HEMATOLOGIC ABNORMALITIES, COAGULOPATHY, AND TRANSFUSION THERAPY
HEMATOLOGIC ABNORMALITIES
Red Blood Cell Disorders
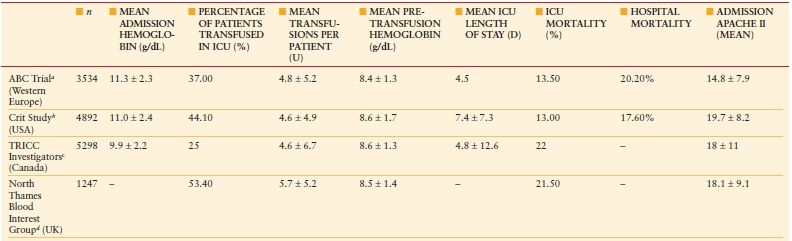
Anemia Anemia is common in critically ill and injured patients admitted to the intensive care unit (ICU), with >90% of patients anemic on ICU day 3.1 Anemia in the ICU is associated with appreciable red blood cell (RBC) transfusion use (Table 10.1).–29 Furthermore, anemia persists after ICU discharge, with >50% of patients still anemic 6 months after ICU discharge.–1012
TABLE 10.1
RESULTS OF EPIDEMIOLOGIC STUDIES ON ANEMIA AND BLOOD TRANSFUSION IN CRITICAL CARE
a Vincent JL, Baron J-F, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002; 288:1499–1507.
b Corwin HL, Gettinger A, Pearl RG, et al. The CRIT study. Anemia and blood transfusion in the critically ill: current clinical practice in the United States. Crit Care Med. 2004; 32:39–52.
c Hebert PC, Wells G, Martin C, et al. A Canadian survey of transfusion practices in critically ill patients. Crit Care Med. 1998; 26:482–487.
d Rao MP, Boralessa H, Morgan C, Soni N, Goldhill DR, Brett SJ, Boralessa H, Contreras M: Blood component use in critically ill patients. Anaesthesia. 2002, 57:530–534
Data are expressed as mean ± standard deviation. ABC, Anemia and Blood Transfusion in Critical Care; APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; TRICC, Transfusion Requirements in Critical Care.
From: Napolitano LM. Scope of the problem: epidemiology of anemia and use of blood transfusions in critical care. Critical Care. 2004; 8(suppl 2):S1–S8.
The World Health Organization defines anemia as Hb concentration <12 g/dL in women and <13 g/dL in men. Etiologies of anemia in the ICU include blood loss related to daily phlebotomy, hemodilution due to crystalloid fluid resuscitation, renal replacement therapies, renal disease, hemorrhage, occult blood loss from the gastrointestinal tract, bone marrow suppression due to diseases, drug-induced anemia, and nutritional deficiencies such as iron, folate, and vitamin B12 deficiency. In ICU patients with anemia and chronic kidney disease, treatment with erythropoietin-stimulating agents (ESAs) is indicated, with target hemoglobin concentrations no higher than 9 g/dL. Adjunctive iron treatment should be strongly considered, as optimal response to ESAs requires supplemental iron.
In most ICU patients, the etiology of anemia is “anemia of inflammation” or “anemia of chronic disease.” Anemia of inflammation develops via three mechanisms: (1) impaired iron regulation, (2) shortened RBC life span, and (3) reduced rate of erythropoiesis related to inappropriate erythropoietin response. Hepcidin is the main iron regulatory hormone, made primarily in hepatocytes, and causes functional iron deficiency, hypoferremia, and iron-restricted erythropoiesis despite normal iron stores by blocking enteral iron absorption and shuttling iron into macrophages where it is unavailable for erythropoiesis (Fig. 10.1). Hepcidin concentrations are high in anemia of inflammation and anemia of chronic disease (ACD).13
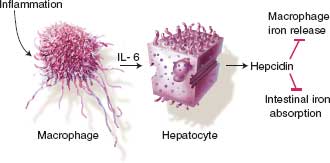
FIGURE 10.1. Hepcidin and the anemia of critical illness. Regulation of hepcidin production in inflammation. Inflammation leads to macrophage elaboration of IL–6, which acts on hepatocytes to induce hepcidin production. Hepcidin inhibits macrophage iron release and intestinal iron absorption, leading to hypoferremia.
ANEMIA: CLINICAL APPROACH TO DIAGNOSTIC EVALUATION
All ICU patients with anemia should undergo a diagnostic evaluation (Algorithm. 10.1) as for any patient with anemia. Determination of the causative factors for anemia will allow appropriate anemia management, which will aid in avoiding RBC transfusion solely for the treatment of anemia.
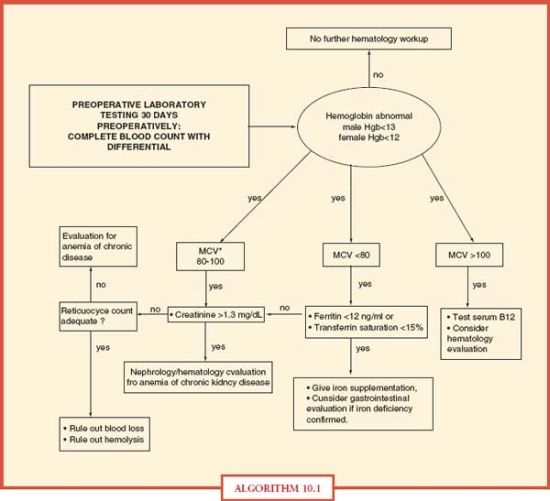
ALGORITHM 10.1 Diagnostic evaluation of anemia in acute care surgery patients. From: Goodnough LT, Shander A, et al. “Detection, evaluation, and management of anemia in the elective surgical patient.” Anesth Analg. 2005; 101(6):1858–1861.
ANEMIA AND DIAGNOSIS OF IRON DEFICIENCY IN THE INTENSIVE CARE UNIT
Iron deficiency is difficult to diagnose in intensive care unit (ICU) patients, since most will have high ferritin levels related to inflammation. In these patients, one should check blood zinc protoporphyrin concentration, which will be high in iron deficiency. In the presence of inflammation, true iron deficiency is defined by a ferritin < 100 ng/mL and a TSAT < 20%, whereas functional iron deficiency is defined by ferritin > 100 ng/mL and a TSAT < 20% (Algorithm 10.2).
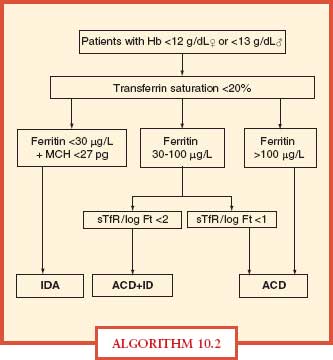
ALGORITHM 10.2 A simplified algorithm for the diagnosis of iron deficiency anemia. ACD, anemia of chronic disease; Hb, hemoglobin; ID, iron deficiency; IDA, iron deficiency anemia; MCH, mean corpuscular hemoglobin; sTfR, serum transferring receptor. From: Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism, part II: iron deficiency and iron overload. J Clin Pathol. 2011; 64:287–296.
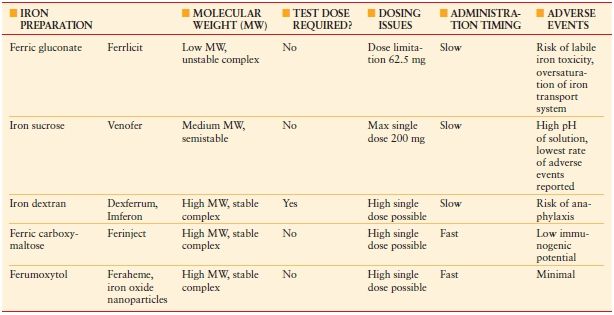
Total iron deficit (TID) can be calculated using the Ganzoni formula: TID (mg) = weight (kg) × (ideal Hb – actual Hb) (g/dL) × 0.24 + depot iron (500 mg). According to this formula, a person weighing 70 kg with a Hb concentration of 9 g/dL would have a body iron deficit of about 1,400 mg. Following the administration of oral iron, it takes 2–2.5 weeks for the Hb to start rising, 2 months for it to return to normal levels, and 6 months for iron stores to be replete. In anemia of inflammation or ACD, enteral iron absorption is problematic due to high hepcidin concentrations, which block enteral iron absorption, and intravenous (IV) iron should be considered. A number of safe IV iron formulations are now available for use (Table 10.2).
TABLE 10.2
INTRAVENOUS (IV) IRON PREPARATIONS AVAILABLE FOR USE
Polycythemia Polycythemia is not common in critical care and is defined as Hgb > 18.5 g/dL (men) > 16.5 g/dL (women) or Hgb > 17 g/dL (men) > 15 g/dL (women) if associated with a sustained increase of >2 g/dL from baseline that cannot be attributed to correction of iron deficiency. The most common cause of polycythemia is hypoxia secondary to pulmonary disease. A low-serum erythropoietin concentration in the polycythemic patient suggests the diagnosis of polycythemia vera. Management of polycythemia is control of the underlying cause (i.e., removal of an erythropoietin-secreting tumor) and limited phlebotomy as indicated if symptoms are present.
Platelet Disorders
Platelet disorders are common in the ICU. Thromboctyopenia occurs often in critical illness, perhaps in up to 41% of patients.14 Thrombocytosis and functional platelet disorders are less common and are found in up to 25% of ICU patients. Systematic evaluation of platelet disorders in critical care is essential to accurate identification and management of the cause. Importantly, thrombocytopenia has been associated with adverse outcomes. In contrast, thrombocytosis has been associated with improved outcomes in the ICU.
Thrombocytopenia Thrombocytopenia is defined as a platelet count of <150,000/mm3 or <150 × 109/L. The normal range for platelet count in adult humans is 150–450 × 109/L. Thrombocytopenia may result from decreased production or increased destruction of platelets. A patient is at risk for spontaneous bleeding when the platelet count fall below 20,000 and may warrant platelet transfusion.
The reported incidence of thrombocytopenia in the critical care setting varies from 23% to 41% and is associated with mortality rates between 38% and 54% in retrospective studies.–1522 Although the incidence of severe thrombocytopenia (platelet counts lower than 50 × 109/L) is lower (10%–17%), the association with adverse outcomes is even stronger. Sepsis and hemodilution are common etiologies of thrombocytopenia in critical illness, but heparin-induced thrombocytopenia (HIT) is one potential etiology that warrants serious consideration in all patients.
DIAGNOSTIC EVALUATION OF THROMBOCYTOPENIA IN THE INTENSIVE CARE UNIT
Systematic evaluation of the numerous potential etiologies of thrombocytopenia in critical care is essential to accurate identification and management of the cause (Table 10.3). Sepsis is the most common etiology of thrombocytopenia in critical illness, accounting for 48% of cases of thrombocytopenia.23 However, >25% of intensive care unit (ICU) patients have more than one cause of thrombocytopenia.8 Drug-induced thrombocytopenias present diagnostic challenges, because many of the multiple medications administered to ICU patients may be the cause.24 One such commonly administered drug is heparin—the most common cause of drug-induced thrombocytopenia due to immune mechanisms.
TABLE 10.3
POTENTIAL ETIOLOGIES OF THROMBOCYTOPENIA
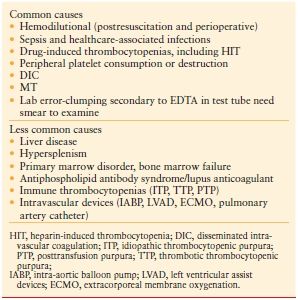
The three most important causes of thrombocytopenia in the ICU are drug-induced thrombocytopenia, HIT, and disseminated intravascular coagulation (DIC). A number of other etiologies of thrombocytopenia may occur in ICU patients. These include autoimmune or alloimmune thrombocytopenia (ITP), posttransfusion purpura, the thrombotic microangiopathies [thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS)], and the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. TTP most commonly presents with a pentad of thrombocytopenia (with purpura), RBC fragmentation, renal failure, neurologic dysfunction, and fever.
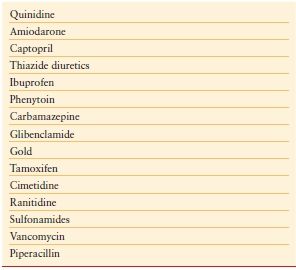
Drugs can induce thrombocytopenia by a number of mechanisms. In addition to those that are directly cytotoxic, thiazide diuretics, interferon, and alcohol can cause thrombocytopenia by inhibiting platelet production in the bone marrow. More commonly, drug-induced thrombocytopenia results from the immunologic destruction of platelets. Drugs can induce antibodies to platelets either by acting as a hapten or by functioning as an innocent bystander. Drugs such as gold salts and interferon can induce an ITP-like disorder. Some common ICU drugs that are associated with thrombocytopenia are detailed in Table 10.4.
TABLE 10.4
COMMON DRUGS THAT CAN INDUCE THROMBOCYTOPENIA
HEPARIN-INDUCED THROMBOCYTOPENIA
Heparin-induced thrombocytopenia (HIT) is an anticoagulant-induced prothrombotic disorder. HIT is an immune-mediated adverse drug effect characterized by platelet activation, hypercoagulability, and increased risk of thrombosis, both venous and arterial. HIT is caused by platelet-activating heparin-dependent antibodies of immunoglobulin G class. HIT should be considered when the platelet count falls to <150 × 109/L (or by >50% from baseline) between days 5 and 14 of exposure to any heparinoid product. Rapid-onset HIT can occur if heparin is given to a patient who already has circulating HIT antibiotics, usually due to heparin given in the last 5–100 days. Thrombocytopenia in HIT is usually moderate – mean platelet count 60 × 109/L – and recovers within a few days of heparin discontinuation. Some patients will develop HITT—HIT and thrombosis (venous or arterial) with associated high rates of limb loss and mortality, ranging from 10% to 20%. A high index of suspicion is required for early recognition of HIT. A clinical scoring system is used to identify patients with HIT, called the “4 Ts” (Table 10.5), with a pretest probability score of 6–8 indicating high risk for HIT.
TABLE 10.5
A CLINICAL SCORING SYSTEM USED TO DIAGNOSE HIT: THE “FOUR T’S”
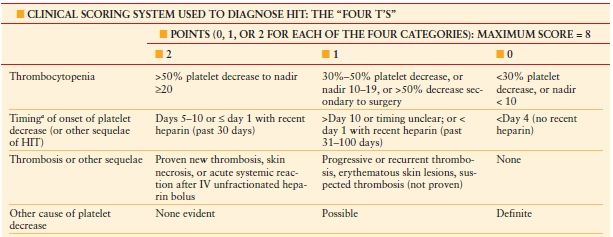
a First day of immunizing heparin exposure considered day 0. Pretest probability score: 6–8 indicates high; 4–5, intermediate; and 0–3, low.
Adapted from Warkentin TE. Heparin-induced thrombocytopenia: diagnosis and management. Circulation. 2004; 110:e454–e458.
Once HIT is strongly suspected in a critically ill patient, prompt discontinuation of all heparin and administration of an alternative nonheparin anticoagulant (commonly a direct thrombin inhibitor, DTI, Table 10.6) should be initiated. The principles of treatment for suspected or confirmed HIT include the “Six A’s” (Table 10.7).25 Do not wait for the laboratory confirmation of HIT prior to initiation of a DTI—this is associated with increased risk for thrombosis and adverse outcome. When evaluating a patient for HIT, one must also consider a number of other potential etiologies of thrombocytopenia (Table 10.8).26
TABLE 10.6
NONHEPARIN ANTICOAGULANTS FOR HIT TREATMENT
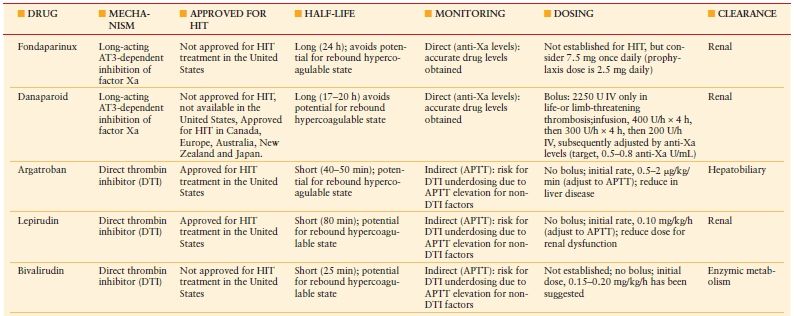
APTT, activated partial thromboplastin time.
Adapted from: Warkentin TE, Greinacher A, Koster A, et al. Treatment and prevention of heparin-induced thrombocytopenia. American College of Chest Physicians evidence-based clinical practice guidelines. 8th ed. Chest. 2008; 133(suppl 6):340S–380S.
TABLE 10.7
THE PRINCIPLES OF TREATMENT FOR SUSPECTED OR CONFIRMED HIT: THE “SIX A’S”
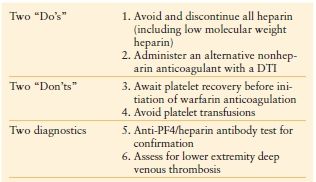
Adapted from: Napolitano LM, Warkentin TE, AlMahameed A, Nasrawy SA. Heparin-induced thrombocytopenia in the critical care setting: diagnosis and management. Crit Care Med. 2006; 34:2898–2911.
TABLE 10.8
COMPARISON OF SELECTED THROMBOCYTOPENIC DISORDERS THAT SHOULD BE CONSIDERED WHEN EVALUATING A PATIENT FOR HEPARIN-INDUCED THROMBOCYTOPENIA (HIT)
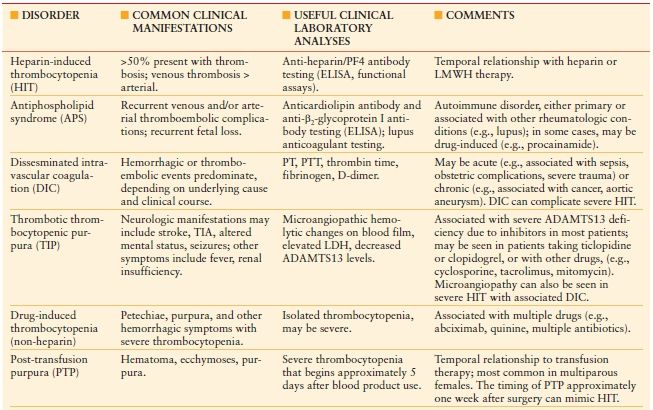
LMWH indicates low molecular weight heparin; DIC, disseminated intravascular coagulation; ELISA, enzyme-linked immunosorbent assay; TIA, transient ischemic attack; PT, prothrombin time; PTT, partial thromboplastin time; LDH, lactate dehydrogenase; ADAMTS13, a disintegrin and metalloproteinase with thrombospondin components–13.
Common clinical manifestations focus on thrombotic versus hemorrhagic symptoms. Comments include relationships to other disorders and/or drugs that need to be considered. HIT has also been reported to occur concomitantly in patients with antiphospholipid syndrome or DIC
From: Ortel TL. Heparin-induced thrombocytopenia: when a low platelet count is a mandate for anticoagulation. Hematology Am Soc Hematol Educ Program. 2009:225–232.
HIT diagnosis is confirmed with either a positive anti-PF4/polyanion-IgG enzyme immunoassay or a positive platelet activation assay (i.e., serotonin-release assay). Once platelet count has normalized, warfarin should be initiated with a low maintenance dose (specifically, no loading dose) and overlapped with the nonheparin anticoagulant until the target international normalized ratio has been reached and for a minimum of 5 days. The duration of warfarin therapy should be standard for venous thromboembolism (VTE), for a minimum of 6 months with consideration for a more extended course depending on clinical circumstances related to the thromboembolic event.
Thrombocytopenia Treatment In the bleeding patient, platelet transfusion is most commonly required. HIT is treated with heparin discontinuation and substitution of an alternative anticoagulant (DTI). Plasma exchange with fresh frozen plasma is the most effective treatment for TTP. HUS is best treated with supportive therapy including dialysis. Corticosteroids and splenectomy are the mainstays of therapy for ITP.
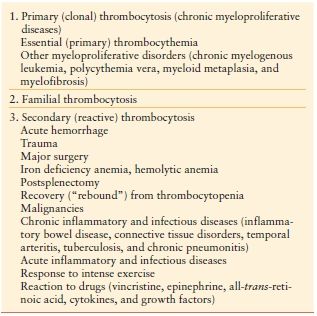
Thrombocytosis Thrombocytosis is defined as a platelet count above the normal value, in general, > 400–600,000/mm3 or > 400–600 × 109/L (normal in adults—150–450 × 109/L). Thrombocytosis is classified into primary and secondary forms. Primary (Clonal) thrombocytosis is due to clonal thrombopoiesis and most often occurs in chronic myeloproliferative or in some myelodysplastic disorders. Secondary (Reactive) thrombocytosis is the most common form of thrombocytosis and is due to a variety of underlying conditions involving an acute phase reaction. These include trauma, surgery, bleeding, malignancy, infection, and inflammatory diseases.–2729 (Table 10.9) Thrombocytosis is common (up to 25% of ICU patients) in the critically ill and is associated with improved outcome.
Thrombocytosis Treatment The greatest challenge in formulating a treatment plan for ICU patients is to correctly diagnose the cause of thrombocytosis. Treatment for patients with reactive thrombocytosis should be directed at the underlying disease. The abnormal platelet count itself does not increase the risk for thrombotic complications. Therefore, antiplatelet or platelet-lowering therapy is not indicated. In contrast, clonal thrombocytosis often needs treatment to reduce platelet counts, especially for high risk patients.30 This group includes those with a history of bleeding or thrombotic complications, age > 60 years, other cardiovascular risk factors or extremely high platelet counts (>1,500 × 109/L).31 Some of the agents employed to decrease platelet counts include hyroxyurea, interferon-α, and anagrelide. Low-dose aspirin (40–325 mg) has been used to reduce the risk of thrombosis in patients with platelet counts < 1,500 × 109/L.32 Platelet apheresis and phlebotomy have been used to decrease platelet counts rapidly in patients with life-threatening complications from thrombocytosis. An algorithmic approach to the management of thrombocytosis is depicted in Algorithm 10.3.33
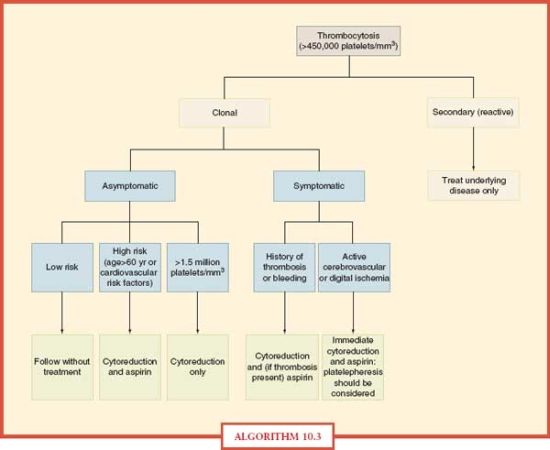
ALGORITHM 10.3 Approach to the management of thrombocytosis. From: Schafer AI. Thrombocytosis: Current Concepts [Review]. N Engl J Med. 2004; 350:1211–1219.
COAGULOPATHY
Hypercoagulable States
Hypercoagulable States (Thrombophilia) Hypercoagulable states should be considered in ICU patients who develop venous or arterial thrombosis. Hypercoagulable states are classified as inherited or acquired. Acquired thrombophilia is related to risk factors or predisposing conditions for thrombosis, including surgery, trauma, malignancy, central venous catheter, use of oral contraceptives, and others. Inherited thrombophilia is a genetic tendency to VTE. Factor V Leiden is the most common cause of inherited thrombophilia, accounting for 40%–50% of cases. The prothrombin 20210A gene mutation and deficiencies in protein S, protein C, or antithrombin account for most of the remaining cases of inherited thrombophilia. Patients with possible hypercoagulable states should undergo diagnostic testing for thrombophilia with a comprehensive panel of diagnostic tests (Table 10.10). Prior to the initiation of diagnostic testing for hypercoagulable states, it is important to assess whether the patient is on medication or has preexisting clinical conditions that may confound interpretation of laboratory tests for evaluation of thrombophilia (Table 10.11).34
TABLE 10.10
DIAGNOSTIC TESTING FOR HYPERCOAGULABLE STATES
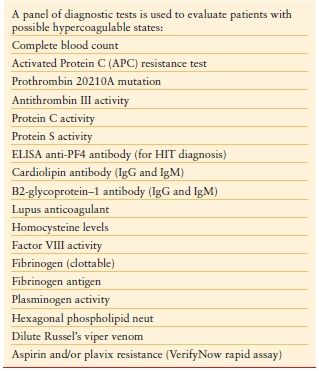
TABLE 10.11
MEDICATIONS AND CLINICAL CONDITIONS THAT CONFOUND INTERPRETATION OF THROMBOPHILIA LABORATORY TESTING
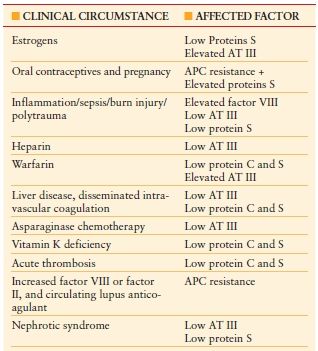
Abbreviations: APC, activated protein C; AT III, antithrombin III.
Prior to diagnostic testing for hypercoagulable states, it is important to assess whether the patient is on medications that may confound interpretation of these laboratory tests.
From: Houbballah R, LaMuraglia GM. Clotting problems: diagnosis and management of underlying coagulopathies. Semin Vasc Surg. 2010; 23(4):221–227.
Treatment of Hypercoagulable States Initial treatment of venous or arterial thrombosis in an ICU patient is systemic anticoagulation. Antithrombin, protein C, or protein S deficiency carry VTE recurrence rates of 5%–15% per year, a relative increase of 2.5 compared with rates in the absence of thrombophilia. Patients heterozygous for factor V Leiden or the prothrombin G20210A mutation are much less prone to VTE recurrence with a relative risk of 1.3–1.4; VTE recurrence rates are fivefold greater with homozygosity or double heterozygosity. A mild/moderate increase in homocysteine level is associated with a 2.5 increased risk for recurrent VTE. Long-term anticoagulant therapy is recommended in selected high-risk hypercoagulable states after a first spontaneous VTE episode in antithrombin, protein C or protein S deficiency, and for patients homozygous or doubly heterozygous for factor V Leiden and/or the prothrombin mutation. For patients with lower risk for recurrence, they are treated with anticoagulation for 6 months, and then imaging for residual thrombosis or D-dimer testing is performed at the end of anticoagulant therapy, and the results are used to determine likelihood of VTE recurrence.
Coagulopathies ICU patients with coagulation defects have a four- to fivefold increased risk for bleeding compared to patients with a normal coagulation status. Diagnostic testing for coagulopathy in critically ill patients requires a panel of comprehensive tests including prothrombin time (PT), activated partial thromboplastin time, thrombin time (TT), fibrinogen, fibrin degradation products, platelet count, and bleeding time (Table 10.12).35
TABLE 10.12
COMPARISON OF HEMOSTATIC TESTING FOUND WITH COMMON MEDICATIONS AND DISEASE STATES IN CRITICALLY ILL PATIENTS
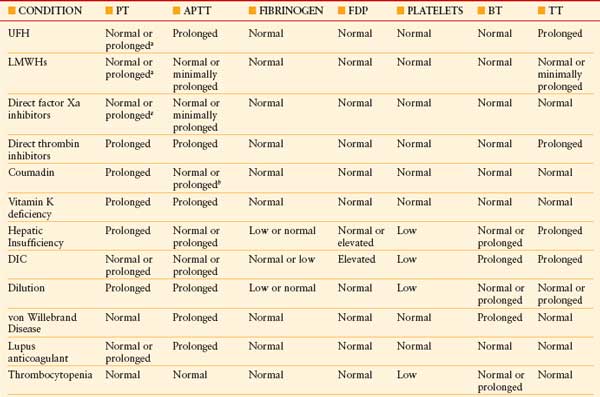
BT, bleeding time; DIC, disseminated intravascular coagulation; FDP, fibrin degradation product; LMWH, low-molecular-weight heparin; TT, thrombin time; UFH, unfractionated heparin. See Table 10.2 for expansion of other abbreviations.
a At supratherapeutic dosages.
b Early in coumadin treatment. From: Wheeler AP, Rice TW. Coagulopathy in critically ill patients, part 2: soluble clotting factors and hemostatic testing. Chest. 2010; 137(1):185–194.
DIC is a syndrome caused by systemic intravascular activation of coagulation with formation of microvascular thrombi with clotting factor consumption and occurs in patients with infection, malignancy, trauma, amniotic fluid embolism, and others. DIC manifests as prolonged plasma clotting times, thrombocytopenia, reduced plasma fibrinogen concentration, raised plasma fibrin-degradation products, and sometimes microangiopathic hemolysis (Algorithm. 10.4). The most frequent cause of DIC is sepsis, with reduced protein C concentrations resulting in decreased endogenous fibrinolysis. ICU patients with severe sepsis or septic shock may manifest abnormalities in coagulation testing, but plasma should not be transfused unless the patient has evidence of clinical bleeding (Surviving Sepsis Guidelines, 2008).36 In cases of DIC, where thrombosis predominates, such as arterial or VTE severe purpura fulminans associated with acral ischemia or vascular skin infarction, therapeutic doses of heparin should be considered. Patients with sepsis-induced DIC may progress to purpura fulminans, a rapidly progressive thrombotic disorder with hemorrhagic infarction of the skin and dermal vascular thrombosis. Protein C concentrations should be measured in patients with purpura fulminans. In patients with severe limb-threatening purpura fulminans and low-protein C levels, replacement with exogenous protein C should be considered. Meningococcal and pneumococcal sepses are common causes of purpura fulminans and DIC. Treatment of DIC is supportive with aggressive treatment of the underlying cause of the DIC. Treatment of bleeding in DIC requires blood component replacement therapy with plasma, platelets, and cryoprecipitate.37
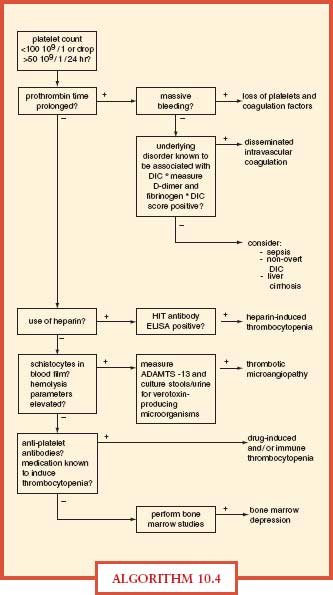
ALGORITHM 10.4 Differential diagnostic algorithm for coagulation abnormalities in the ICU. DIC, disseminated intravascular coagulation; ELISA, enzyme-linked immunosorbent assay; HIT, heparin-induced thrombocytopenia. From: Levi M, Opam SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006; 10(4):222.
Acute Traumatic Coagulopathy and Hemostatic Resuscitation
Hemorrhage is a major cause of trauma deaths and coagulopathy exacerbates hemorrhage. Trauma patients with severe hemorrhage have early hyperfibrinolysis in addition to coagulopathy (dilutional and consumptive).–3840 Uncontrolled hemorrhage in trauma leads to the lethal triad of acidosis, hypothermia, and coagulopathy. Most severely injured patients are coagulopathic at hospital admission, before resuscitation interventions (Algorithm. 10.5).
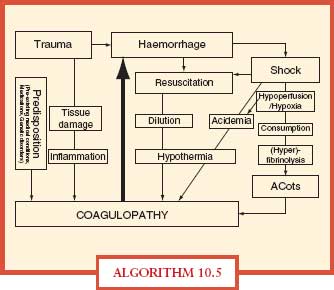
ALGORITHM 10.5 Acute coagulopathy of trauma. The main contributing factors and potential mechanisms implicated in the acute coagulopathy of trauma. Hemorrhage in the severely injured patient may lead to shock that causes acidemia and hypothermia further triggering coagulopathy, typically termed the “lethal triad.” Injudicious fluid resuscitation is closely linked to dilutional coagulopathy and hypothermia. Trauma in combination with shock causing hypoperfusion and hypoxia can also cause the “Acute Coagulopathy of Trauma-Shock” (ACoTS) associated with anticoagulation and hyperfibrinolysis. The clinical significance of inflammation for the development of acute traumatic coagulopathy still has to be fully elucidated. From Wafaisade A, Wutzler S, Lefering R, et al. Trauma Registry of DGU. Drivers of acute coagulopathy after severe trauma: a multivariate analysis of 1987 patients. Emerg Med J. doi:10.1136/emj.2009.088484 and Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008; 65:748–54.
Prompt reversal of coagulopathy using “hemostatic resuscitation” with early use of blood component therapy is advocated as the optimal practice for patients requiring massive transfusion (MT). It is recognized that increased early use of plasma is associated with increased risk for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS), but is associated with decreased mortality. An emerging consensus for hemostatic resuscitation in patients requiring MT is as follows:
- Expedite the control of hemorrhage to prevent consumptive coagulopathy and thrombocytopenia and reduce the need for blood products.
- Limit isotonic crystalloid infusion to prevent dilutional coagulopathy and thrombocytopenia.
- Hypotensive resuscitation (SBP, 80–100 mm Hg) until definitive hemorrhage control is established
- Transfuse blood products in a 1:1:1 ratio of RBCs/FFP/platelets (one five-pack of pooled platelets counted as 5 U).
- Frequent laboratory monitoring (arterial lactate to assess adequacy of resuscitation, ionized calcium, and electrolytes)
TRANSFUSION THERAPY
Indications for Transfusion of Blood Components in the Intensive Care Unit
Red Blood Cell Transfusion RBC transfusion is common in intensive care unit (ICU) patients, with 40%–50% receiving RBC transfusion during the ICU stay. The Transfusion Requirements in Critical Care (TRICC) trial confirmed that ICU patient 30-day mortality was not different in patients randomized to a restrictive (transfuse if hemoglobin < 7 g/dL) or liberal (transfuse if hemoglobin < 10 g/dL) transfusion strategy.41 Guidelines for RBC transfusion in ICU patients recommend transfusion when hemoglobin < 7 g/dL in most ICU patients (see Guideline Executive Summary, Table 10.13). In ICU patients with acute cardiac ischemia, the recommendation is to transfuse RBCs if hemoglobin < 8 g/dL.42
TABLE 10.13
EXECUTIVE SUMMARY FROM CLINICAL PRACTICE GUIDELINE: RBC TRANSFUSION IN ADULT TRAUMA AND CRITICAL CARE


From: Napolitano LM, Kurek S, Luchette FA, et al; American College of Critical Care Medicine of the Society of Critical Care Medicine; Eastern Association for the Surgery of Trauma Practice Management Workgroup. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37(12):3124–3157.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree