreatic and biliary ducts predispose to the development of these false diverticula. Patients typically present with symptoms related to biliary or pancreatic ductal obstruction including cholangitis, jaundice, or pancreatitis. Patients with duodenal diverticula have a higher incidence of choledocholithiasis and pigment and bilirubinate stones. Duodenal diverticula should be considered in the differential diagnosis for patients with pancreatitis of unclear etiology.–135138
Asymptomatic or incidentally found duodenal diverticula should not be resected. The patient should be informed of the incidental finding and potential sequelae. Choledocholithiasis in association with a duodenal diverticulum should be managed conventionally as in patients without duodenal diverticula. Inflammation, perforation into the retroperitoneum or peritoneal cavity (Fig. 35.13), and hemorrhage occur less frequently but may necessitate intervention acutely.
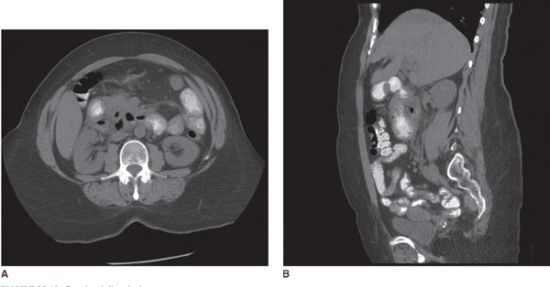
FIGURE 35.13. Duodenal diverticulum.
Surgery for duodenal diverticula is associated with significant morbidity and mortality related to the patient’s age, comorbidities, and technical challenges of the operation itself. The surgical approach begins with a complete Kocher maneuver and identification of the pancreaticobiliary structures and their relationship to the diverticulum. Cannulation of the ampulla of Vater with a metal probe or red rubber catheter helps to maintain orientation and avoid injury. Diverticulectomy with primary closure is the procedure of choice when the ductal structures are not involved and when there is no retroperitoneal contamination. For duodenal diverticula distal to the ampulla, options include diverticulectomy with jejunal serosal patch repair or segmental resection with end-to-end anastomosis. Roux-en-Y biliary bypass may be necessary when the ampulla is involved. In cases of retroperitoneal contamination secondary to a perforated duodenal diverticulum, options include antrectomy with Billroth II reconstruction or duodenal exclusion with placement of gastrostomy and jejunostomy tubes. To avoid biliary stasis and future diagnostic dilemmas, cholecystectomy should be performed in patients requiring surgery for duodenal diverticula.
Intraluminal or “windsock” diverticula are large, congenital, saccular diverticula in the second portion of the duodenum. They are associated with a myriad of other congenital anomalies including annular pancreas, choledochocele, malrotation, and superior mesenteric artery (SMA) compression syndrome. Cardiac and urogenital anomalies are also common. Obstruction is the most frequently encountered complication associated with an intraluminal diverticulum. The preferred treatment for symptomatic intraluminal diverticula is duodenotomy and excision.139 Endoscopic excision has also been performed successfully.140,141
Jejunoileal Diverticula
Jejunoileal diverticula occur in 1%–3% of the general population and comprise only 25% of all small bowel diverticula, but are the most likely to be symptomatic.142 They are often multiple, with 80% occurring in the jejunum, 15% in the ileum, and 5% along the full length. Patients typically present at age 50 or greater. Patients may present acutely with perforation, obstruction, hemorrhage, or inflammation causing peritonitis. More commonly, patients present with symptoms related to the intestinal dysmotility that is the underlying pathophysiology for the development of these acquired diverticula. Signs and symptoms related to stasis and malabsorption may include abdominal pain, steatorrhea, megaloblastic anemia, and neuropathy. Small bowel follow through establishes the diagnosis. CT with oral contrast has the added benefit of assessment of inflammation or subtle signs of perforation as well as the anatomic relationship of diverticula to surrounding structures. Diagnosis is often made at laparoscopy or laparotomy. In patients with symptoms related to malabsorption due to bacterial overgrowth, medical therapy with metronidazole and amoxicillin clavulanate, and nutritional support with vitamin supplementation is successful in 75% of cases. Surgical intervention may be indicated in patients in whom medical therapy fails. Segmental resection of the involved diverticula is the treatment of choice for symptomatic diverticula. Diverticula encountered incidentally during laparoscopy or laparotomy should not be excised.142–144
Meckel’s Diverticulum
Meckel’s diverticula, true diverticula on the antimesenteric side of the bowel, are the most common congenital anomaly of the small bowel and account for 25% of all small bowel diverticula (Fig. 35.14). Failure of the vitelline (omphalomesenteric) duct to obliterate may manifest as an ileoumbilical fistula, vitelline duct cyst, but most commonly as a Meckel’s diverticulum. Its blood supply is derived from a persistent right vitelline artery from the SMA. The defining features of a Meckel’s diverticulum are described by the “rule of two”: 2% of the population, 2 inches long, within 2 feet of the ileocecal valve, and two types of heterotopic mucosa. It typically presents within the first 2 years of life but occasionally presents in adult patients. Half of Meckel’s diverticula contain heterotopic mucosa with 75% being gastric mucosa and 15% pancreatic. It is the most common cause of lower GI bleeding in pediatric patients. Acid secretion from heterotopic mucosa within the diverticulum leads to ulcer formation on the opposite side of the bowel wall which may bleed. A “Meckel’s scan,” used to identify uptake of radioisotope by gastric mucosa, may be helpful in the workup of GI bleeding but plays no role in the workup of an acute abdomen. The most common presentation of a symptomatic Meckel’s diverticulum in adults is bowel obstruction which may be secondary to intussusception, volvulus, or incarceration within an inguinal hernia (Littre’s hernia).145,146 Meckel’s diverticulitis occurs in 25% of symptomatic patients and is often found at laparoscopy for what is initially thought to be appendicitis. In cases of obstruction or localized inflammation, stapled diverticulectomy or wedge excision with primary closure is adequate. With significant inflammation or with a wide-based diverticulum, segmental bowel resection is preferred. With hemorrhage, segmental resection is necessary to include the bleeding ulcer on the opposing bowel wall. Appendectomy should be performed to avoid future diagnostic dilemmas.
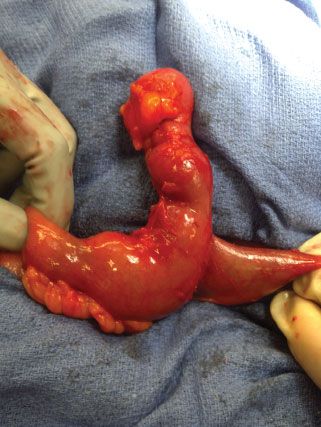
FIGURE 35.14. Meckel’s diverticulum.
Incidentally found Meckel’s diverticula in the pediatric population should be removed. Removal of an incidentally discovered Meckel’s diverticulum in adults is controversial. Relative indications for removal include evidence of heterotopic tissue, signs of prior diverticulitis, or the presence of a mesodiverticular band. The initial indication for operation and implications for future planning should also be considered. Presently, most surgeons do not favor removing a Meckel’s diverticulum found incidentally in an adult patient.143,147
INFLAMMATORY AND INFECTIOUS DISEASES OF THE COLON
An understanding of the management of inflammatory and infectious diseases of the colon is essential for the acute care surgeon. Multiple pathologies of the colon will prompt surgical consultation and consideration of operative management; these include colonic ischemia, bleeding, obstruction, diverticulitis, infectious colitides, and acute, severe manifestations of inflammatory bowel disease. This section will focus on inflammatory and infectious pathophysiology of the colon and will review current strategies of diagnosis and management. Under conditions of health, the complex microbiome of the colonic wall and lumen exists in symbiotic harmony. However, in the setting of the diseased colon, the colonic microbiome can result in life-threatening infection that may require source control, including surgical management.
Anatomy/Physiology
The colon extends from the end of the ileum to just above the peritoneal reflection at the junction of the sigmoid colon and rectum. Embryologically, the colon is derived from two origins.149 The cecum, ascending, and proximal transverse colon are derived from the midgut and receive blood supply from the SMA, while the distal transverse colon and beyond are derived from the hindgut and receive blood supply from the inferior mesenteric artery (IMA) (Fig. 35.15). In general the venous and lymphatic drainage parallel the arteries. Grossly, the colon is 115–150 cm in length and ranges in diameter from 7.5 cm at the cecum to 2.5 cm at the rectosigmoid junction.149 The colon rotates during development and attaches laterally on the right as the ascending colon and on the left as the descending colon. The mesentery at these locations most often fuses to the posterior peritoneum. The transverse and sigmoid colons have long, mobile peritoneum and allows significant variability in the location of these structures.
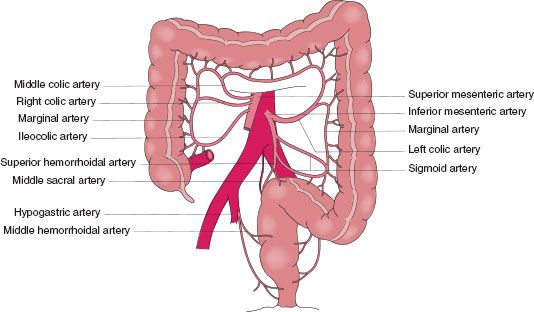
FIGURE 35.15. Arterial anatomy of the colon. The right colon and proximal transverse colon, which are embryologically derived from the midgut, have blood supply from the SMA, while the distal transverse, descending, and sigmoid colon, which are derived from the hindgut, have blood supply from the IMA. Rectal blood supply is from the IMA and the internal iliac arteries.(Reprinted from Greenfield LJ, Mulholland MW. Greenfield’s Surgery: Scientific Principles and Practice. 4th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2006, with permission. Figure 65.3. Arterial blood supply of the colon.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree