Drugs for Control of Rhythm
Peter J. Kudenchuk
Antiarrhythmic agents have the potential for benefit as well as harm. Their benefit is maximized when rhythm diagnosis is accurate and when thought is given to targeted treatment.
Classification of antiarrhythmia drugs
Risk–benefit for administration: proarrhythmia consideration
Use of individual antiarrhythmia agents
Using Antiarrhythmics
Antiarrhythmic agents have the potential for benefit as well as harm. Their benefit is maximized when rhythm diagnosis is accurate and when thought is given to targeted treatment. In patients who are hemodynamically compromised by an acute arrhythmia, the approach of choice is to treat first with immediate cardioversion. Conversely, the approach of choice in patients who are hemodynamically stable in the face of an acute arrhythmia is to think first and then treat accordingly. Algorithms are intended to promote such thoughtful approaches to management. Because of the focus on emergency care, only agents that are available in parenteral form are presented in this discussion, not all of which are available in such a formulation within the United States. Recommended dosing guidelines for the drugs discussed, if approved for use in the United States, are taken from published prescribing sources. For drugs not approved
for use in the United States, doses are derived from the published studies that evaluated these medications. It is advisable for the reader to refer to these sources for confirmaton of these doses or any change that might have trans-pired since publication of this text.
for use in the United States, doses are derived from the published studies that evaluated these medications. It is advisable for the reader to refer to these sources for confirmaton of these doses or any change that might have trans-pired since publication of this text.
Preferred Routes of Drug Administration
Peripheral or Central IV
A peripheral vein is the first choice for intravenous (IV) access in patients, particularly during cardiac arrest. Central line access (internal jugular or subclavian) requires interruption of chest compressions. Peak drug concentrations, however, are lower and circulation times are longer when drugs are administered via peripheral sites compared with central sites.1,2,3 When given via a peripheral vein, drugs require 1 to 2 minutes to reach the central circulation; this delay is appreciably shorter with a central venous route. Peripheral venous cannulation, however, is easier to learn, results in fewer complications, and does not require interruption of cardiopulmonary resuscitation (CPR).
If peripheral venous access is used during resuscitative efforts, IV drugs should be administered by bolus injection; follow with a 20-mL bolus of IV fluid and elevation of the extremity to facilitate transport into the central circulation.4 Unless there are contraindications, if peripheral veins are inaccessible and experienced providers are available, placement of a central line should be considered. Placement of central lines can cause a pneumothorax and potentially increase the risk of bleeding complications for patients who subsequently receive fibrinolytic therapy.
Endotracheal Drug Administration
If an endotracheal tube has been placed before venous access is achieved, lipid-soluble drugs such as epinephrine,5,6 lidocaine, and atropine7,8 can be administered via the tube; absorption occurs in the lungs. Much of the evidence for the efficacy of endotracheal administration of drugs stems from studies under normal or near normal circulatory conditions.9 Notably, there is no clinical evidence that endotracheal administration of drugs at recommended doses is necessarily effective under cardiac arrest conditions. Because pulmonary blood flow is compromised during cardiac arrest and CPR, transalveolar drug absorption is minimal. Therefore, even higher doses of medications may be needed to achieve the same serum levels as when such agents are administered intravenously.10
Because administration of resuscitation drugs via the trachea results in lower blood concentrations than when they are given intravenously, it has traditionally been taught to give all endotracheal medications at 2 to 2.5 times the recommended IV dose, diluted in 10 mL of normal saline or distilled water. Lung absorption is greater with distilled water as the diluent than with normal saline, but distilled water has a greater adverse effect on PaO2. The technique for administration is to pass a catheter beyond the tip of the endotracheal tube, stop chest compressions, inject the drug solution quickly down the catheter, follow immediately with several quick lung insufflations to create a rapidly absorbed aerosol, and then resume chest compressions.5
The endotracheal administration of drugs that are not lipid-soluble, such as calcium and sodium bicarbonate, may result in airway injury; therefore, they should not be administered in this manner. While endotracheal use of other drugs such as naloxone and vasopressin is permissible, dosing is uncertain.
Intraosseous Drug Administration
Intraosseous access uses the marrow (medullary) cavity of bones such as the tibia or sternum as a “noncollapsible vein” for parenteral access in both children and adults. This is regarded as a viable alternative to conventional IV line placement under emergency conditions where vascular access is not otherwise possible. The technique requires special training and is described in detail elsewhere.11 Virtually all fluids and medications that can be administered intravenously can be given by intraosseous means without dose modification and can achieve as rapid an entry into the central circulation, with concentrations and peak effects comparable to those of IV administration.12 If properly performed, complications from intraosseous access are uncommon; however, they can include extravasation of fluid, fractures at the insertion site, compartment syndrome, fat emboli, cellulitis, and local infection.
Basic Principles of Antiarrhythmic Therapy
No antiarrhythmic agent has yet been proven to improve survival after cardiac arrest. Nonetheless, such drugs are useful for the control of shock-refractory arrhythmias and to restore and maintain sinus rhythm. Their benefits must be balanced against their potential risks. Proarrhythmias are serious tachyarrhythmias or bradyarrhythmias generated by antiarrhythmic agents. All antiarrhythmic agents have some degree of proarrhythmic risk, and sicker hearts appear more susceptible to such effects. Tachyarrhythmias, such as torsade de pointes, account for most proarrhythmia events. Other manifestations of proarrhythmia can include frequent premature ventricular contractions (PVCs) and incessant ventricular tachycardia (VT).
The interactions between antiarrhythmic agents are also complex because of their differing effects on a variety of ionic channels in the heart. Sequential use of two or more antiarrhythmic drugs compounds the adverse effects, particularly for bradycardia, hypotension, and torsades de pointes. Therefore, it is best to avoid use of more than one agent unless absolutely necessary. In most patients, when an appropriate dose of a single antiarrhythmic medication fails to
terminate an arrhythmia, it is wiser to turn to electrical cardioversion rather than a second antiarrhythmic medication.
terminate an arrhythmia, it is wiser to turn to electrical cardioversion rather than a second antiarrhythmic medication.
Patients with clinical congestive heart failure or depressed left ventricular (LV) function should also be treated cautiously with antiarrhythmic therapy. In these patients, many antiarrhythmic agents depress LV function further, often precipitating or worsening congestive heart failure. In addition, the risk of proarrhythmia is higher.
Because of its broad antiarrhythmic spectrum, lesser negative inotropic effect, and low incidence of proarrhythmia, amiodarone continues to dominate the management of tachycardias. If amiodarone fails to produce the desired response, the preferred next intervention is to attempt early electrical cardioversion.
Classification of Antiarrhythmic Drugs
Vaughan Williams Classification
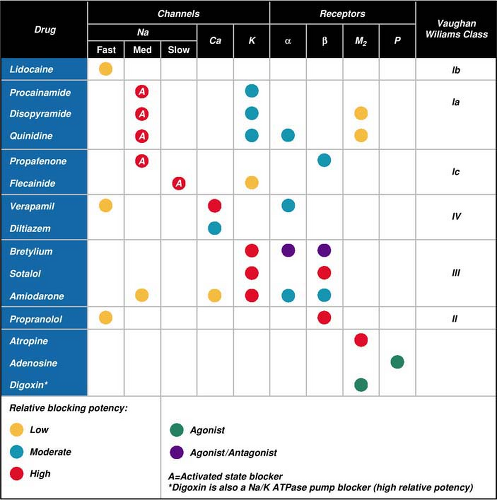
Traditionally antiarrhythmic drugs are classified according to site of action or electrophysiologic effects. The Vaughan Williams classification is the most widely used of these classification schemes.13 The system is practical, although it oversimplifies the complex cellular-level, ionic-channel processes involved in rhythm genesis. The Vaughan Williams system labels antiarrhythmics based on the ion channels or receptors that they block. It classifies a drug according to (1) whether the blocked ionic channel is sodium, potassium, or calcium; (2) whether the drug blocks beta-adrenergic receptors; and (3) the effect of the drug on conduction and repolarization (Table 25-1). Most advanced cardiac life support (ACLS) drugs for rhythm and rate reviewed below are classified according to the Vaughan Williams system (Table 25-2). The following information about scientific evidence, drug indications, and drug doses refers to the parenteral forms of all drugs. During emergencies, antiarrhythmics should be administered by the parenteral route.
Sicilian Gambit
The Sicilian Gambit is a classification of antiarrhythmic agents by the European Society of Cardiology. It is more accurate but more cumbersome14,15 than the Vaughan Williams system. In the Sicilian Gambit, antiarrhythmic drugs are grouped according to their multiple channel-blocking effects (Fig. 25-1).
The Sicilian Gambit reports the work of a group of basic and clinical investigators who met in Taormina, Sicily, to consider the classification of antiarrhythmic drugs. Paramount to their considerations were
Their dissatisfaction with the options offered by existing classification systems for inspiring and directing research, development, and therapy
The disarray in the field of antiarrhythmic drug development and testing following the Cardiac Arrhythmia Suppression Trial (CAST)
The desire to provide a framework for consideration of antiarrhythmic drugs that will encourage scientific advances and possess the flexibility to grow as advances are made
For more information, the interested reader is encouraged to read the full report,3 which contains
A discussion of the shortcomings of the current system for antiarrhythmic classification
A review of the molecular targets of antiarrhythmic effects (including channels and receptors)
A consideration of the mechanisms responsible for arrhythmias, including identification of “vulnerable parameters” that might be most accessible to drug effect
Clinical considerations when deploying antiarrhythmic drugs
Amiodarone
IV amiodarone is a complex drug with effects on sodium, potassium, and calcium channels as well as alpha- and beta-adrenergic blocking properties. The drug is useful for treatment of atrial and ventricular arrhythmias. In patients with severely impaired heart function, IV amiodarone is preferable to other antiarrhythmic agents because of its greater efficacy and lower incidence of proarrhythmic effects. Uses and indications include:
Cardiac arrest due to persistent VT or ventricular fibrillation (VF)
Hemodynamically stable VT, polymorphic VT (where torsades de pointes is not suspected), and wide-complex tachycardia of uncertain origin
Ventricular rate control of rapid atrial arrhythmias in patients with severely impaired LV function when digitalis has proved ineffective
Pharmacologic conversion of supraventricular arrhythmias that are refractory to other agents
Control of rapid ventricular rate due to accessory pathway conduction in preexcited atrial arrhythmias
Cardiac Arrest
Amiodarone is a potent and effective antiarrhythmic agent for the termination of hemodynamically unstable ventricular arrhythmias, including treatment of cardiac arrest due to VF and pulseless VT.16,17,18 The major drawbacks to its use are a somewhat cumbersome manner of administration in an emergency setting (it is packaged in 150-mg [3-cc] glass ampules, is not available in prefilled syringes, requires dilution prior to administration, and calls for care to prevent foaming of its diluent in this process), and the incidence of hypotension and bradycardia.
Two high-quality randomized trials of shock-resistant out-of-hospital cardiac arrest—one of which compared amiodarone against placebo and another against lidocaine—demonstrated that it improved the likelihood of successful resuscitation and admission alive to hospital but did not have a significant effect on survival to hospital discharge.18,19 In both of these trials, amiodarone, by design, was administered relatively late in resuscitation. Its potential to improve long-term survival if given earlier in the course of resuscitation is untested.
Table 25-1 • Classification of Antiarrhythmic Drugs Modified from the Vaughan Williams System | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Other Arrhythmias
IV amiodarone is an effective antiarrhythmic agent for virtually any tachyarrhythmia, but it is most commonly used for ventricular arrhythmias and atrial fibrillation or flutter with and without preexcitation.19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 It has not been studied specifically for the pharmacologic termination of hemodynamically stable VT, but it is likely to be effective based on experience of its success in treating hemodynamically unstable VT and VF.16,17,44,47,48,49,50,51,52,53,54,55
Dosing and Administration
IV amiodarone is administered as 150 mg over 10 minutes, followed by an infusion of 1 mg/min for 6 hours and then 0.5 mg/min. Supplementary infusions of 150 mg can be repeated as necessary for recurrent or resistant arrhythmias to a maximum manufacturer-recommended total daily dose of 2 g. One study found amiodarone to be effective in the pharmacologic conversion of atrial fibrillation when it is administered at relatively high doses of 125 mg/hr for 24
hours (total dose 3 g).56 In cardiac arrest due to pulseless VT or VF, IV amiodarone is initially administered as a 300-mg rapid infusion diluted in a volume of 20 to 30 mL of saline or dextrose in water. Based on extrapolation from studies in patients with hemodynamically unstable VT, supplementary doses of 150 mg by rapid infusion may be administered for recurrent or refractory VT/VF, followed by an infusion of 1 mg/min for 6 hours and then 0.5 mg/min, to a maximum daily dose of 2 g.
hours (total dose 3 g).56 In cardiac arrest due to pulseless VT or VF, IV amiodarone is initially administered as a 300-mg rapid infusion diluted in a volume of 20 to 30 mL of saline or dextrose in water. Based on extrapolation from studies in patients with hemodynamically unstable VT, supplementary doses of 150 mg by rapid infusion may be administered for recurrent or refractory VT/VF, followed by an infusion of 1 mg/min for 6 hours and then 0.5 mg/min, to a maximum daily dose of 2 g.
Table 25-2 • Major Pharmacologic Effects of ACLS Medications Arranged According to the Vaughan Williams Classification | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
IV amiodarone has bradycardic and vasodilatory (hypotensive) effects, which can destabilize patients who may already have a tenuous hemodynamic status.16,57,58 The hypotensive effect is felt to be related to its diluent rather than to amiodarone itself and perhaps to histamine release. Hypotension is most commonly seen during the initial rather than repeat dosing of IV amiodarone. The major adverse effects from amiodarone can be prevented by slowing the rate of drug infusion, or they can be treated as necessary with fluids, pressors, chronotropic agents, or temporary pacing.
Adenosine
Adenosine is an endogenous purine nucleoside that depresses the activity of the atrioventricular (AV) and sinus nodes. Adenosine produces a short-lived pharmacologic response because it is rapidly metabolized by enzymatic degradation in blood and peripheral tissue. The half-life of adenosine is <5 seconds.
Indications
Adenosine is indicated for paroxysmal supraventricular tachycardia (PSVT) whether it is due to AV-nodal reentry or AV reentry mediated by an accessory pathway.59 These forms of PSVT involve a reentry pathway including the AV node as at least one limb of the circuit. Adenosine is effective in terminating these arrhythmias. If the arrhythmias are not due to reentry involving the AV node (such as atrial flutter, atrial fibrillation, or other atrial arrhythmias), adenosine will not terminate the arrhythmia but may produce transient AV block, which may clarify the diagnosis. However, because of its ultrashort half-life, adenosine is not appropriate for ventricular rate control of AF or atrial flutter.
Adenosine is also not an effective agent for common forms of ventricular arrhythmias and is contraindicated in preexcited atrial arrhythmias such as AF or atrial flutter.60,61,62,63,64 In the past, use of adenosine was advocated to discriminate VT from supraventricular tachycardia (SVT) with aberrancy in hemodynamically stable wide-complex tachycardia of uncertain origin. This led to instances of worsened hypotension after inappropriate treatment with adenosine for VT.62 Such practice is discouraged, and adenosine is recommended for use only when a supraventricular origin is strongly suspected.
Dosing
The recommended initial dose of adenosine is a 6-mg rapid bolus over 1 to 3 seconds.65 The dose should be followed by a 20-mL saline flush. Rapid (bolus) administration is required because the effect of adenosine on nodal tissue is concentration-dependent; slower infusion is more likely to result only in hypotension due to its systemic vasodilatory effects. If no response is observed within 1 to 2 minutes, a 12-mg repeated dose should be administered in the same manner. Experience with larger doses is limited, but patients taking theophylline are less sensitive to adenosine and may require such larger doses.
Side effects with adenosine are common but transient; flushing, dyspnea, and chest pain are the most frequently observed.66 Adenosine carries the risk of causing angina, bronchospasm, proarrhythmia, and acceleration of accessory pathway conduction.62 Because of the short half-life of
adenosine, PSVT may recur once its effects have abated. Recurrent PSVT may be treated with additional doses of adenosine or with a longer-acting AV-nodal blocking agent (calcium channel blocker or beta-blocker). The systemic hypotensive effects of adenosine are mitigated by successful conversion of PSVT. In the event that the arrhythmia is not terminated, drug-induced hypotension, though transient, may require treatment.
adenosine, PSVT may recur once its effects have abated. Recurrent PSVT may be treated with additional doses of adenosine or with a longer-acting AV-nodal blocking agent (calcium channel blocker or beta-blocker). The systemic hypotensive effects of adenosine are mitigated by successful conversion of PSVT. In the event that the arrhythmia is not terminated, drug-induced hypotension, though transient, may require treatment.
Adenosine has several important drug interactions. Therapeutic concentrations of theophylline or related methylxanthines (caffeine and theobromine) block the receptor responsible for the electrophysiologic and hemodynamic effects of adenosine. Dipyridamole blocks adenosine uptake and potentiates its effects. The effects of adenosine are also prolonged in patients on carbamazepine and those with denervated transplanted hearts. Dose adjustment or alternative therapy should be selected in such patients.
Atropine
Atropine sulfate reverses cholinergic-mediated decreases in heart rate, systemic vascular resistance, and blood pressure.67 Atropine is useful in treating symptomatic sinus bradycardia. It may be beneficial in the presence of AV block at the nodal level (Mobitz I second-degree AV block) or ventricular asystole but should not be used when infranodal (Mobitz type II) block is suspected. Its use in the latter setting may worsen rather than enhance AV conduction.
Dosing
The recommended dose of atropine sulfate for asystole and slow pulseless electrical activity is 1 mg IV, repeated in 3 to 5 minutes if asystole persists. For bradycardia, the dose is 0.5 mg every 3 to 5 minutes to a total dose of 0.04 mg/kg. A
total dose of 3 mg (0.04 mg/kg) results in full vagal blockade in humans. Because atropine increases myocardial oxygen demand and can initiate tachyarrhythmias, the administration of a total vagolytic dose of atropine should be reserved for asystolic cardiac arrest. Doses of atropine sulfate <0.5 mg may be parasympathomimetic and further slow the cardiac rate. Atropine can be administered intravenously or by intraosseous means, and it is also well absorbed via the endotracheal route of administration.
total dose of 3 mg (0.04 mg/kg) results in full vagal blockade in humans. Because atropine increases myocardial oxygen demand and can initiate tachyarrhythmias, the administration of a total vagolytic dose of atropine should be reserved for asystolic cardiac arrest. Doses of atropine sulfate <0.5 mg may be parasympathomimetic and further slow the cardiac rate. Atropine can be administered intravenously or by intraosseous means, and it is also well absorbed via the endotracheal route of administration.
Atropine should be used cautiously in the presence of acute coronary ischemia or myocardial infarction (MI), because excessive increases in rate may worsen ischemia or increase the zone of infarction. VF and VT rarely follow IV administration of atropine. Atropine is not indicated in bradycardia from AV block at the His–Purkinje level (second-degree AV block type II and third-degree AV block with new wide-QRS complexes). In such instances atropine can accelerate sinus rate and worsen AV conduction.
Beta-Adrenergic Blockers
Beta-adrenergic blockers inhibit sympathetic tone on the sinus node and AV node, resulting in sinus bradycardia and slowing of AV-nodal conduction.68 This makes the drugs useful for termination of PSVT due to AV-nodal reentry or AV reentry mediated by an accessory pathway as well as for rate control of atrial arrhythmias (such as AF, atrial flutter, ectopic atrial tachycardia, and multifocal atrial tachycardia) without preexcitation. Inhibition of sympathetic tone may also have a beneficial effect on recurrent ventricular arrhythmias.69 Beta-adrenergic blockers also have potential benefits outside the scope of this discussion, as in patients with acute coronary syndromes.