Obtaining Blood Cultures
Obtaining Blood Cultures
I. INTRODUCTION
A. The management of infectious disease is a crucial skill for the intensivist. Epidemiological studies suggest that more than 70% of adult patients in an ICU receive antibiotics, and about 50% demonstrate overt signs of infection during their stay. Infection is a major contributor to mortality among the most critically ill patients, especially following trauma, burns, and extensive surgical insults.
B. Critically ill patients have numerous potential sources of infection as outlined in Table 12.1. Table 12.2 summarizes the common bacterial pathogens referred to herein.
C. Chapter 27 reviews empiric and disease-specific treatment.
D. Please see the video regarding how to obtain blood cultures.
II. HOSPITAL-ACQUIRED INFECTION (HAI) increases health care costs, extends hospital stays, and contributes to excess mortality. A sizeable percentage of these infections are preventable through multifaceted measures; accordingly, HAI has become the focus of worldwide surveillance and practice-improvement measures.
A. The estimated prevalence varies, but approximately 4% to 5% of hospital patients are affected by HAI. Patients in an ICU are about three times more likely to contract HAI than are patients on a general care ward.
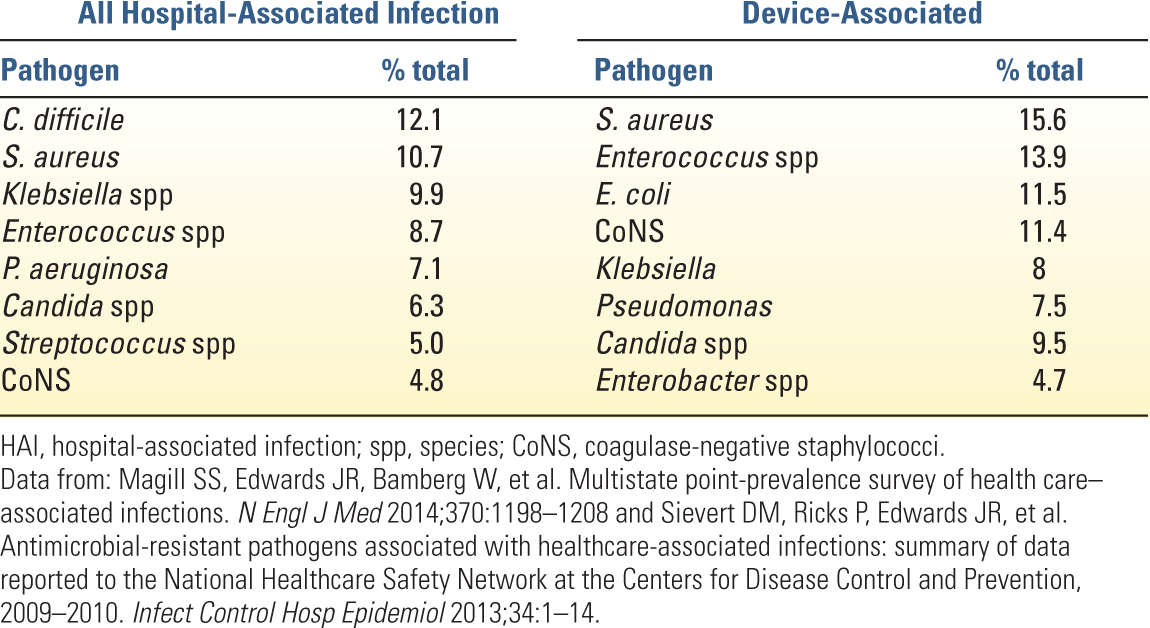
1. Device-associated infections, including central line–associated blood stream infection, catheter-associated urinary tract infection, and ventilator-associated pneumonia, have come under intense scrutiny despite contributing to only about 25% of all HAI. Efforts led by the CDC and other worldwide regulatory bodies are targeted at both surveillance and development of preventative strategies.
2. All critical care practitioners must be familiar with guidelines aimed at reducing the incidence of HAI (see Selected Readings).
3. Efforts at incentivizing improved hospital performance are continuing to develop and largely based around pay for performance models linked to HAI, including surgical site infections.
4. Prevention strategies have been bolstered by the JCAHO mandate that hospitals have an active program for the surveillance, prevention, and control of HAI. Strategies include the following:
a. Infection control measures including universal precautions, isolation, and hand hygiene are fundamental (see Chapter 40).
b. Surveillance should be implemented to gauge trends, identify problematic organisms, and alert clinicians to new trends in resistance or areas for improvement.
c. Limiting the duration of care in an ICU helps to reduce patient exposure to the highest risk environment for HAI.
d. The physical design of an ICU can help to reduce foot traffic, maximize availability of hand hygiene stations, facilitate easy cleaning, and provide adequate space for the routine care of isolated patients.
e. Invasive procedures and devices should be minimized to the extent possible.
f. Device-specific procedures should be implemented and monitored for the placement and maintenance of intravenous lines, urinary catheters, etc. Clinicians must be familiar with established protocols for their placement, use, and maintenance (see Selected Readings).
g. Routine cleaning of the ICU environment, including multiuse diagnostic equipment, helps to reduce colonization.
h. Decolonization of patients via daily chlorhexidine baths is somewhat controversial but has been proven beneficial in several studies.
5. Educating clinicians about responsible antibiotic prescribing is of the utmost importance.
B. Patterns of nosocomial pathogens are shifting as gram-negative organisms are beginning to overtake gram-positives as the most common pathogens worldwide (Table 12.3). However, broad epidemiological data are not substitutes for local trends in infection and resistance, which should be tracked to improve empiric treatment.
C. Although not the focus of similar regulatory attention at present, gastrointestinal infection is the third most common HAI (17%) behind pneumonia (22%) and surgical site infection (22%). Clostridium difficile is the main contributor to these underappreciated GI infections and warrants heightened clinical suspicion.
| Potential Sites of Infection in Intensive Care Unit Patients | |
Central nervous system | Epidural or brain abscess, encephalitis, meningitis |
Head and neck | Sinusitis, pharyngitis, parotitis |
Chest | Pneumonia, tracheobronchitis, endocarditis, empyema, mediastinitis, pericarditis |
Abdomen | Peritonitis, cholecystitis, cholangitis, appendicitis, diverticulitis, C. difficile colitis |
Genitourinary | Pyelonephritis, cystitis, prostatitis |
Musculoskeletal and vascular | Cellulitis, abscess, pressure ulcers, osteomyelitis, deep venous thrombosis |
Surgical | Wound, implanted hardware, anastomotic failure, abscess |
Device-associated infections | Intravascular catheter, urinary catheter, epidural or spinal catheter, intracranial pressure monitor, any drain site or tract |
| Classification of Common Pathogenic Bacterial Genera and Species | |
Gram-positive aerobic cocci | Staphylococcus |
Gram-positive aerobic bacilli | Bacillus, Corynebacterium, Listeria |
Gram-negative nonenteric bacilli | Acinetobacter, Pseudomonas, Burkholderia, Neisseria, H. influenzae, Legionella |
Gram-negative enteric bacilli | (Enterobacteriaceae family) E. coli, Klebsiella, Enterobacter, Proteus, Citrobacter, Serratia, Salmonella |
Gram-positive anaerobic cocci | Peptostreptococcus |
Gram-positive anaerobic bacilli | Bacteroides |
Gram-negative anaerobic bacilli | Clostridium (sporulating), Actinomyces, Lactobacillus |
III. ANTIBIOTIC RESISTANCE AND INFECTION PREVENTION
A. Resistance represents a growing global challenge. Critically ill patients are highly susceptible to HAI owing to environmental colonization, immunosuppression, malnutrition, and the use of invasive devices. Coupled with higher rates of resistant organisms in critical care settings (Fig. 12.1), it is unsurprising that intensivists and their patients must frequently combat this problem.
1. The etiologies of bacterial resistance are diverse and differ widely depending on organism; however, they are the result of selective pressures that favor survival of robust organisms. Widespread antibiotic use, ineffective or inappropriate treatment, environmental contamination, incomplete patient isolation, and inadvertent transmission all contribute to the development and spread of resistant organisms.
2. Epidemiological studies suggest that more than 50% of nosocomial pathogens demonstrate some degree of resistance. The CDC estimates that the worldwide impact of antibiotic resistance in the United States alone exceeds $20 billion in addition to excess mortality and the societal impact of prolonged treatment among affected patients.
IV. Attention to basic principles of antimicrobial therapy is a key component of reducing antibiotic resistance and improving clinical outcomes.
A. The diagnosis of infection in the ICU is a nontrivial task. Fever, leukocytosis, respiratory disturbances, hemodynamic perturbations, and other markers of inflammation are nonspecific. Patients demonstrating these symptoms should also be evaluated for noninfectious sources of inflammation. In short, not all patients with fever are infected.
B. Testing in the setting of a suspected infection should be directed toward identification of the source and obtaining specimens for culture and susceptibility. Ideally, specimens should be collected before the initiation of antibiotic therapy to improve their yield. Colonizing organisms often complicate culture results. Quantitative analysis and consultation with the microbiology lab can be useful adjuncts. Quite frequently the source of infection and associated pathogens are never identified.
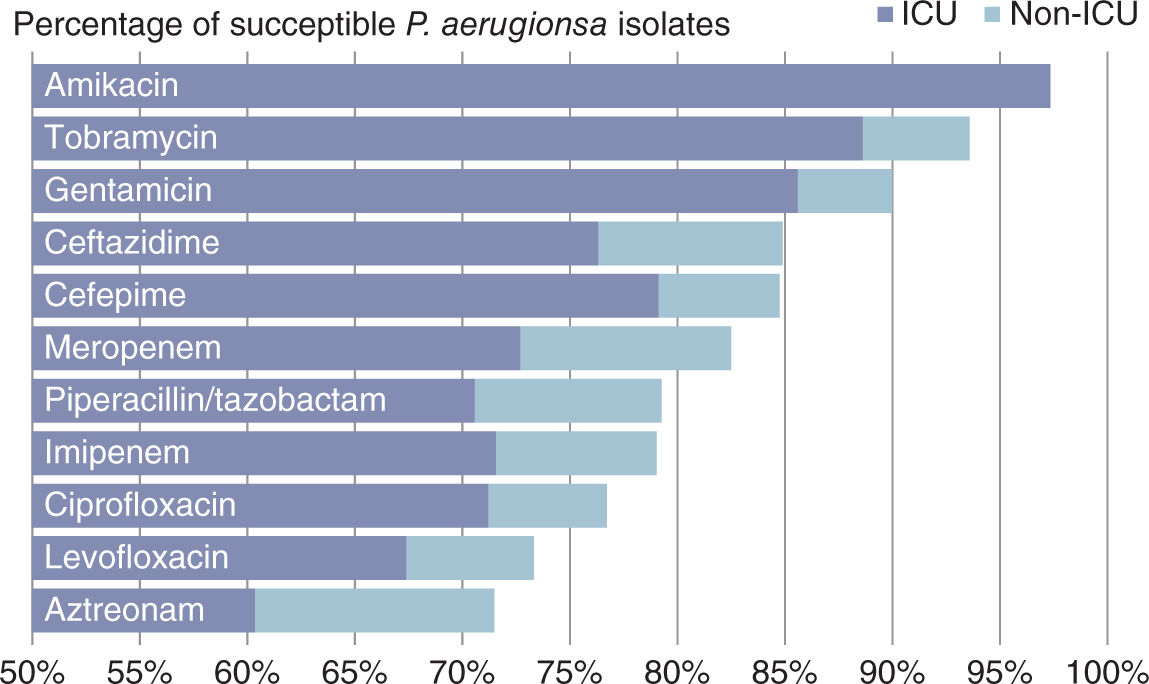
FIGURE 12.1 Percentage of susceptible Pseudomonas aeruginosa isolates in US ICU versus non-ICU health care settings (2009–2011). (Data from: Sader HS, Farrell DJ, Flamm RK, et al. Antimicrobial susceptibility of gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009–2011). Diagn Microbiol Infect Dis 2014;78:443–448.)
C. Empiric antibiotics must be selected on the basis of suspected site of infection, likely pathogens at that site, local resistance patterns, prior antibiotic therapy (if any), and any comorbidities that may heighten risks of toxicity.
1. The initiation of empiric therapy should not be delayed unreasonably for diagnostic testing in the setting of a high clinical suspicion for sepsis, meningitis, or pneumonia. Increased latency from diagnosis to treatment leads to heightened mortality with these clinical entities.
2. Do not overlook the potential contribution of anaerobic and fungal organisms to clinically significant infections, especially in the setting of prior or ongoing antibiotic therapy.
D. Documentation of suspected infection, rationale for drug selection, and duration of proposed treatment is critical and reduces confusion during the course of treatment and turnover among the care team.
E. Continuous Reassessment of antibiotic therapy is complicated by the fact that many critically ill patients will be slow to improve despite selection of appropriate therapy.
a. Deescalation can be accomplished in one of three ways:
1. Transitioning from a broad-spectrum to a narrower-spectrum agent following the return of culture and sensitivity data
2. Shortening the duration of therapy on the basis of clinical response when the location of infection can be identified
3. In the familiar scenario of unhelpful culture data but obvious infection, antimicrobials can be discontinued in a stepwise fashion on the basis of the likely pathogen involved as judged by local patterns, clinical experience, and patient response.
b. Consider oral therapy in patients with a functioning GI tract that are responding to treatment. Fluoroquinolones, linezolid, and fluconazole have excellent oral bioavailability.
c. Adverse effects may not emerge until well into a course of therapy.
d. Superinfection is a well-recognized consequence of broad-spectrum antimicrobial therapy, namely, with C. difficile and Candida.
e. Persistent fever despite other signs of resolving infection should prompt reevaluation of other causes rather than escalation or unnecessary continuation of therapy.
F. Limit the Duration of treatment whenever possible on the basis of type of infection and patient response. The ideal duration of treatment for many infections has not been concretely determined. Empiric therapy often must be stopped empirically.
V. ANTIBACTERIALS comprise a wide range of pharmaceuticals with variable microbial targets. A review of the most clinically relevant agents is presented herein.
A. Aminoglycosides (gentamicin, tobramycin, amikacin) provide unique bactericidal activity among the inhibitors of protein synthesis. Owing to toxicity, their role is largely confined to a few specific indications often in combination with other agents.
1. Spectrum: Aminoglycosides are most effective against aerobic gram-negative bacilli, including Pseudomonas (see Fig. 12.1), Acinetobacter, and members of the Enterobacteriaceae.
2. Resistance to aminoglycosides is multifactorial but due largely to modifying enzymes. Amikacin is the least affected by these enzymes and often reserved for the treatment of resistant organisms.
3. Considerations
a. Aminoglycosides penetrate poorly into the CNS, secretions, pleural fluid, infected tissue, and anaerobic environments owing to their cationic structure and need for active transport.
b. Nephrotoxicity almost invariably develops within the first week of treatment and can progress to nonoliguric renal failure that reverses upon discontinuation of therapy. Superimposed hypovolemia and chronic kidney disease, both common in the ICU, contribute to increased susceptibility.
c. The incidence of ototoxicity, especially with concomitant loop diuretic administration, has been estimated at 3% to 15% and may manifest insidiously as ataxia, disequilibrium, or nystagmus.
d. Rare but potentially life-threatening neuromuscular blockade has been reported in the setting of myasthenia gravis, hypocalcemia, or hypomagnesaemia. Aminoglycosides also potentiate nondepolarizing neuromuscular blockers.
e. Aerosolized tobramycin and gentamicin have been studied for treatment of Pseudomonas in patients with cystic fibrosis and bronchiectasis. Although the role for inhalational administration in the ICU is less clear, it may be useful to help minimize toxicity or treat resistant isolates.
4. Dosage must be adjusted on the basis of renal function and age, and monitoring of serum levels is recommended.
B. Anaerobic Agents
1. Metronidazole works via radical-mediated DNA disruption to exert bactericidal effects against anaerobic bacteria and is used in the treatment of Clostridium difficile and Bacteroides. It lacks efficacy against aerobes, facultative organisms, and nonsporulating gram-positive anaerobic bacilli. Listeria and Lactobacillus are resistant. Metronidazole has excellent oral bioavailability and is largely well-tolerated, with nausea and dyspepsia being the most common side-effects. Despite over five decades of clinical use, the estimated rate of anaerobic resistance is less than 5%.
2. Clindamycin interferes with bacterial protein synthesis and is bacteriostatic. Owing to its activity against both aerobic gram-positive cocci and most anaerobes, clindamycin can be used for treatment of skin or soft tissue infections. However, its strong association with the development of C. difficile and rising rate of resistance among Bacteroides spp. limit clindamycin’s utility in a critical care setting.
3. Tigecycline is the sole member of the novel glycylcylines and demonstrates activity against not only anaerobes but also most multidrug-resistant (MDR) organisms. Initial enthusiasm was tempered by an association between tigecycline and increased mortality in patients treated for VAP. Subsequent analyses suggested that the underlying cause was likely worsening infection secondary to treatment failure, which is puzzling given its spectrum of activity. Tigecycline is largely reserved for salvage therapy at present when faced with resistant organisms, especially when anaerobes are involved.
C. β-Lactams comprise a diverse and frequently utilized group of antibiotics that inhibit bacterial cell wall synthesis.
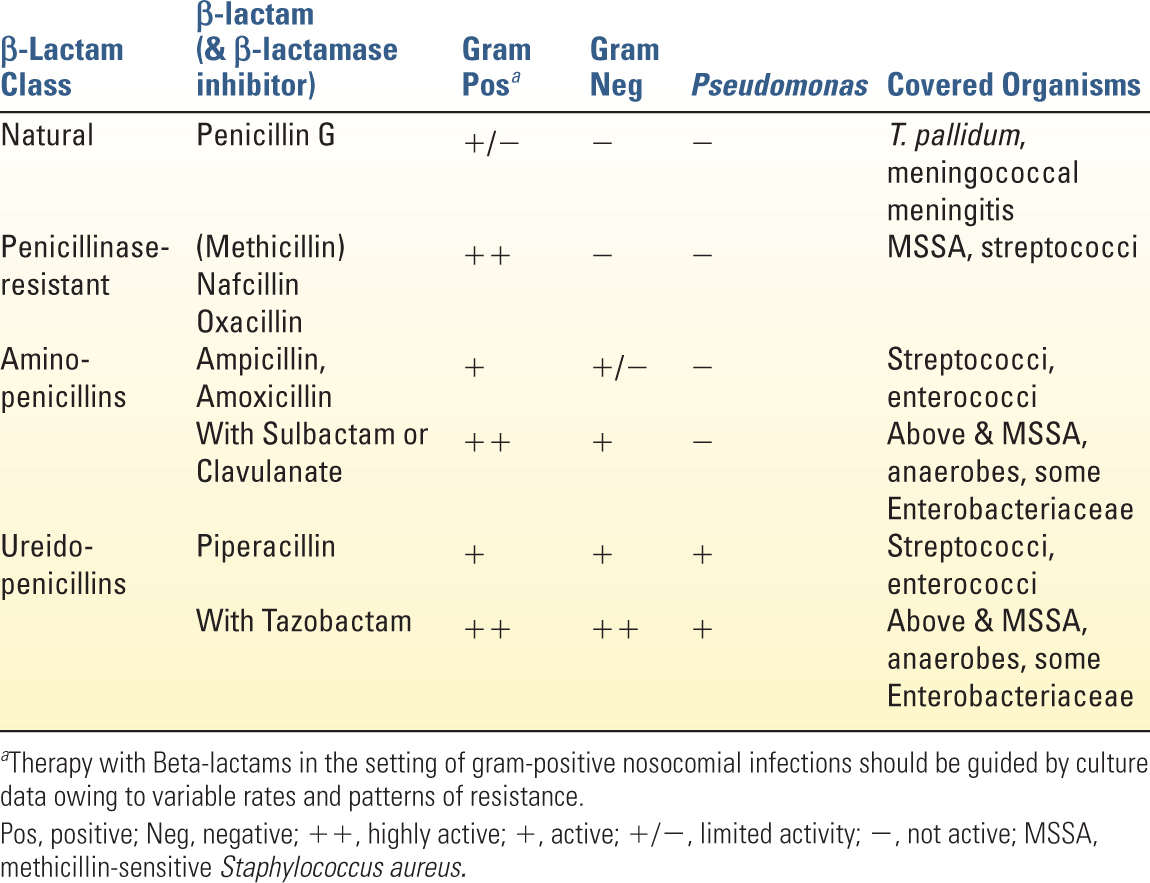
1. The numerous penicillins currently used in clinical practice are reviewed in Table 12.4. They are often used in combination with β-lactamase inhibitors to overcome microbial resistance.
a. Spectrum: While the penicillins possess a relatively broad spectrum of microbial coverage, patterns of resistance mandate sensitivity-directed treatment when considering their use for nosocomial infections. Nafcillin or oxacillin are used to treat methicillin-sensitive S. aureus (MSSA). The combination of ampicillin/sulbactam (Unasyn) offers broad coverage of gram-positives, Enterobacteriaceae, and anaerobes in the setting of maxillofacial pathology. Piperacillin in combination with tazobactam (Zosyn) offers the broadest spectrum of parenteral penicillin/β-lactamase combinations and is the most widely used penicillin in the ICU. As a part of empiric treatment regimens, it offers broad gram-positive and gram-negative coverage with efficacy against B. fragilis and (to a diminishing extent as outlined in Fig. 12.1) Pseudomonas.
b. Resistance is a major hurtle that limits the clinical utility of many penicillins. Methicillin-resistant S. aureus (MRSA), and other gram-positive organisms, expresses modified penicillin binding proteins (PBPs) with low affinity for β-lactams. Many gram-negative organisms produce inducible β-lactamases that are able to render many β-lactam compounds ineffective. While the development of β-lactamase inhibitors has extended the utility of the penicillins, resistance continues to develop. For example, piperacillin-tazobactam is vulnerable to extended-spectrum β-lactamases (ESBLs), which are not inhibited by tazobactam.
c. Considerations
1. Hypersensitivity is the most common adverse reaction following penicillin administration and can range in severity from a rash to angioedema and anaphylaxis. Patients who are candidates for penicillin therapy but report a history of allergy should be questioned to elucidate the details of the adverse reaction. Skin testing for penicillin allergy is unreliable. Accordingly, desensitization via escalating doses may be considered when the benefits to treatment outweigh the possible risks in patients with a true history of severe hypersensitivity. These protocols should be conducted by specialists in an ICU setting with the assumption that fulminant anaphylaxis could develop.
2. Intravenous administration of all penicillins has been associated with phlebitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree