Infectious disease specialists are often consulted for infections in the critically ill given the degree of diagnostic and therapeutic complexity. That being said, the “first-responder” provider needs to familiarize him-/herself with fundamental management principles. This chapter emphasizes the fundamentals of clinical presentation, microbiology, and diagnostic and therapeutic approaches for infections in the critically ill.
Chapter 29 provides a review on the sepsis syndrome and has not been separately discussed here. Infection prophylaxis is beyond the scope of this chapter and likewise has not been included. Unless otherwise indicated, the potential antibiotic regimens mentioned represent initial empiric therapy. Subsequent therapy should be tailored to culture and susceptibility data as they become available.
I. THORACIC INFECTIONS: This section focuses on infections of the lungs, pleura, and thoracic cavity that may either be the presenting diagnosis or discovered during the ICU stay.
A. Community-Acquired Pneumonia (CAP) is an infection of the lower respiratory tract that is acquired in the community. While most cases can be managed in the outpatient setting with oral antibiotics, a subset of severe cases requires critical care and often even mechanical ventilation and is associated with significant morbidity and mortality. Advanced age, multiple comorbidities, and immune suppression are common host factors, whereas high virulence and large organism load are agent-related factors associated with severe CAP. While fever, chills, cough, expectoration, and chest pain are common, symptoms of air hunger, confusion, and signs of tachypnea, accessory muscle use, hypoxemia despite oxygen supplementation, hypotension, and evidence of high leukocytosis, bandemia, and lactic acidosis should arouse suspicion for severe CAP and lower the threshold for ICU admission.
1. Microbiology. The distribution of organisms varies slightly between CAP and severe CAP. The most frequent pathogen in both categories is Streptococcus pneumoniae. The other common causes of CAP that often require ICU care are nontypeable Hemophilus influenza, Pseudomonas aeruginosa, Staphylococcus aureus (including community-acquired methicillin-resistant strains), Legionella pneumophila, Chlamydia pneumoniae, and respiratory viruses, namely, influenza. Moraxella catarrhalis and P. aeruginosa usually cause CAP in patients with bronchiectasis or chronic bronchitis. Certain fungi like Histoplasma capsulatum, Coccidioides immitis, and Mycobacteria (M. tuberculosis) may cause CAP in regions where they are endemic, and some like Pneumocystis jirovecii cause CAP in specific immune-suppressed populations (e.g., AIDS).
2. Diagnosis. Findings on chest radiography (CXR) may be variable depending on the pathogen and the underlying condition of the host. Depending on the etiological agent, infiltrates may be interstitial or parenchymal, which may be unilobar or multilobar. Infiltrates may not be apparent on initial CXR in the setting of hypovolemia and “blossom” following rehydration on subsequent films. Despite all efforts, the causative microorganism will not be identified in close to 40% cases of severe CAP. Yet, a specific microbial etiology is desirable and must be searched for through sputum sampling or bronchoalveolar lavage (BAL), serological testing (e.g., Mycoplasma pneumoniae, C. immitis), sputum sampling for immunoassays or PCR (respiratory viruses), and urine for specific antigens (S. pneumoniae, L. pneumophila Serotype I and Histoplasma). Gram stain of sputum or BAL fluid may provide important clues regarding the potential causative organism early on based on abundance, morphology, and presence of neutrophils, which findings can be subsequently confirmed on culture. Blood cultures may also be positive in severe CAP with certain bacterial etiologies. If a significant pleural effusion accompanies pneumonia, the pleural fluid should be analyzed with gram stain and culture, pH, lactate dehydrogenase, glucose, and protein concentration. These tests allow ruling out empyema, which if present, warrants drainage or decortication.
3. Triage. Several well-validated severity scores have been developed to triage patients with CAP such as the Pneumonia Severity Index, which is complex and uses up to 20 variables, or the CURB-65, which is a simpler score that is easier to apply in practice (see Fig. 28.1) (American Thoracic Society; Infectious Diseases Society of America, 2005). While a CURB-65 score of 4 or 5 should prompt ICU admission in most cases, scores such as these should only supplement clinical gestalt.
4. Treatment. Initial management of CAP is based on established guidelines coupled with acknowledgement of host and epidemiological factors and Gram-stain results, when available. Outcome is improved with early administration of antibiotics, especially in severe CAP. Clinical presentation does not reliably predict the pathogens involved. The potential for antibiotic resistance should influence the choice of antibiotics (e.g., S. pneumoniae may be penicillin resistant). Early empiric antibiotic regimens should include coverage of typical as well as atypical microorganisms. Patient-specific risk factors for pseudomonas should be considered, which include structural lung diseases, frequent severe COPD exacerbations, recent antibiotic use, and chronic alcoholism. Therapy should be subsequently tailored on the basis of results of cultures, susceptibility, and serology where applicable.
Potential empiric regimens for severe CAP (in the ICU) include the following:
a. A β-lactam third- or fourth-generation cephalosporin such as cefotaxime, ceftriaxone, cefepime, or ampicillin/sulbactam plus an intravenous (IV) macrolide such as azithromycin.
b. A third- or fourth-generation cephalosporin plus a fluoroquinolone such as levofloxacin.
c. A β-lactam/β-lactamase inhibitor such as ampicillin/sulbactam plus IV macrolide or fluoroquinolone.
d. If pseudomonas is a possibility, an antipseudomonal and antipneumococcal β-lactam/β-lactamase inhibitor such as piperacillin/tazobactam, or a carbapenem such as imipenem or meropenem should be used along with either azithromycin, levofloxacin or ciprofloxacin.
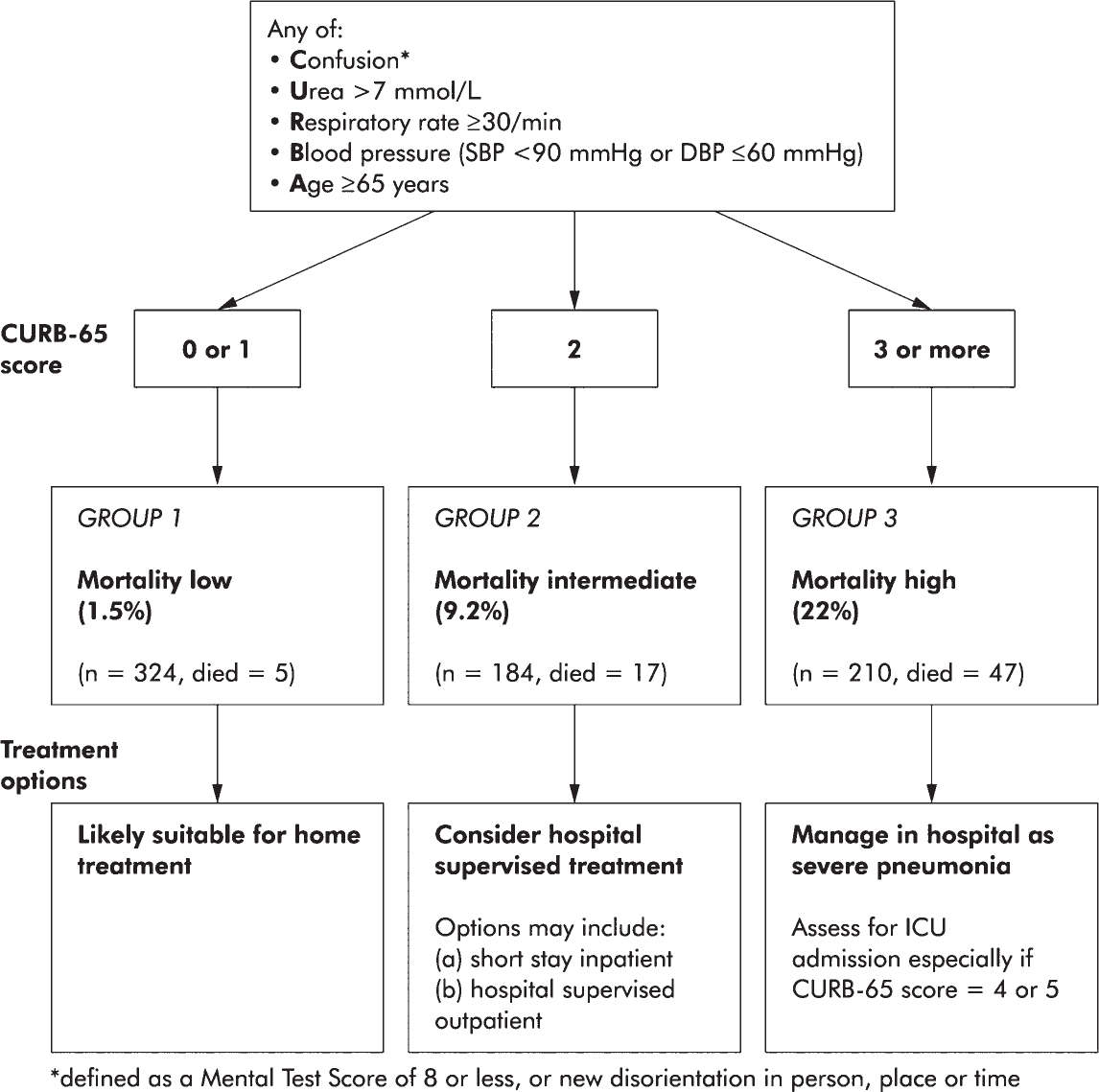
FIGURE 28.1 Severity assessment in a hospital setting: the CURB-65 score. One-step strategy for stratifying patients with CAP into risk groups according to risk of mortality at 30 days when the results of blood urea are available. (From Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003 May;58(5):377–382.)
e. Ciprofloxacin should only be used in combination with an antipneumococcal β-lactam/β-lactamase inhibitor because it does not have antipneumococcal coverage.
f. If methicillin-resistant Staphylococcus aureus (MRSA) is a possibility, add vancomycin or linezolid.
B. Hospital-acquired pneumonia (HAP) is defined as pneumonia that occurs at least 48 hours after admission to the hospital and did not appear to be incubating at the time of admission. It carries the highest risk of mortality among nosocomial infections. Health care–associated pneumonia (HCAP) is a broader term that encompasses pneumonias acquired in a health setting and not limited to the hospital—a definition that is less specific and diminishing fast in its appeal. Ventilator-associated pneumonia (VAP) is a subset of HAP that occurs more than 48 to 72 hours after intubation. VAP affects between 9% and 27% of intubated patients and is associated with high attributable mortality. Bacteria enter the lungs through various routes, including aspiration of oropharyngeal secretions or gastric contents, inhalation of droplets or aerosolized microorganisms from health care surroundings, hematogenous seeding, direct inoculation from colonized hospital personnel and contaminated equipment or devices.
1. Microbiology. The microorganisms causing nosocomial pneumonia differ substantially from those causing CAP. Infections can be polymicrobial. Common pathogens include gram-negative bacilli, such as P. aeruginosa, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and less commonly Serratia marcescens, Enterobacter cloacae, Stenotrophomonas maltophilia, and Burkholderia cepacia or gram-positive cocci such as S. aureus. Antimicrobial resistance is on the rise among these organisms, which may be preexisting or may develop de novo under selective pressure of antibiotics. MRSA is a common cause of hospital acquired pneumonia, particularly in patients with diabetes mellitus and those colonized with the microorganism. Risk factors for multidrug-resistant (MDR) infections include being hospitalized for >5 days, use of antibiotics during the preceding 90 days, a high frequency of antibiotic resistance in the specific hospital/unit, and immune-suppressive disease or therapy.
2. Diagnosis of VAP can be difficult because many conditions (e.g., sepsis, ARDS, CHF, atelectasis, thromboembolic disease, pulmonary hemorrhage, etc.) that are common in critically ill patients demonstrate similar clinical signs. The clinical criteria to diagnose VAP include the presence of new or progressive radiographic infiltrates in addition to one or more of the following: fever, purulent secretions, leukocytosis, tachypnea, diminished tidal volume, and hypoxemia. Radiographic signs alone are too nonspecific (see Fig. 28.2) (Bratzler, 2013). Acknowledging the difficulties in making the diagnosis of VAP using the existing definition of VAP, a new three-tier approach of ventilator-associated condition (VAC), infection-related ventilator-associated complication (IVAC), and possible or probable VAP has been proposed by the CDC but are currently limited to epidemiological surveillance and performance and quality assessments (Centers for Disease Control and Prevention, 2013).
a. Lower respiratory tract sampling is the cornerstone of VAP management. This is achieved by any of the following:
1. Deep tracheal aspiration: Deep suction using a catheter introduced through the ET tube or tracheostomy
2. Bronchoalveolar lavage (BAL): Wedging the tip of the bronchoscope in a segmental bronchial orifice and infusion of sterile saline followed by aspiration of the infusate-secretion mixture
3. Protected specimen brush (PSB): Introduction of a sampling brush via the bronchoscope that is covered by a protective sheath to minimize trans-bronchoscopic contamination
a. Results must be interpreted in light of time of antibiotic administration relative to sampling. Whenever possible, empiric antibiotics must be administered postsampling to maximize culture yield.
b. While BAL and PSB sampling were shown not to provide a survival benefit over deep tracheal aspiration, they do provide greater microbiological specificity, which potentially allows early antibiotic de-escalation and diminishes de novo resistance development and should be attempted whenever possible, if the risk is low and trained personnel are available.
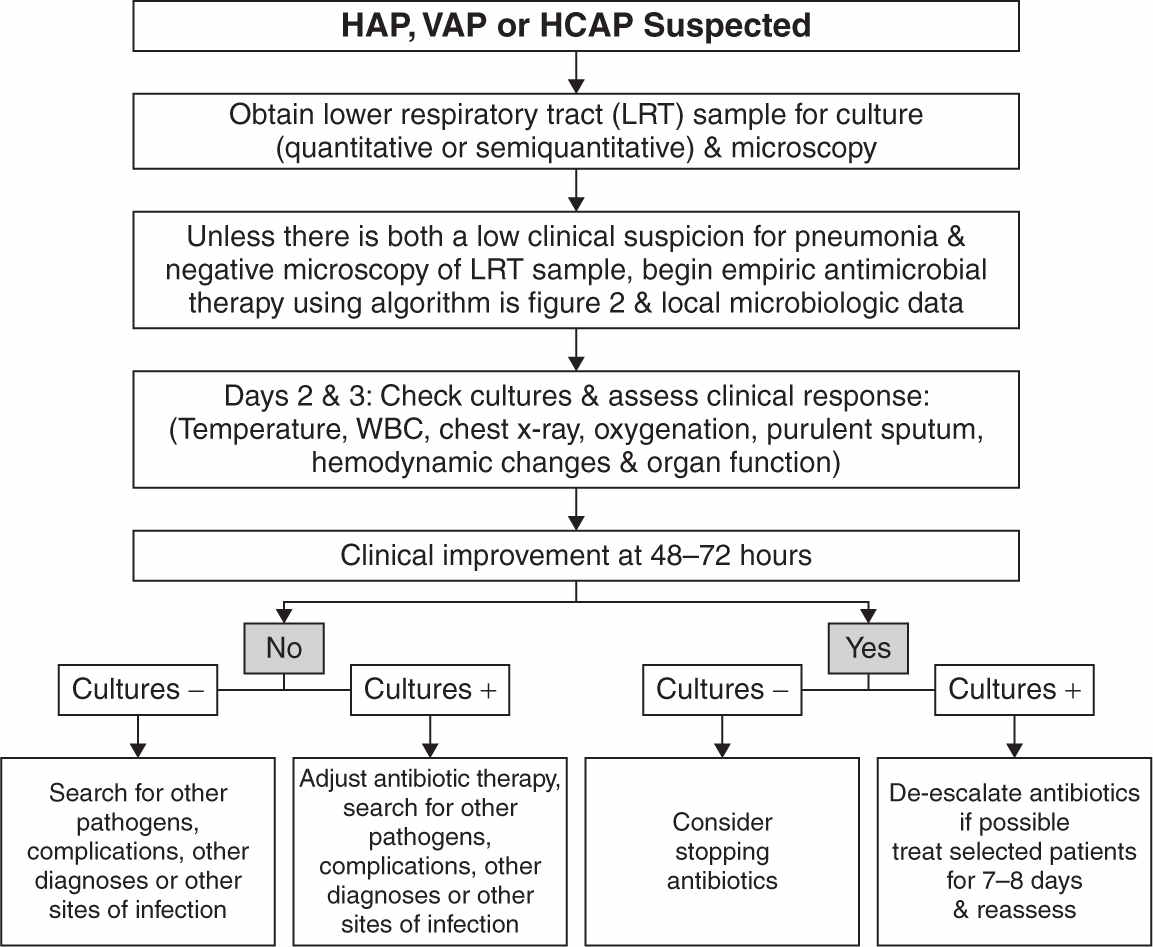
FIGURE 28.2 Summary of the management strategies for a patient with suspected hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), or health care–associated pneumonia (HCAP). The decision about antibiotic discontinuation may differ depending on the type of sample collected (PSB, BAL, or endotracheal aspirate), and whether the results are reported in quantitative or semiquantitative terms (see text for details). (From American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416.)
b. Gram stain: This is a valuable tool especially if empiric antibiotics may have sterilized respiratory samples. In these situations, morphological identification of bacterial “carcasses” may be the only available hint while making decisions for targeted therapy. Abundance of neutrophils on gram stain increases the likelihood of VAP.
c. Semiquantitative cultures: These are reported as none, mild, moderate, or abundant bacterial growth. The latter two strata are suggestive of VAP, but discrimination between colonization and infection using such ordinal strata is challenging.
d. Quantitative cultures: Bacterial growth of >100,000 CFU/mL in cultures from deep tracheal aspirate, >10,000 CFU/mL from BAL cultures, and >1,000 CFU/mL from PSB cultures are considered reasonable cutoffs for discriminating between VAP and colonization. Severely ill cases may warrant treatment despite failure to meet these cutoffs for VAP. While they may minimize resistance development by limiting inadvertent antimicrobial use, quantitative cultures have not shown to improve clinical outcomes. Severely ill cases warrant treatment despite failure to meet these cutoffs for VAP.
3. Treatment. Principles for managing VAP include use of early, appropriate empiric antibiotics in adequate doses, de-escalation of initial antibiotic therapy based on lower respiratory tract culture data and patient response, and avoiding unduly long durations of therapy. Use of a unit-specific broad-spectrum antibiotic regimen can reduce the incidence of inappropriate initial therapy to <10%.
a. Uncomplicated mild to moderate disease (i.e., without respiratory failure, hemodynamic instability, or signs of injury to other organs) occurring early during hospitalization (<5 days) is often treated with a single antibiotic; such as a third- or fourth-generation cephalosporin, a carbapenem, or a fluoroquinolone in patients who are allergic to penicillin. Numerous studies suggest that monotherapy is safe and effective when used properly and may decrease the likelihood of infection due to antibiotic-resistant microorganisms. If anaerobes are a possibility, a β-lactam/β-lactamase inhibitor combination such as ampicillin/sulbactam, piperacillin/tazobactam, or ticarcillin/clavulanate can be used as monotherapy. Alternatively, clindamycin or metronidazole may be added to a β-lactam or fluoroquinolone for adequate anaerobic coverage.
b. Severe HAP (i.e., respiratory failure, hemodynamic instability, extrapulmonary organ damage) is treated with combination therapy. Combination therapy should also be considered when mild to moderate pneumonia occurs later in the hospitalization, occurs in patients with significant comorbidities, or occurs in patients with recent antibiotic exposure. These situations increase the likelihood that pneumonia is caused by P. aeruginosa, other multidrug-resistant enteric gram-negative bacilli such as Acinetobacter spp., Enterobacter spp., Klebsiella spp., and/or MRSA. Generally, combination therapy includes any empiric monotherapy agent mentioned previously along with a fluoroquinolone or an aminoglycoside. Linezolid or vancomycin should be added if there is a possibility of MRSA pneumonia. Linezolid may be favorable in patients who have an MRSA infection with an MIC >1 mcg/mL as these infections have been associated with slower clinical response and higher rates of vancomycin failure. Daptomycin is inactivated by surfactant and should not be used to cover MRSA in suspected or confirmed cases of VAP. Aerosolized aminoglycoside or polymyxin antibiotics treatment may be considered as an adjunct to IV therapy in MDR gram-negative pneumonias in patients who are not improving with systemic therapy alone. However, adjunctive aerosolized antibiotics have so far only shown to provide improved microbiological eradication, not clinical outcomes necessarily.
C. Lung abscess results from focal necrosis of the pulmonary parenchyma leading to a fluid-filled cavity. The most frequent predisposing factor for lung abscess is aspiration, followed by periodontal disease and gingivitis. Bronchiectasis, pulmonary infarction, septic embolization from bacteremia, right-sided endocarditis, or septic venous thrombosis (e.g., Lemierre’s syndrome) also predispose to lung abscess.
1. Microbiology. Bacteria causing aspiration pneumonia and subsequent abscess formation differ depending on whether aspiration occurs in the community or health care setting. Anaerobic organisms appear to be key players. Community-acquired aspiration pneumonia is typically caused by S. pneumoniae, H. influenzae, community-acquired S. aureus, and Enterobacteriaceae, and the health care–associated variety is caused by gram-negative bacteria flora. Abscesses resulting from hematogenous spread of infection are usually peripheral, multifocal, and monomicrobial, most commonly caused by S. aureus. Mycobacterium tuberculosis, Nocardia, parasites (e.g., amebae and paragonimus spp.), and fungi are less frequent causes of lung abscess.
2. Diagnosis of lung abscess may be made on the basis of chest radiograph or chest computed tomography (CT). The distinction between pleural and peripheral parenchymal-based collections is clearer using CT. Cultures of expectorated sputum are unreliable. While transtracheal and transthoracic aspiration are ways to sample lung abscesses, these are rarely performed.
3. Treatment. With some exceptions, witnessed aspiration rarely requires antibiotics. Exceptions include patients whose stomachs are colonized with enteric flora (such as patients with small bowel obstruction), severely immune compromised, or continue to remain severely ill after the aspiration episode. Lung abscesses require prolonged (2–4 months) antibiotic therapy. Antibiotic choices will depend on culture isolates. Historically, antibiotics with anaerobic coverage were used to treat aspiration pneumonia. However, more recent studies that used protected-brush bronchoscopy to obtain samples failed to isolate pathogenic anaerobes. Postural drainage is an important aspect of the management, and bronchoscopy can be helpful in facilitating drainage or removing foreign bodies. Occasionally, a lung abscess is treated with surgical resection; however, this is not a first-line therapy. Complications of lung abscess include empyema, bronchopleural fistula formation, and bronchiectasis.
D. Empyema usually originates from intrapulmonary infection such as lung abscess or pneumonia, but pathogens also can be introduced into the pleural space from extrapulmonary sites as in trauma or thoracic surgery.
1. Microbiology. The most common bacterial cause of empyema is S. aureus. Enteric and nonenteric gram-negative bacteria (including Legionella pneumophila), gram-positive bacteria, anaerobic bacteria, fungi, and M. tuberculosis also can cause empyema.
2. Diagnosis requires direct analysis of pleural fluid for pH, total protein, red blood cells (RBC), and leukocyte (WBC) count and differential, Gram stain and bacterial cultures (anaerobic and aerobic), and possibly fungal and mycobacterial smear and culture in the right setting. Pleural fluid with pus or pH <7.2 and/or Gram-stain evidence of bacteria on pleural fluid sampling is highly suggestive of an empyema.
3. Treatment. An empyema is refractory to medical therapy alone. It must be treated with a combination of antibiotics and drainage via a thoracostomy tube. Occasionally, open drainage or decortication by video-assisted thoracoscopic surgery (VATS) of the empyema sac may be required.
E. Mediastinitis. The commonest presentation of mediastinitis in the modern day is as a postoperative complication of cardiac or thoracic surgery. Some cases may be associated with bacteremia, penetrating chest trauma, retropharyngeal abscess extension, and viscus rupture (e.g., Boerhaave syndrome).
1. Microbiology. Mediastinitis following cardiothoracic surgery that does not involve the esophagus is generally monomicrobial and is most often due to S. Aureus. Mediastinitis from disruption of the esophagus is usually polymicrobial and is caused by mixed anaerobic bacteria (Peptococcus spp., Peptostreptococcus spp., Fusobacterium spp., and Bacteroides spp.), gram-positive bacteria, enteric and nonenteric gram-negative bacteria, and fungi (C. albicans, C. glabrata). Other rare etiologies include Legionella, Mycoplasma, Histoplasma, and M. tuberculosis.
2. Diagnosis. Clinical signs and symptoms commonly include fever, leukocytosis, and chest pain. It is often difficult to differentiate superficial postoperative wound infection from wound infection with mediastinitis that is usually accompanied with signs of toxicity. Wound crepitus and sternal dehiscence are more specific but not always present. Chest CT may reveal widening of the mediastinum, complicated pleural effusions, and subcutaneous or mediastinal emphysema. However, most findings are difficult to distinguish from postoperative changes in the early postoperative period and results must be interpreted in light of clinical findings.
3. Treatment. In most cases, a combination of antibiotics and surgical intervention, including drainage, debridement, and removal or repair of the infected material and tissue must be initiated rapidly. In some situations, contained rupture or small perforations of the esophagus can be treated conservatively with careful monitoring. Broad empiric antibiotic coverage should be employed initially. Antibiotics should then be adjusted on the basis of results of intraoperative cultures. Coverage for head and neck sources (including esophageal disruption) should include antibiotics against gram-positive and gram-negative aerobes, as well as obligate and facultative anaerobes. Combination therapy with penicillin G or clindamycin plus agents against gram-negatives (such as a third-generation cephalosporin or a fluoroquinolone) is effective. Metronidazole will also provide adequate anaerobic coverage. Broadly active β-lactams such as ticarcillin/clavulanate, piperacillin/tazobactam, imipenem/cilastatin, or meropenem also offer reasonable early coverage. Empiric coverage for postsurgical mediastinitis should include an antistaphylococcal agent such as nafcillin or vancomycin (for MRSA or for patients who are allergic to β-lactams).
II. INTRA-ABDOMINAL INFECTIONS: These arise either from sources within the gastrointestinal (GI) tract, through contiguous spread from the urogenital/reproductive tract, through hematogenous or lymphatic seeding, or introduced from the outside (as occurs with trauma or surgery). Infections are predominantly polymicrobial. They include enteric gram-negative rods (E. coli, Klebsiella, Enterobacter, and Proteus spp.), P. aeruginosa, aerobic gram-positive cocci (Enterococcus and Streptococcus spp.), and anaerobes (Clostridium, Bacteroides, Fusobacterium, and Peptostreptococcus spp.).
Management of intra-abdominal infections depends on the cause and extent of involvement.
A. Microflora of Abdomen and Pelvis
1. GI tract. Normally, concentrations of bacteria increase progressively from the stomach through the small bowel and colon. Bacteria in the stomach and proximal small bowel include Streptococcus spp. and Lactobacillus spp. as well as anaerobes such as Peptostreptococcus spp. The concentrations of enteric gram-negative rods such as E. coli and anaerobic gram-negatives such as Bacteroides spp. increase progressively as one moves distally toward the colon. Colonic bacteria include enteric gram-negative rods, Enterococcus spp., Lactobacillus spp., and anaerobes such as Bacteroides spp., Clostridium spp., and Peptostreptococcus spp.
Factors that influence the quantity or quality of the GI microflora include the following:
a. pH (antacids, histamine-2 blockers, proton-pump inhibitors)
b. Antimicrobial use
c. GI dysmotility
d. Small-bowel obstruction, ileus, or regional enteritis
e. Bowel resection or intestinal diversion procedures
f. Hospitalization or residence in a chronic care facility prior to developing infection
2. Genital tract microflora include gram-positive aerobic bacteria such as Streptococcus spp., Lactobacillus spp., and Staphylococcus spp. as well as anaerobic bacteria such as Peptostreptococcus spp., Clostridium spp., and Bacteroides spp.
B. Peritonitis. Peritoneal infections can occur following penetrating abdominal trauma, contamination of peritoneal dialysis catheters, perforation of abdominal viscus, or even without a surgically correctable cause in specific populations.
1. Spontaneous bacterial peritonitis (SBP) is defined as peritonitis due to bacteria without a surgically correctable cause. It almost exclusively occurs in patients with end-stage liver disease (ESLD) from cirrhosis. The pathogenesis is postulated to translocation of gut bacteria across edematous intestinal mucosa and lymphatic or hematogenous seeding in the milieu of third spacing of fluid into the peritoneal cavity under high portal pressures. Infection of ascitic fluid that has accumulated as a result of congestive heart failure, malignancy, or hypoproteinemia in the absence of liver disease is exceedingly rare. Fever, abdominal pain, and confusion are the commonest symptoms. However, patients with SBP may be asymptomatic or have only minor symptoms.
a. Microorganisms. SBP is predominantly a monomicrobial condition. Enteric gram-negative bacteria are the commonest cause, followed by streptococci. Anaerobes rarely cause SBP. The presence of anaerobic bacteria or mixed flora suggests the possibility of secondary peritonitis.
b. Diagnosis. Early diagnosis is key; in the absence of treatment, most cases will progress to septic shock, which carries a dismal prognosis in the ESLD population. A diagnostic paracentesis must be performed prior to the administration of antibiotics if SBP is suspected. Ascites fluid should, at minimum, be sent for cell counts, Gram stain, and culture. A polymorphonuclear leukocyte (PMN) count of more than 250 or positive ascitic fluid cultures is highly suggestive of SBP. However, cultures of ascites fluid are often negative despite a cell count that is consistent with SBP. Use of blood culture bottles (anaerobic and aerobic) to culture ascites fluid may increase the likelihood of detecting the bacteria. Blood cultures should also be obtained.
c. Treatment. Empiric antibiotic therapy should be initiated prior to obtaining the results of cultures if the ascites fluid PMN counts are greater than 250 cells/mm3. Antibiotics should be modified appropriately when culture and sensitivity data become available. Potential empiric regimens include the following:
1. Cefotaxime is the primary agent for SBP. High doses (2 g IV every 4 hours) are used for life-threatening SBP.
2. A β-lactam/β-lactamase inhibitor combination, such as piperacillin/tazobactam or ticarcillin/clavulanate.
3. Ceftriaxone
4. Fluoroquinolone (use an alternative agent in patients taking fluoroquinolones for SBP prophylaxis or at high risk of fluoroquinolone resistance)
5. Ertapenem
6. If resistant enteric gram-negatives are a possibility, imipenem or meropenem should be considered.
2. Secondary peritonitis usually results from perforation or necrosis of a solid viscus (ruptured appendix, diverticulitis, duodenal ulcer), anastomotic leaks following bowel surgery or suppurative infections of the biliary and female reproductive tracts.
a. Diagnosis is often made with assistance of upright plain abdominal radiograph films that may show free air under the diaphragm. A CT scan with oral contrast usually identified the area of perforated viscus from extravasation of contrast material. Exploratory laparotomy may be necessary to diagnose and treat the source of peritonitis.
b. Treatment usually involves a combination of surgery and placement of drainage catheters and broad-spectrum antibiotics that are active against gram-negative and gram-positive bacteria and anaerobes. Conservative management may be attempted in cases of a small walled-off perforation or when operative mortality obviates any chance of a benefit. Knowledge of local resistance patterns and known colonization by drug-resistant microorganisms should influence empiric antibiotic choices. Antibiotic choices differ on the basis of severity and setting in which infection occurs.
1. Mild to moderate community-acquired peritonitis. This occurs very early in the hospitalization and without recent antibiotic therapy in patients who are not in chronic care facilities and is unlikely to result from antibiotic-resistant bacteria. Potential regimens include the following:
i. A third-generation cephalosporin (ceftriaxone, cefotaxime) or fluoroquinolone plus an agent against anaerobes (clindamycin or metronidazole). Ampicillin-sulbactam is not recommended for empiric treatment because of high rates of community-acquired E. coli resistance).
ii. Monotherapy with a carbapenem
iii. Fluoroquinolone plus an agent against anaerobes such as metronidazole
iv. Severe, high-risk, community-acquired or health care–associated peritonitis.
2. Peritonitis that develops during hospitalization, in a chronic nursing facility, or in the context of recent therapy with antibacterial agents may be caused by antibiotic-resistant microorganisms. Potential regimens include the following:
i. Monotherapy with a carbapenem (imipenem/cilastatin or meropenem)
ii. A third- or fourth-generation cephalosporin (ceftazidime or cefepime) or fluoroquinolone plus metronidazole. Although, fluoroquinolones as the only gram-negative coverage should be avoided in this setting and reserved for hospitals that demonstrate >90% sensitivity to E. coli. If ceftazidime is used, an additional agent with gram-positive coverage should be considered as ceftazidime has but modest activity against gram-positive bacteria. The regimen should include agents effective against enterococci such as ampicillin or vancomycin (in setting of β-lactam allergy or high rates of penicillin resistance). Linezolid or daptomycin should be used instead of ampicillin or vancomycin if infection with vancomycin-resistant enterococci (VRE) is a possibility.
C. Intra-abdominal abscesses may result from persistence of bacteria after secondary peritonitis or hematogenous spread of extra-abdominal infection. Locally, these may originate from the GI, upper urinary, or female genital tract. Abscesses should be suspected in the setting of focal abdominal pain and tenderness with high fevers, chills, and/or high leukocytosis that persist despite coverage with empiric antibiotics. Untreated, they may rupture, cause peritonitis, septic shock, and multiple-organ dysfunction syndrome.
1. Microorganisms commonly cultured from abscesses include Bacteroides spp. (especially B. fragilis), enteric gram-negative bacteria, gram-positive bacteria such as Enterococcus spp. and S. aureus, as well as candida.
2. Diagnosis. CT is useful for diagnosing and localizing abscesses. Ultrasonography can be done at the bedside and can be particularly useful in diagnosis of right-upper-quadrant (RUQ), renal, and pelvic abscesses. Low specificity of indium-labeled WBC and gallium scans limit their utility. Rarely, exploratory laparotomy must be performed in cases of high clinical probability with low yield on imaging.
3. Treatment of intra-abdominal abscess includes drainage and antibiotics. The method of drainage (percutaneous under CT or ultrasound guidance vs. operative) depends on a variety of factors including the abscess location, whether the abscess is associated with perforation or gangrene, and the presence of loculations when it makes drainage even with multiple catheters unlikely. Often culture data are available to guide antibiotic selection. For initial empiric coverage, it is reasonable to use one of the combinations suggested previously for treating secondary peritonitis in hospitalized patients. Enterococcus spp. discovered in abscesses as part of a polymicrobial process is usually considered relatively avirulent (in immune-competent individuals) and it is acceptable to hold off on providing specific coverage for the same. Serial imaging can be used to monitor treatment, and in cases where a drain is in place, an abscessogram may be performed (imaging following retrograde instillation of contrast into the drain).
D. Infections of the Hepatobiliary System
1. Acute cholecystitis results from cystic duct obstruction. When this is due to a stone it is called calculous cholecystitis. This can be encountered in the ICU when severe sepsis and septic shock related to gangrene or perforation occur. Acalculous cholecystitis (accounting for a tenth of all acute cholecystitis cases, but more commonly seen in the ICU) occurs in the setting of critical illness, endothelial injury, gall bladder hypoperfusion, stasis edema, and cystic duct luminal narrowing. Complications such as gangrene and perforation as well as untreated mortality are higher in the acalculous variety.
a. Complications include GB gangrene and perforation with subsequent peritonitis, emphysematous cholecystitis, and empyema of the GB, all of which can lead to septic shock if left untreated.
b. Microbiology. Bacteria include enteric gram-negative bacteria such as E. coli, Klebsiella, Proteus, and Enterobacter spp., gram-positive bacteria such as Enterococcus spp., and anaerobes such as Clostridium and Bacteroides spp. Emphysematous cholecystitis is caused by Clostridium spp. and gram-negative bacteria like E. coli and Klebsiella spp.
c. Diagnosis. Acute cholecystitis produces more continuous pain and usually manifests with peritoneal signs like rebound tenderness and Murphy’s sign not seen with biliary colic that is accompanied by pain-free intervals and an absence of systemic toxicity and peritoneal signs. Abdominal ultrasonography has reasonable sensitivity and specificity. It may reveal gallstones, GB wall thickening, a dilated gallbladder, or a pericholecystic fluid collection. In ambiguous cases, cholescintigraphy (HIDA scan) can demonstrate nonfilling of the GB. Morphine administered prior to the test may result in a false-negative HIDA scan.
d. Treatment of acute calculous cholecystitis includes antibiotics and surgery. The timing of surgery depends on a number of factors. Surgery is often performed on an urgent basis when the more severe complications of acute cholecystitis as just described occur. Surgery may be delayed for stabilization of the patient or for optimizing the patient with serious medical conditions. Cholecystitis can be treated with the antibiotic regimens previously described for secondary peritonitis. For acalculous cholecystitis, the treatment of choice is percutaneous cholecystostomy tube and antibiotics. These usually cause rapid improvement in clinical and laboratory parameters. In the absence of such improvement, gangrene and/or perforation must be suspected and cholecystectomy is indicated, especially if the risk of untreated complicated acalculous cholecystitis outweighs the surgical risk in these critically ill patients.
2. Acute cholangitis is usually caused by partial or complete common bile duct (CBD) obstruction. This obstruction may be due to calculus, strictures, or an obstruction or extrinsic compression by malignant neoplasms of the biliary tract or pancreas.
a. Diagnosis. The classic presentation is Charcot’s triad of jaundice, fevers, chills, and RUQ pain. Blood cultures are often positive. No further diagnostic tests are needed if this triad is present and one can move directly to treatment. In the absence of all features of the classic presentation, a RUQ ultrasound may reveal biliary duct dilatation and CBD stone. In early cases, not much time may have lapsed to cause discernible CBD dilatation or the stones may be too small to be seen on ultrasound, in which case magnetic resonance cholangiopancreatography (MRCP) is helpful.
b. Treatment differs depending on whether there is partial or complete CBD obstruction. Nonsuppurative cholangitis, which results from partial CBD obstruction, will often respond to antibiotic therapy alone. Suppurative cholangitis, which is due to complete CBD obstruction causing pus under pressure, bacteremia, and septic shock, must be treated as early as possible, with a combination of antibiotics and decompression. Cholangitis can be treated with the antibiotic regimens described previously for secondary peritonitis. Decompression may be achieved endoscopically (endoscopic retrograde cholangiopancreatography), via a naso-biliary tube or percutaneously using a T-tube (esp. among poor surgical candidates).
3. Liver abscess. Abscesses can be solitary or multiple. Manifestations range from fever, leukocytosis, and RUQ pain to sepsis. Liver abscesses can result from seeding from the blood stream and portal pyemia or from local extension of biliary tract infection.
a. Microbiology. There is a wide spectrum of microbial etiologies that are difficult to predict beforehand and underscore the importance of sampling. Infections are monomicrobial or polymicrobial, which may include mixed aerobic, anaerobic, enteric bacteria and candida. Multiple monomicrobial abscesses with S. aureus or Streptococcus anginosus group are indicative of hematogenous spread and potential sources must be investigated. A large number of liver abscess are accompanied with bacteremia and blood cultures must be drawn in each case prior to empiric antibiotics. Patients with a liver abscess from or with recent travel to regions where amebiasis is endemic should raise suspicion of this etiology.
b. Diagnosis of liver abscess is generally made by CT scan or ultrasonography, and a microbiological diagnosis is obtained by percutaneous aspiration or concurrently positive blood cultures.
c. Treatment includes drainage and antibiotics. Drainage yields diagnostic and therapeutic benefits. For smaller abscesses, this can be done by needle aspiration but larger ones may require placement of a drain. Abscess arising from the biliary tract or peritoneum should be treated with antibiotics directed against the organisms involved in the initial infection. Abscess suspected to be of hematogenous origin should be treated empirically with regimens that include agents active against gram-positive bacteria or the specific organism when this is known. In most other cases, empiric broad-spectrum therapy with coverage of at least enteric gram-negative organisms and anaerobes should be used such as ampicillin-sulbactam or amoxicillin-clavulanate monotherapy or even levofloxacin plus metronidazole. Anaerobes are difficult to grow, and, given their high prevalence in liver abscesses, many recommend presumptive therapy with a regimen that includes anaerobic coverage even if they are not cultured.
E. Splenic abscess is rare, but has high mortality if left untreated. It usually results from hematogenous spread, but can result from splenic trauma or contiguous spread. The diagnosis of splenic abscess should prompt a search for bacterial endocarditis as the source.
1. Microbiology. The most common organisms isolated on culture are Streptococcus spp. followed by S. aureus. Salmonella spp. and, rarely, anaerobic bacteria also cause splenic abscess.
2. Left-upper-quadrant pain, fever, leukocytosis, and a left-sided pleural effusion suggest the diagnosis. A splenic abscess can often be mistaken for splenic infarcts on imaging, which has important therapeutic implications. These can often coexist.
3. Treatment. Splenic abscess is usually treated with antibiotics and with splenectomy rather than percutaneous drainage. The duration of antibiotic therapy is extended to 4 to 6 weeks when concurrent endocarditis is discovered.
F. Clostridium difficile–Associated Diarrhea (CDAD): One in every five patients admitted to a US hospital acquires C. difficile, a toxigenic spore-forming, gram-positive bacillus, as part of their colonic flora. While only a fraction develop clinical disease, they form a sizable reservoir and contribute to propagation of CDAD. While most cases are acquired in the health care setting, severe CDAD cases acquired in the community are on the rise. One must not rule out CDAD when evaluating a patient with consistent clinical features and an absence of antibiotic and health care exposure. That being said, the commonest modifiable risk factor remains antibiotic exposure, especially clindamycin, fluoroquinolones, broad-spectrum penicillins, and cephalosporins. Advanced age, comorbidities, immune suppression, gastric acid suppression, and health care exposure are other important risk factors. In the recent years, a hypervirulent strain (NAP1) has been implicated in epidemics of severe CDAD in the United States and other parts of the world. Interestingly, the same strain does not typically cause severe disease outside of epidemics. Antibiotics alter the normal flora, providing an environment for the conversion of C. difficile spores to vegetative forms leading to rapid replication and toxin production.
1. Clinical features: Nosocomial diarrhea (as frequent as 15 bouts of watery stool) occurring more than 3 days after hospitalization in patients that are receiving antibiotics should be assumed to be CDAD until proven otherwise. Other features may include bloody diarrhea, abdominal cramps and tenderness, toxic megacolon, bowel perforation, and peritonitis. Leukocytosis can be marked, sometimes in excess of 50,000 cells/µL.
2. Diagnosis. Although the C. difficile cytotoxicity assay is still considered the gold standard for diagnosis of CDAD, it is labor intensive, time-consuming, and not practical for wide clinical use. The commercially available enzyme immunoassay (EIA) on liquid/semisolid stool samples for C. difficile glutamate dehydrogenase (GDH) is highly sensitive and is a good screening tool. The stool EIA to detect toxin A and toxin B are more specific and used commonly in diagnostic algorithms.
a. Recently, the role of C. difficile PCR on stool samples has expanded across hospitals nationwide. While some solely rely on PCR for its high sensitivity, specificity, and rapid turnover (as early as 1 hour), others consider routine use of C. difficile PCR to be cost-prohibitive and restrict its use for confirmation when two EIA tests yield discordant results. As a large proportion of hospitalized individuals are colonized with C. difficile, testing on formed stool produces high rates of false-positive results and is discouraged.
b. CT imaging in fulminant CDAD may show diffuse colonic thickening and pericolic stranding. Flexible sigmoidoscopy with visualization of “pseudomembranes” can be helpful in making the diagnosis if serologic tests are inconclusive or an urgent diagnosis of fulminant disease is warranted.
3. Treatment. Timely discontinuation of causative antibiotics is a common therapeutic dictum for C. difficile, regardless of severity. Therapeutic options, otherwise, vary with disease severity and frequency. Criteria for severe disease are still based on expert opinion (Cohen, 2010). However, features considered as indicative of severe disease include WBC count >15,000, presence of shock despite fluid resuscitation, severe lactic acidosis, acute kidney injury (Cr >1.5 times premorbid level), and toxic mega colon.
a. Metronidazole (500 mg oral or IV; 3 times a day) is comparable to oral vancomycin (125 mg every 6 hours) for initial therapy for nonsevere disease. Higher doses of PO vancomycin provided no additional benefit.
b. Initial recurrences of nonsevere CDAD can be treated with PO vancomycin; although fidaxomicin was shown to lower further recurrences and may be a preferred choice for that reason. Some suggest adjunctive rifaximin for recurrences but evidence is limited to a few case series.
c. For initial severe CDAD, vancomycin PO 125 mg every 6 hours is the drug of choice. The dose may be pushed to 500 every 6 hours in severe cases. The drug usually concentrates well in the colon; however, in the setting of profound ileus or inability to tolerate PO medication, vancomycin 500 mg 4 times a day may be administered in the form of an enema. Colonic perforation is a rare but serious adverse effect of this route of administration. Treatment is usually continued for 10 to 14 days. Surgical consultation is indicated in patients who have signs of shock or organ failure, or who have not responded to 24 to 72 hours of maximal medical therapy. The surgical treatment of choice is a total abdominal colectomy with an end ileostomy.
d. Experimental therapies include the nitazoxanide, IV immunoglobulin, and monoclonal antibodies against toxins A and B, but sufficient evidence is lacking. Replenishment of GI flora with probiotics (Lactobacillus spp., Saccharomyces boulardii) for prevention or treatment of CDAD are also not routinely recommended due to limited data and an increased risk of bloodstream infections, especially in the immune compromised. There is growing evidence to support fecal microbiota (stool) transplants from healthy subjects for recurrent, refractory, or severe CDAD. Attempts are being made at standardization of sample storage, delivery media, and administration protocols that will hopefully allow more robust inferences of efficacy in the near future.
III. SKIN AND SOFT TISSUE INFECTIONS
A. Postoperative wound infections. Multiple factors influence the development and severity of wound infections. The incidence of postoperative wound infection due to antibiotic-resistant bacteria increases with the length of hospitalization prior to surgery.
1. Microbiology. Microorganisms often reflect the site of origin and are altered by recent treatment with antibiotics, prolonged preoperative hospitalization, and coexisting diseases. Clean surgical wound infections are most often caused by S. aureus, coagulase-negative Staphylococcus, and Streptococcus spp. Severe wound infections that occur in the first 48 hours after surgery may be caused by Clostridium or group A streptococcus (Streptococcus pyogenes). The distributions of microorganisms in contaminated wounds can often reflect the origin of contamination (respiratory, GI, or genitourinary [GU] tract).
2. Clinical Presentation and Diagnosis. Wound infections vary in severity from superficial infections of the skin and subcutaneous tissues to deep and severe infections involving the underlying fascia and/or muscles. Superficial wound infections are most frequently manifested by erythema, warmth, and swelling. Fever and purulence are variably present. Diagnosis is essentially clinical. Surface wound swabs are not entirely helpful. They may grow skin flora in culture. Deeper sampling (such as during debridement in the OR) provides greater specificity for the offending microorganism.
3. Prevention. Detection and treatment of infection at other sites, limiting the duration of hospitalization before surgery, proper surgical technique, and proper preoperative scrubbing of the patient and the surgical team are important measures. While recommendations vary for clean procedures that do not involve placement of foreign material, prophylactic antibiotics are routinely administered for clean procedures involving placement of foreign material and for all procedures that enter, or are complicated by spillage from internal organs. Prophylactic antibiotics that can be rapidly infused should be given within the 30 minutes prior to incision, and for clean or clean-contaminated operations should be discontinued within 24 hours of surgery to minimize the risk of colonization with antibiotic-resistant organisms. Antibiotics such as vancomycin and ciprofloxacin require a longer time for infusion and should be started at least 120 minutes prior to incision to ensure that a maximum amount of drug is infused. Longer courses of antibiotics are generally given for contaminated or dirty wounds. Choices of prophylactic antibiotics are guided by site and type of surgery, duration of hospitalization prior to surgery, and recent use of antibiotics. Many institutions have established specific guidelines for prophylactic antibiotics.
B. Treatment
1. Mild superficial wound infections may be treated with removal of sutures or staples and opening of the wound to drain fluid collections.
2. Severe wound infections are usually treated with a combination of parenteral antibiotics and surgical debridement. As stated earlier, cultures of fluid or tissue collected in a sterile fashion should be used to guide antimicrobial therapy. Initial empiric antibiotic coverage will be dictated by the clinical setting. First-generation cephalosporins offer reasonable coverage for uncomplicated postoperative wound infections. Clindamycin is an alternative in patients allergic to β-lactams. Vancomycin, linezolid, daptomycin, or ceftaroline should be reserved for cases where there is a reasonable possibility that the infection is caused by MRSA. Gram-negative coverage should be considered for infections originating in the GI, GU, and respiratory tracts.
C. Necrotizing soft tissue infections: These can be classified on the basis of microbial etiology as polymicrobial (also referred to as Type I) or monomicrobial (Type 2). They are also classified on the basis of the tissue plane involved as necrotizing fasciitis or myonecrosis or both. These life-threatening infections have the propensity to spread rapidly and cause severe systemic toxicity early in the course of infection. The mortality due to necrotizing soft tissue infections approaches 100% in the absence of timely surgical control or if unresectable portions of the body are involved. Timely administration of appropriate antimicrobial therapy, while essential, is not able to arrest the process without debridement due to poor antibiotic delivery to necrotic tissue.
1. Microbiology
a. Necrotizing fasciitis. Streptococcus spp. are most commonly isolated from wound cultures. Polymicrobial infections with anaerobes, enteric gram-negatives, and Streptococcus spp. also occur.
b. Myonecrosis. Clostridial myonecrosis (gas gangrene) is a severe, fulminant skeletal muscle infection caused by Clostridium spp. Exotoxins released by bacteria are important in the pathogenesis of clostridial myonecrosis. Nonclostridial myonecrosis is generally polymicrobial due to Streptococcus spp., enteric gram-negative rods (E. coli, K. pneumoniae, Enterobacter spp., etc.), and anaerobic bacteria.
2. Diagnosis. Early features include pain out of proportion to the local external findings and systemic toxicity. Crepitus may be present due to gas in soft tissues.
3. Treatment
a. Debridement. Immediate recognition and prompt surgical exploration and debridement are critical. Frequent surveillance of the wound is essential and repeated surgical debridement is often necessary.
a. Antibiotics are chosen on the basis of the presentation and the likely source of infection. Gram stain of intraoperative wound samples can guide initial therapy. Empiric therapy should be broad and include coverage of Streptococcus spp. and Staphylococcus spp., enteric gram-negative bacteria, and anaerobes. Clindamycin is included in regimens for treatment of suspected exotoxin producing necrotizing soft-tissue infections because it acts as a bacterial protein synthesis inhibitor.
a. The role of hyperbaric oxygen in necrotizing fasciitis and that of intravenous immune globulin in superantigen-mediated toxic shock like syndrome from necrotizing fasciitis are still unclear.
IV. URINARY TRACT INFECTIONS
Urinary tract infections (UTIs) in the ICU either present as the reason for admission in the form of urosepsis from community- or hospital-acquired pathogen or as cystitis/urethritis that develops during the ICU stay among patients with indwelling urinary catheters. They are responsible for 40% of nosocomial infections and cause up to 30% cases of gram-negative bacteremia in hospitalized patients. Fungal UTIs are discussed in Section VIII.
A. Predisposing Factors: Indwelling urinary catheters remain the leading cause in the ICU. Other factors include neurologic or structural abnormalities of the urinary tract and nephrolithiasis.
B. Microbiology: In most cases, the infection is of the ascending variety. In these cases, the organisms most commonly encountered are gram-negative rods including E. coli, Klebsiella, Proteus, and Enterobacter spp. or gram-positive cocci like Enterococci and S. saprophyticus. Serratia and Pseudomonas spp. are additional causes of catheter-related infections. Bacteriuria from S. aureus should raise suspicion of hematogenous seeding and prompt a search for bacteremia. Contiguous peritoneal infection can predispose to perinephric and renal abscesses. Urethritis can also be caused by Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas, Candida spp., and herpes simplex virus (HSV).
C. Diagnosis. Analysis of the urinary sediment for leukocytes in conjunction with urine cultures and clinical features can be useful in distinguishing colonization from true infection. Urinary sediment that contains WBC casts suggests that the infection involves the kidneys or tubules.
D. Specific UTIs
1. Cystitis is infection of the bladder characterized by dysuria and frequency, cloudy or bloody urine, and localized tenderness of the urethra and suprapubic regions. More severe symptoms such as high fever, nausea, and vomiting suggest upper urinary tract involvement.
2. Acute pyelonephritis is a pyogenic infection of the renal parenchyma and pelvis. It is characterized by costophrenic angle tenderness, high fevers, shaking chills, nausea, vomiting, and diarrhea. Laboratory analysis reveals leukocytosis, pyuria with leukocyte casts, and occasional hematuria. Bacteria are often visible on Gram stain of unspun urine. Evaluation of the urinary tract should be considered because a significant proportion of pyelonephritis is associated with structural abnormalities. Treatment includes antibiotics and removal or correction of the source. Complications include papillary necrosis, urinary obstruction causing hydro or pyonephrosis, and frank sepsis/septic shock.
3. Renal and perinephric abscesses are uncommon and usually are due to ascending infection from the bladder and ureters. Major risk factors include nephrolithiasis, structural urinary tract abnormalities, urologic trauma or surgery, and diabetes mellitus. Most abscesses in the urinary tract are usually bacterial but may be caused by Candida spp. in some cases. Renal and perinephric abscesses may present nonspecifically with fever, leukocytosis, and pain over the flank, groin, or abdomen). Urine cultures may be negative, particularly if the patient has already received appropriate antibiotics. Diagnosis can be confirmed by abdominal ultrasound or CT scan. Treatment is drainage and antibiotics.
4. Prostatitis is an infrequent infection in the ICU that can occur as a result of bladder catheterization. Symptoms and signs include fevers, chills, dysuria, and an enlarged, tender, and boggy prostate on per rectal examination.
E. Treatment of UTIs. Prior to receiving culture results, empiric broad therapy should be initiated that cover likely organisms. Fluoroquinolones or third- or fourth-generation cephalosporins are a reasonable empiric choice for most patients. However, fluoroquinolones should be avoided in patients with recent fluoroquinolone use, admitted from long-term care facilities, or use in institutions with high rates of resistance. Antimicrobial therapy must be tailored to species and susceptibility data when they become available. Duration of treatment is uncertain; however, 7 to 14 days is an accepted range. Often those with resistant organisms are treated for 14 days.
V. INTRAVASCULAR CATHETER-RELATED INFECTIONS can be localized to the site of insertion (exit-site infections) or can be systemic (catheter-related bloodstream infections or CRBSI). Important risk factors for CRBSI include total parenteral nutrition (TPN), prolonged central line use, and femoral location. Fever is the most common presenting feature, and localized signs of infection at the insertion site are often absent. Inflamed exit site or unexplained fever among patients with central lines should prompt assessment for CRBSI.
A. Microbiology. The most common pathogens are coagulase-negative Staphylococcus (CoNS) followed by S. aureus. Since CoNS are also the commonest organisms to contaminate blood cultures, CRBSIs caused by them are often difficult to diagnose. A variety of gram-negative and other gram-positive bacteria are less frequent causes. Candida spp. account for close to 10% of CRBSI and are commonly associated with TPN administration.
B. Management
1. Depending on the type and extent of infection and microorganism involved, treatment options may range between systemic, topical or no antibiotic therapy, antibiotic lock therapy, and removal of the catheter.
2. Indications for catheter removal:
a. Septic shock
b. CRBSI due to S. aureus, P. aeruginosa, Candida, or Mycobacterium spp.
c. CRBSI with persistent bacteremia despite 3 days of appropriate antibiotic therapy
3. In general, there is a lower threshold to removing temporary catheters. It is considered safe to insert a new catheter (including peripherally inserted or tunneled varieties) if blood cultures remain negative 72 hours after catheter removal.
4. The choice of antibiotics is dictated by the clinical situation and culture data. Empiric therapy is often started with vancomycin if there are systemic signs of infection or if preliminary blood culture results indicate gram-positive bacteremia. Sometimes an additional agent is added to cover gram-negatives or Candida spp. when suspicion for these is high. Further therapy should be tailored to the specific organism identified.
5. Duration of systemic antibiotics upon removal of catheter: 7 to 10 days (14 days minimum in cases of S. aureus). One may wait to start antibiotic therapy in CRBSI where only blood cultures drawn from the line grow a microorganism that is not S. aureus, P. aeruginosa, or Candida spp. Longer duration of antibiotics may be necessary if there is evidence of endocarditis, venous thrombosis, or presence of an implanted device.
6. When central-line salvage is attempted, antibiotics may be administered systemically for 1 to 2 weeks with or without antibiotic lock therapy. The latter strategy targets microorganisms within the biofilm by allowing the antibiotic to dwell within a long-term catheter since removal of these often presents management problems. Isolated exit-site infection may be treated with appropriate topic antimicrobials. However, a 7-day course of targeted systemic antibiotic therapy is recommended if purulence is observed at the exit site.
7. Catheter exchange over a guide wire in response to CRBSI is not routinely recommended. If an exchange is performed and catheter tip cultures are positive, the rewired catheter should be removed and a new line placed at a fresh site. A strong suspicion that the catheter is the source of fever or septic complications should prompt a change in site and, at a minimum, blood cultures.
VI. INFECTIVE ENDOCARDITIS (IE). IE is caused by microbial invasion of the endocardium. It most commonly involves the cardiac valves, but can also occur in the septal or mural myocardium. IE is classified on the basis of course, as acute or subacute, or type of valve affected, as native valve (NVE) or prosthetic valve endocarditis (PVE).
PVE that occurs within 2 months of valve replacement (early PVE) results from colonization of the valve by microbes at the time of surgery and most commonly is caused by Staphylococcus spp. Late PVE is similar to NVE. Microorganisms gain entry into the bloodstream via direct inoculation during airway, GU, GI, and dental procedures or from an existing focus of infection such as pneumonia or dental abscess.
A. Predisposing factors for IE include IV drug use, previous IE, cicatricial complications of rheumatic heart disease, and congenital heart disease. However, endocarditis can occur in previously normal hearts as well. Intravascular devices such as central venous catheters, pacemaker wires, hemodialysis shunts, and prosthetic valves are additional risk factors.
B. Microbiology: IE is most commonly caused by bacteria, but can be caused by fungi, viruses, and rickettsiae.
1. Gram-positive bacteria. Streptococcus spp. are common, particularly the viridans group (such as S. sanguis, S. mutans, and S. intermedius). Enterococcus spp. also causes endocarditis, particularly in elderly patients who have undergone GU procedures. Staphylococcus spp., particularly S. aureus, is a common cause of NVE in drug users. S. aureus endocarditis is usually severe and commonly complicated by inflammatory damage to valvular and perivalvular structures, myocardial and valve ring abscesses, emboli, and metastatic lesions (e.g., lung, central nervous system [CNS], and splenic abscess). Identification of Streptococcus bovis as the causative organism should prompt a workup for a GI source such as colon cancer. Contrary to their reputation as blood culture contaminants, coagulase-negative staphylococci often cause PVE that is accompanied by significant valvular destruction.
2. Gram-negative bacteria infrequently cause IE. It is often severe, with an abrupt onset and high mortality. A characteristic NVE affecting structurally abnormal valves is caused by a group of bacteria collectively called HACEK (Haemophilus, Actinobacillus, Cardiobacterium, Eikenella, and Kingella spp.) and is characterized by a delayed growth in blood cultures, subacute course, large vegetations, and frequent embolic events. Brucella spp. is a common cause in areas where it is endemic.
C. Diagnosis. A severe systemic illness with high fevers and chills along with a new heart murmur should raise suspicion for acute endocarditis. The subacute variety may be less evident and is difficult to diagnose clinically.
1. Physical examination commonly includes a heart murmur, petechiae, and splinter hemorrhages in the nail beds. More specific but less common findings include retinal hemorrhages (Roth’s spots), painful red or purple nodules on digital pads (Osler’s nodes), and painless red macules on the palms or soles (Janeway lesions). Often complications may be discovered before IE is diagnosed, such as stroke, osteomyelitis, or metastatic abscesses.
2. Blood cultures: Three or more sets of blood cultures should be obtained within the first 24 hours if IE is suspected. Rarely, blood cultures are negative, particularly when IE is due to intracellular organisms such as rickettsiae, anaerobic bacteria, the HACEK group of bacteria, and fungi. Special media may be necessary to isolate the responsible microorganism underscoring the need to inform the laboratory when these pathogens are suspected.
3. Echocardiography is an important tool for diagnosing and managing IE. While transthoracic echocardiography (TTE) is less sensitive than transesophageal echocardiography (TEE) for detecting vegetations, it is a reasonable first choice, especially for NVE in nonobese patients that are not mechanically ventilated. Echocardiography can be used to follow the progression of vegetations and to identify and follow complications such as valvular insufficiency, valve ring or myocardial abscesses, pericardial effusions, and heart failure.
The Modified Duke Criteria is a helpful guide to make a diagnosis in less obvious cases of IE (Tables 28.1 and 28.2).
D. Treatment: Patients with acute IE are often critically ill and in addition to usual supportive care, early empiric antibiotics and prompt management of complications form the cornerstones of treatment of acute IE. Initial blood cultures must be obtained prior to the first dose of antibiotics.
1. Antibiotics
a. NVE: Vancomycin is a reasonable empirical choice, as it would cover streptococci, staphylococci, and most strains of enterococci. Gentamicin may be added if streptococci or enterococci are clinically suspected.
b. PVE: Empirical therapy usually includes vancomycin, gentamicin, and either a fourth-generation cephalosporin or a carbapenem. Rifampin is added subsequently when staphylococcal PVE is identified.
| Definition of Infective Endocarditis According to the Proposed Modified Duke Criteria, with Modifications Shown in Boldface | |
Definite infective endocarditis
Pathologic criteria
1. Microorganisms demonstrated by culture or histologic examination of a vegetation, a vegetation that has embolized, or an intracardiac abscess specimen; or
2. Pathologic lesions; vegetation or intracardiac abscess confirmed by histologic examination showing active endocarditis
Clinical criteriaa
1. Two major criteria; or
2. One major criterion and three minor criteria; or
3. Five minor criteria
Possible infective endocarditis
1. One major criterion and one minor criterion; or
2. Three minor criteria
Rejected
1. Firm alternate diagnosis explaining evidence of infective endocarditis; or
2. Resolution of infective endocarditis syndrome with antibiotic therapy for >4 d; or
3. No pathologic evidence of infective endocarditis at surgery or autopsy, with antibiotic therapy for >4 d; or
4. Does not meet criteria for possible infective endocarditis, as above
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







