REGIONAL ANESTHESIA
Neuraxial Blocks for Regional Anesthesia Claims
A total of 443 claims associated with nonobstetric neuraxial (epidural/spinal blockade) anesthesia in the surgical setting were identified in the ASA Closed Claims Database from 1990 or later out of a total of 652 nonobstetric regional anesthesia claims (excluding acute and chronic pain claims). Mean age in the nonobstetric neuraxial anesthesia group was 57 years (range, 0.25–94), and approximately half (52%) of patients were ASA physical status 1 to 2 and half (48%) were ASA physical status 3 to 4. No patients with an ASA physical status 5 were present in this dataset. Neuraxial anesthetic claims were evenly split between male and female gender (51% vs. 49%) and one-third (31%) of patients were considered obese. The type of anesthetic technique with these claims included 45% subarachnoid blocks, 45% lumbar epidural blocks, 1% caudal epidural blocks, 5% thoracic epidural blocks, and 2% combined subarachnoid/epidural blocks. Although temporary injury accounted for the largest proportion of neuraxial anesthetic claims (45%), over one-third of claims were associated with high severity injuries (death or permanent brain damage, 37%) and 16% of claims with permanent nerve injury (Fig. 36-1).
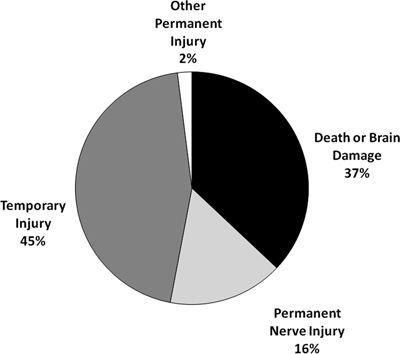
FIGURE 36-1. Outcomes in neuraxial regional anesthetics (n = 443). Neuraxial blocks for obstetrics and acute and chronic pain were excluded from this analysis.
Block-related mechanisms of injury were associated with 41% of all neuraxial anesthesia claims with the remaining 59% of claims including (in decreasing percentages of claims) no event (9%), other anesthetic events (6%), cardiovascular events (14%), respiratory events (9%), unknown events (4%), surgical events or patient condition (8%), medication problems (4%), and equipment problems (7%). Block technique (50%, n = 88), neuraxial cardiac arrest (21%, n = 37), dural puncture (9%, n = 17), and high spinal/epidural block (8%, n = 14) were the most common causes of block-related injury and accounted for 87% of claims in this category. Block technique referred to a complication thought to be caused by the technical performance of the block and most commonly was associated with needle or catheter damage to nerves or surrounding structures.
Block-related mechanisms of injury accounted for more than one quarter (n = 48, 29%) of the high severity claims associated with death or brain damage (n = 163). Neuraxial cardiac arrest (n = 34, 21%) and high spinal/epidural block (n = 11, 7%) were the most common block-related causes of death or brain damage (Fig. 36-2). Non–block-related mechanisms of injury for the high severity claims associated with death or brain damage were most commonly associated with cardiovascular events (e.g., air/fat pulmonary emboli, stroke, hypotension, and myocardial infarction, 33%) and respiratory events (e.g., inadequate ventilation, airway obstruction, bronchospasm, and aspiration, 20%). Other events including surgical events or patient condition, wrong drug or dose, unknown events, multiple events, and no event accounted for the remainder of non–block-related events.
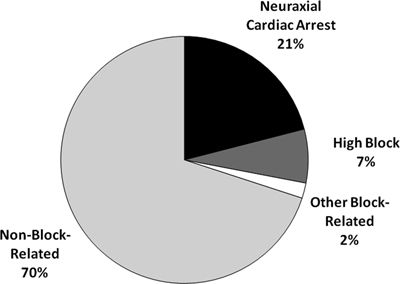
FIGURE 36-2. Mechanism of injury for neuraxial claims associated with death or brain damage (n = 163).
Neuraxial Cardiac Arrest in Regional Anesthesia Claims
Neuraxial cardiac arrest (Box 36-1) accounted for 21% of all block-related causes of injury, and it was associated with the largest proportion of neuraxial anesthesia claims with death or brain damage (n = 34). Outcomes with neuraxial cardiac arrest in the ASA Closed Claims Database are predominately high severity with >90% resulting in death or brain damage. Mean age in these claims was 54 years, and 30% were ASA physical status 1 to 2, 54% were ASA physical status 3, and 8% were ASA physical status 4.
BOX 36-1 Neuraxial Cardiac Arrest
1. Risk factors:
 Mid- to upper thoracic block
Mid- to upper thoracic block
 Baseline bradycardia
Baseline bradycardia
 Male gender
Male gender
2. Occurs with spinal or epidural blockade at any time during the operation.
3. May be preceded by sudden bradycardia or may present as sudden asystole.
4. Neuraxial block patients must be monitored with EKG and pulse oximetry.
5. Vigilance should be heightened during the prone position or during positioning changes on the table.
6. Resuscitation drugs and airway equipment must be immediately available throughout case.
Neuraxial cardiac arrest claims were examined for associated factors as both capnography and pulse oximetry were widely available from 1990 onward (Table 36-1). Two-thirds occurred following subarachnoid block and 11% were after epidural anesthesia with accidental subarachnoid block (Table 36-1). Sedation was frequently utilized in these cases (76%) from the 1990s, and it has previously been implicated with neuraxial cardiac arrest with inadequate oxygenation and/or ventilation.15 However, hypoxia and inadequate ventilation were not noted to precede these arrests as 81% of claims were associated with the use of pulse oximetry and 32% with capnography. Cardiac arrest was frequently identified by the presence of cyanosis, but there is little evidence to suggest that inadequate oxygenation and/or ventilation is a common event preceding neuraxial cardiac arrest.16,17 Monitoring with electrocardiogram and pulse oximetry is useful for providing immediate warning of bradycardia that may precede asystole in neuraxial cardiac arrest.
TABLE 36.1 Associated Factors for 1990s Neuraxial Cardiac Arrest Claims (n = 37) in Regional Anesthesia Claims
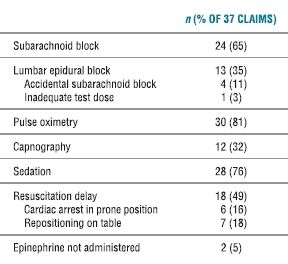
Unfortunately, half of these claims (49%) were associated with delays in resuscitation and were caused by delay in recognition of the event and/or delay in administration of appropriate resuscitation drugs. Six cases were in the prone position when cardiac arrest developed. Seven cases were in the midst of a position change on the OR table or moving between the OR table and the patient gurney when cardiac arrest developed. It is unclear if these position changes worsened a precarious physiologic state, or if they caused a delay in recognition of the cardiac arrest because of distraction or missing monitors, or delay in treatment for returning to the supine position. Anesthesiologists must be vigilant for this complication and promptly utilize resuscitative maneuvers if monitors are to be effective at prevention.
However, monitoring may not be as useful at preventing neuraxial cardiac arrest when there is a rapid and sudden onset of bradycardia/asystole that may not allow adequate time for treatment before cardiac arrest ensues.16,17 Despite appropriate and timely resuscitative efforts, 17 of 19 claims resulted in death or brain damage. These poor outcomes may be explained by Rosenberg et al.18 study that demonstrated that cardiac arrest in dogs during total spinal anesthesia is difficult to treat because of the presence of intense sympathetic blockade, which decreases circulating blood volume, reduces coronary perfusion pressure, and renders cardiopulmonary resuscitation (CPR) ineffective.18 Additionally, other studies from this group in dogs have demonstrated that spinal anesthesia prevents a rise in epinephrine and norepinephrine catecholamine levels during cardiac arrest compared to controls without spinal anesthesia.19 Therefore, both severe vasodilation and a defective neuroendocrine response to stress are thought to contribute to the poor outcome during neuraxial cardiac arrest. Cases of neuraxial cardiac arrest that promptly respond to therapy and result in no sequelae have been reported, but are unlikely to result in claims.16,20 Consequently, the success and failure rates of prompt resuscitation, specific resuscitation drugs, and types of monitoring cannot be determined from this database.
The two most commonly proposed mechanisms to explain neuraxial cardiac arrest are (i) low-filling of the left ventricle causing a paradoxical bradycardic response via stretch/mechanoreceptors, commonly referred to as the Bezold-Jarisch reflex, and (ii) blockade of the cardiac accelerator fibers with sympathetic blockade >T4.17 Patients with baseline bradycardia and male gender have been shown to have an increased occurrence of severe bradycardia (<40 beats per minute) under neuraxial blockade, and the bradycardic episodes are widely distributed throughout the time course of cases of variable duration.17 Monitoring of electrocardiogram and pulse oximetry should be utilized for patients with neuraxial anesthetics for the entire duration of the case, and resuscitation drugs and equipment should be immediately available.
High Spinal/Epidural Block
High spinal/epidural blocks accounted for 2% (n = 14) of all nonobstetric neuraxial anesthesia claims in 1990 or later and 8% of the 180 neuraxial anesthesia claims associated with a block-related mechanism of injury. An outcome of death or brain damage was associated with 79% (n = 11) of these high block claims. Five of these 14 claims were associated with subarachnoid blockade, and 9 claims with epidural blockade (7 lumbar, 1 thoracic, and 1 caudal). An accidental subarachnoid block was thought to be associated with all of the epidural claims. There was no significant association with a specific local anesthetic. Mean age of patients was 52 years (range, 0.25–76 years), and no difference was observed with respect to gender (7 female: 7 male). The type of surgery associated with high spinal/epidural blocks included eight lower extremity procedures, two urologic procedures, two gynecologic procedures, and two abdominal operations. CPR was utilized in six claims.
Aspiration of the epidural catheter and use of a 3 mL test dose of local anesthetic with epinephrine should be utilized with epidural anesthetics to rule out both intravascular and intrathecal injections. Failure to wait the appropriate amount of time after the test dose may lead to a falsely negative result. Injection of a test dose of local anesthetic through the epidural needle prior to catheter placement is not adequate for testing the placement of the catheter. Increased vigilance for an accidental intrathecal injection during the entire course of an epidural anesthetic is essential for prompt diagnosis and resuscitation to avoid hypoxia and/or cardiovascular collapse.
Permanent Nerve/Spinal Cord Injuries in Neuraxial Regional Anesthesia Claims
Permanent nerve injuries were associated with 16% (n = 71) of the neuraxial anesthesia claims. Of these 71 permanent nerve injuries, 77% (n = 55) were judged as block-related mechanisms of injury, 8% (n = 6) related to surgical technique, 4% (n = 3) related to patient condition, and 10% (n = 7) related to other or unknown mechanisms (Fig. 36-3). Damage to the lumbosacral nerve roots and thoracolumbar spinal cord accounted for 93% of the permanent nerve injuries. The two most common causes of block-related permanent nerve damage to the neuraxis (n = 55) were hematoma with or without block needle trauma (n = 27) and anterior spinal artery syndrome/infarct (n = 8). There were 15 neuraxial claims associated with cauda equina symptoms, of which 13 utilized lidocaine, 1 chloroprocaine, and 1 without injection of local anesthetic caused by block needle trauma. Blocks associated with permanent cauda equina injury were subarachnoid (n = 8), combined spinal-epidural (n = 2), unintentional intrathecal injection (n = 1), and lumbar epidural without evidence of intrathecal injection (n = 3).
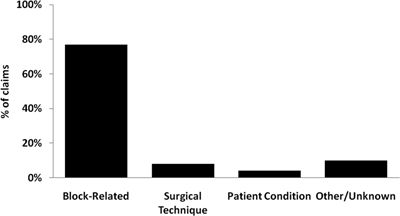
FIGURE 36-3. Permanent nerve damage in neuraxial anesthesia claims (n = 71).
Intrathecal lidocaine has been reported to cause either transient neurologic symptoms or persistent cauda equina symptoms since the early 1990s.21,22 Lidocaine has also been shown to cause dose-related neurotoxicity when infused epidurally in a rat model in concentrations as low as 2%, though a strong clinical association has not been detected.23 Recent experimental evidence indicates that low concentrations of lidocaine induce apoptosis, a form of programmed cell death in cell cultures, via the mitochondrial pathway.24 At higher concentrations, toxicity is caused by cell necrosis in cell cultures.
Neuraxial Hematoma in Neuraxial Regional Anesthesia Claims
Surgical procedures for the 27 neuraxial hematoma claims were vascular (48%, n = 13), orthopedic (33%, n = 9), and abdominopelvic (19%, n = 5; Box 36-2). Not surprisingly, the most common associated factor for epidural/spinal hematoma was the presence of either preoperative (iatrogenic or intrinsic), intraoperative, or postoperative coagulopathy in 59% (n = 16) of these claims. Other associated factors included needle trauma to the spinal cord/conus medullaris (22%, n = 6) and catheter removal on anticoagulation (15%, n = 4). Of the cases with data available, symptom onset was postoperative day 0 in 33% (n = 9), postoperative day 1 in 19% (n = 5), and later than postoperative day 1 in 19% (n = 5) of patients (eight cases without symptom onset data). There was a delay of 1 or more days from symptom onset to diagnosis/treatment in at least 41% (n = 11) of cases. The most common symptom was failure of the block to resolve in the recovery room or ward after surgery (33%, n = 9) or increased motor block (22%, n = 6). Back pain was present in only 19% (n = 5) of claims.
BOX 36-2 Neuraxial Hematoma in Regional Anesthesia Claims
1. Risk factors:
 Perioperative coagulopathy, usually iatrogenic
Perioperative coagulopathy, usually iatrogenic
 Vascular surgery
Vascular surgery
 Needle trauma at or above the L1 level
Needle trauma at or above the L1 level
2. Symptoms:
 Failure of block to resolve or increased motor block out of proportion to local anesthetic (most common)
Failure of block to resolve or increased motor block out of proportion to local anesthetic (most common)
 Increased sensory block
Increased sensory block
 Back pain (least common)
Back pain (least common)
3. Diagnosis:
4. Treatment:
 Prompt surgical evacuation
Prompt surgical evacuation
 Time interval from onset of symptoms to decompression and neurologic deficits prior to decompression predict neurologic recovery.
Time interval from onset of symptoms to decompression and neurologic deficits prior to decompression predict neurologic recovery.
a CT of the spine without myelography is not recommended for detection of spinal epidural hematoma.
Previous studies have shown that neuraxial anesthesia for vascular surgery has been associated with significant patient benefits including reduced graft thrombosis and improved graft blood flow.25–27 However, a recent review in the Cochrane Database examining four randomized controlled trials of neuraxial anesthesia compared to general anesthesia for lower limb revascularization found no significant differences between groups for mortality rate, myocardial infarction, or amputation.28 In this review, patients in the neuraxial anesthesia group did have a significantly lower incidence of pneumonia compared to the general anesthesia group. Prospective studies of complications associated with neuraxial anesthetics and vascular surgery have demonstrated a very low incidence of epidural/spinal hematoma.29–31 Consequently, regional anesthesia for vascular surgery will continue to be used, but anesthesiologists and other health care providers should have increased vigilance for neuraxial hematomas in these patients. Patients with neuraxial blocks out of proportion to the local anesthetic being utilized (especially increased motor block) should be evaluated immediately for the presence of a neuraxial hematoma. Magnetic resonance imaging (MRI) is the most useful radiologic study for detecting these lesions. Epidural hematomas may also be detected by CT myelog raphy, but CT of the spine without myelography can easily miss a spinal epidural hematoma. Neurologic recovery from epidural/spinal hematomas is thought to be partially dependent on time to decompression so prompt diagnosis and treatment are essential to a good outcome. Injuries from neuraxial hematoma caused by the wrong level of needle insertion during subarachnoid block may partially be caused by the anatomic variability among patients in the end of the spinal cord and in the alignment of the iliac crests with lumbar interspaces.32,33 Needle insertion at the most caudad suitable interspace may reduce these types of complications, particularly in obese patients where landmarks may be difficult to palpate.
Temporary Injuries in Neuraxial Regional Anesthesia Claims
Temporary injuries accounted for the largest proportion of neuraxial anesthesia claims (45%, n = 198). Nerve damage was the most common cause of temporary injury (44%) followed by back pain (17%), headache (10%), emotional distress (6%), and inadequate analgesia (3%, Fig. 36-4).
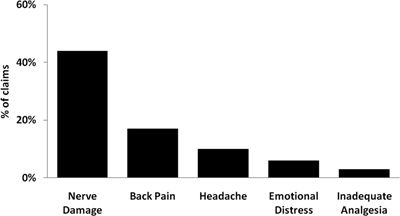
FIGURE 36-4. Temporary injuries associated with neuraxial anesthesia claims (n = 198).
Standard of care associated with nonobstetric neuraxial anesthetic claims was judged as less than appropriate in 45% of death or brain damage claims, 34% of permanent nerve injury claims, and only 18% of temporary injury claims. Payments also trended with severity of injury as death or brain damage claims had a median payment (in 2008) of $536,000 (range $3,350–$8,220,000); permanent nerve injury claims had a median payment of $457,000 ($7,000–$2,647,000); and temporary injury claims had a median payment of $63,700 (range $1,000–$1,876,000).
Peripheral Nerve Blocks in Regional Anesthesia Claims
Peripheral nerve blocks for surgical anesthesia were associated with 135 claims in 1990 or later excluding acute and chronic pain claims and accounted for 21% of all nonobstetric regional anesthesia claims (n = 652). Axillary blocks were the most common peripheral nerve block (36%) followed by interscalene blocks (30%), intravenous regional blocks (15%), ankle blocks (6%), supraclavicular blocks (4%), and miscellaneous blocks (10%). Outcome severity for peripheral nerve blocks was associated with death or brain damage in only 16% of claims, with permanent nerve damage in 13% of claims, and with temporary injury in 70% of claims.
The mechanism of injury for claims associated with death or brain damage (n = 22) was block-related in only five claims (23%), all related to intravascular injection/absorption. Non–block-related events included cardiovascular (n = 10, 45%), respiratory (n = 3, 9%), medication (n = 2, 9%), and other (n = 2, 9%). Regional techniques utilized in these claims were interscalene block (n = 10), axillary blocks (n = 6), ankle block (n = 2) intravenous regional blocks (n = 1), and miscellaneous blocks (n = 3).
Permanent nerve damage associated with peripheral nerve blocks occurred in 17 of 135 claims (13%, Table 36-2). Temporary injuries accounted for the majority of peripheral nerve block claims (n = 95) and included nerve damage (n = 46), pneumothorax (n = 8), emotional distress/fright (n = 7), and miscellaneous causes (n = 34). The mechanism of injury was designated as block-related in 44% of all peripheral nerve block claims (27% block technique, 6% unintentional intravenous injection/absorption, 6% pneumothorax, 2% needle trauma to nerve, 1% high block from unintentional intrathecal injection, 1% inadequate analgesia, and 3% unexplained block-related).
TABLE 36.2 Permanent Nerve Injuries Associated with Peripheral Nerve Blocks for Surgical Anesthesia (n = 17)
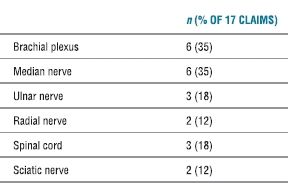
Total sums to >17 and >100% due to multiple nerve injuries in some claims.
These results are consistent with data from prospective studies that have demonstrated relatively few high severity injuries associated with peripheral nerve blocks.34–36 The voluntary reporting system established by Auroy et al.34 over a 10-month period in France found only 7 high severity injuries out of 23,784 upper extremity blocks, and no cases were associated with death.34 Lower extremity blocks were associated with slightly more high severity injuries in this study with 15 complications out of 20,162 blocks. It is unclear why there are so few claims associated with lower extremity blocks compared to upper extremity blocks in the Closed Claims Database, but it may be attributable to different practice patterns between France and the United States (e.g., possibly more neuraxial anesthetics are used for lower extremity procedures in the United States).
As almost half of the peripheral nerve block claims were associated with block technique as the mechanism of injury, improved tools or techniques to more accurately locate nerves and avoid surrounding tissues are needed. Several small studies have demonstrated the benefits of ultrasound in performance of peripheral nerve blocks, including reduced onset time for block and improvement in quality and duration of block.37,38 It remains unclear if ultrasound-guided regional anesthesia will reduce the incidence of significant block-related injuries as randomized controlled trials are lacking (Chapter 17).6,39
Liability associated with peripheral nerve blocks was similar to neuraxial anesthetics and was related to severity of injury. The highest percentage of less than appropriate care (41%), the highest percentage of claims with payment (86%), and the highest median payments ($543,750—range $18,000–$1,875,000) were associated with the death or brain damage group. Overall, permanent nerve injury claims had a 41% payment rate with only 12% judged to be less than appropriate care and a median payment of $290,000 (range $74,000–$990,000). Temporary injury claims had a 24% payment rate with only 14% of claims judged with less than appropriate care and a median payment of $31,000 ($600–$636,500).
Summary for Regional Anesthesia Claims
Over one-third of the neuraxial regional anesthesia claims in the nonobstetric surgical anesthesia were associated with death or brain damage, and 16% with permanent nerve damage. Block-related mechanisms of injury accounted for over one-quarter of death or brain damage claims, with neuraxial cardiac arrest and high block as the two most common causes. Outcome after neuraxial cardiac arrest in this database remains poor despite the use of pulse oximetry and capnography, although successful resuscitations would be less likely to result in claims. Occurrence of neuraxial cardiac arrest in the prone position or during position changes on the table was associated with one third of these claims. Accidental intrathecal blocks comprised over half of the high spinal/epidural block claims. Although neuraxial hematoma is rare based on large prospective studies, this complication continues to occur in association with either intrinsic or iatrogenic anticoagulation and usually results in permanent damage to the neuraxis. Outcome from these three complications may be improved with heightened vigilance and rapid diagnosis and treatment.
Claims associated with peripheral nerve blocks are predominately of temporary severity of injury. Permanent nerve injury occurred in only 13% of the peripheral nerve block claims, and <4% of claims associated with death or brain damage were associated with a block-related mechanism of injury. Large randomized trials will need to determine if the use of ultrasound for peripheral nerve blocks will decrease the number of permanent nerve injuries compared to conventional techniques.
 CHRONIC PAIN MANAGEMENT
CHRONIC PAIN MANAGEMENT
Cervical Interventional Procedures—Review of the Literature
The role of interventional procedures for chronic pain management is controversial and efficacy is not well defined.40,41 There is no strong evidence to support the use of any type of injection therapy (epidural, facet joint, or local sites) for subacute or chronic low back pain in patients without radicular pain.42 Most common among interventional therapeutic pain treatments are the use of epidural injection of steroids to treat acute radicular pain often associated with disk herniation or spinal stenosis and facet injections to treat chronic neck or low back pain usually associated with facet degeneration. Increased utilization of interventional therapies and surgeries has not been associated with improved health status among patients with low back pain.43 Evidence from randomized, placebo-controlled trials showing benefits of most nonsurgical interventional therapies for back pain is limited.44 Despite the lack of proven efficacy, Medicare data demonstrated that overall growth in interventional techniques from 1998 to 2005 was 179%.45 Between 1994 and 2001, there was a 271% increase in lumbar epidural steroid injections and a 231% increase in facet injections in a Medicare population.46
Transforaminal epidural steroid injections or selective nerve root blocks are frequently used both in the treatment of cervical radiculopathy and for diagnostic purposes. With increased utilization of interventional pain treatments, unanticipated complications have been reported, particularly an increase in neurologic injuries, including spinal cord infarction and stroke, which have been associated with interventional pain treatment.47–50 Serious reported complications include anterior spinal artery syndrome,51,52 quadriplegia,53 ischemic stroke,54,55 and death.56 Catastrophic neurologic complications may occur as a result of radicular arterial uptake of injectate, arterial perforation leading to dissection/thrombosis, and needle-induced vasospasm.48 Nahm et al.57 reported that the highest incidence of intravascular injection during transforaminal injections was at the cervical level. This may occur because of differences in the content of the vessels in the intervertebral foramina resulting from different types of arterial supply to the spinal cord at various spinal levels. In the thoracolumbar levels, spinal arterial branches arise from the aorta and iliac vessels while in the cervical spine, arterial branches to the cord arise from the vertebral, ascending cervical, superior intercostals, and deep cervical arteries (see Figure 12-2).
Anterior spinal artery syndrome and cerebellar ischemia are devastating complications of cervical transforaminal injections. Reports of anterior spinal artery syndrome or brain injury occurring during cervical transforaminal steroid injections raise safety concerns.50,51,56,58–61 A potential hazard to the safe performance of cervical transforaminal injections would be the presence of a vessel that communicates with the anterior spinal artery in the posterior aspect of the foramen. Ascending and deep cervical arterial branches enter the external opening of the posterior intervertebral foramen near the classic target area for transforaminal epidural injections.62 These branches occasionally supply anterior radicular and segmental medullary arteries to the spinal cord. Because these arteries are contributors to anterior spinal artery flow, injection into or injury to these vessels may explain the occurrence of ischemic neurologic events (Chapter 28).
Although interlaminar cervical injections are generally considered safer than transforaminal injections, serious complications can also occur with the interlaminar approach.63 Cervical spine anatomy is not analogous to the lumbar spine, and the distance to the dura and the dimensions of the cervical epidural space varies.64,65 Some authors recommend not injecting higher than C6-7 interspace as the epidural space is largest in this area66 and, if a preprocedure MRI shows significant canal narrowing at the level of pathology, performing the injection below that level or avoiding the injection entirely.53 Epidural procedures in the cervical region may be more prone to complications because of the narrow distance between the ligamentum flavum and the spinal cord.63 Severe neurologic injuries have been reported after interlaminar injections, caused by needle trauma to the spinal cord or nerves.67–71 Fluoroscopic guidance is widely used and advocated to obtain accurate needle positioning but cannot guarantee prevention of dural puncture or spinal cord penetration either by the interlaminar72 or transforaminal approach.73
Cervical Interventional Procedures: ASA Closed Claims Analysis
Summary of Results
A second analysis of the 294 chronic pain malpractice claims collected between 2005 and 2008 by the ASA Closed Claims Project was published in Anesthesiology in 2011.12 This work compared 64 claims for cervical procedures (22%) to all other chronic pain claims collected during this period. Most (83%) of the claims related to cervical procedures that occurred between 2000 and 2006.
The mechanism of injury in these claims and the use of sedation in spinal cord injury claims were of particular interest in this analysis. Neuraxial injury was defined by the anatomic location where the injury occurred (including epidural, intrathecal, or other areas) and by the mechanism of the injury. Injuries may result from compressive lesions, ischemia/infarction, direct trauma, or other mechanisms. Clinical manifestations of neuraxial injuries consisted of quadriplegia or quadriparesis, paraplegia or paraparesis, unilateral tract signs unilateral (including corticospinal tract as manifested by ipsilateral hemiparesis, spinothalamic tract manifested by contralateral pain or temperature loss, or dorsal column usually manifesting as ipsilateral proprioception), bilateral tract signs, reticulospinal, and gray matter injuries.
Of the 64 cervical procedures involved in these claims, almost all were blocks or injections (91%) with four claims (6%) for radiofrequency ablation. Forty-three of the 58 blocks (67%) were epidurals (of which 41 were steroid injections—12 transforaminal and 28 interlaminar), seven (11%) were stellate ganglion blocks, and six (9%) were trigger point injections. The indication for these procedures was cervical radicular pain in 50%, neck pain of musculoskeletal origin in 28%, complex regional pain syndrome in 11%, and spinal stenosis in 5%. Patients in malpractice claims who underwent a cervical procedure were more likely to be women (73%; p < 0.011) and healthier (p < 0.001) than other chronic pain patients.
Almost 60% of patients who had cervical procedures experienced spinal cord injuries compared to 11% of those receiving other chronic pain treatment (p < 0.001, Fig. 36-5) with the majority (91%) occurring during epidural procedures (20 of these were interlaminar injections and 10 were transforaminal injections). Of interest, other procedures such as trigger-point injections, stellate ganglion block, and facet injection were also associated with spinal cord injury. The majority of the 38 spinal cord injuries (87%) resulted in permanent disabling injuries and manifested as quadriplegia/quadriparesis (27%), paraplegia/paraparesis (18%), and hemiplegia/hemiparesis (9%). Fifty-three percent of the spinal cord injuries resulted from direct needle trauma, 16% were a result of cord infarction after intra-arterial injection, and 8% were a result of hematoma caused by cord compression. Care was deemed less than appropriate in 52% of claims. Payment was made in 51% of claims, and the median payment for cervical procedures was $388,600 (range, $642–$2,681,720).
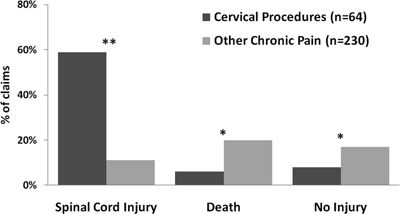
FIGURE 36-5. Cervical procedure outcomes compared to other chronic pain treatment. *p < 0.01; **p < 0.001.
 MRI–T2 weighted image (most sensitive and specific)
MRI–T2 weighted image (most sensitive and specific) CT Myelography
CT Myelography 


