Bradycardia
Peter J. Kudenchuk
In managing patients with a bradycardia, the critical first action is to evaluate whether the heart rate is hemodynamically significant and sufficiently slow to account for serious and related symptoms.
Definition and approach to the patient with a bradycardia
Differential diagnosis of bradycardia
Emergency treatment considerations for patients with symptomatic bradycardias
Initial treatment, including “expert consultation advised”
Considerations for treatment in special circumstances: acute coronary syndrome (ACS), cardiac transplantation
Cardiac Rhythm and Escape Rhyhms
It is the character of the escape rhythm and hemodynamic impact of the resulting heart rate that determines the emergent or nonemergent consequences of an abnormality in impulse formation or conduction.
The normal cardiac rhythm comprises of two electrical processes, impulse formation and impulse conduction. If either of these fails, an escape rhythm (originating from a subsidiary site of impulse formation) can serve as a backup pacemaker. Impulse formation originates in specialized tissue in the sinus node and is dependent on phase 4 (spontaneous depolarization) of the action potential. Phase 4 of the action potential is heavily dependent on autonomic tone, with parasympathetic (vagal) tone typically slowing impulse formation and sympathetic tone accelerating it. In addition to the sinus node, automatic tissues in other portions of the heart serve as subsidiary pacemakers, which can take control of impulse formation should the sinus node fail, but at a slower rate. Impulse formation in junctional automatic tissue, for example, typically occurs at a rate of 40 to 60 beat per minute (bpm); His automaticity at a rate of 40 bpm; and ventricular automaticity at 30 to 40 bpm. Should impulse formation fail at higher levels, the activity of these otherwise suppressed automatic sites may become manifest.
Impulse conduction is the transmission of an impulse, formed in the atria through the atrioventricular (AV) node and specialized His–Purkinje system, to depolarize the ventricles. Impulse conduction may be slowed or blocked completely at any of these levels, resulting in a spectrum of abnormalities ranging from first-degree AV block and bundle-branch block to complete AV block.
Should either impulse formation or impulse conduction fail completely, the consequences of such failure are dependent upon whether an escape rhythm is robust. Complete AV block, for example, may be associated with a junctional escape rate of 40 to 60 bpm with minimum symptoms or with a slow ventricular escape rate of 30 to 40 bpm and hemodynamic compromise. Importantly, it is the character of the escape rhythm and hemodynamic impact of the resulting heart rate that determines the emergent or nonemergent consequences of an abnormality in impulse formation or conduction.
Disorders of Impulse Formation
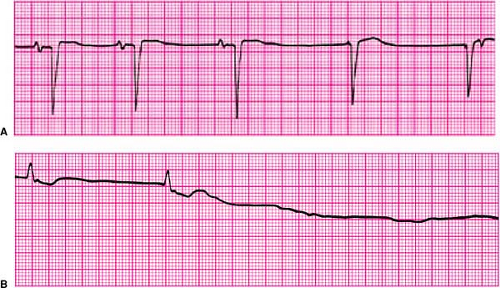
Disorders of impulse formation include sinus bradycardia, sinus pause (Fig. 23-1A), and sinus arrest (Fig. 23-1B); they represent impaired automaticity.1 Such disorders are evidenced by failure to generate P waves at an appropriate rate, abrupt pauses between successive P waves, or complete absence of P waves for a protracted period of time. Criteria that define these disorders are somewhat arbitrary. For
example, sinus bradycardia is defined as a heart rate <60 bpm, yet many persons, particularly trained athletes, may be completely asymptomatic with resting heart rates that may average in the range of 40 bpm. Similarly, brief pauses in sinus rhythm of up to 3 seconds may be seen during sleeping periods without adverse consequences. Therefore, bradycardia should become a concern when patients develop symptoms as a result of inappropriately slow heart rates or experience symptomatic pauses in rhythm, provoking significant activity limitations, dizziness, or syncope.
example, sinus bradycardia is defined as a heart rate <60 bpm, yet many persons, particularly trained athletes, may be completely asymptomatic with resting heart rates that may average in the range of 40 bpm. Similarly, brief pauses in sinus rhythm of up to 3 seconds may be seen during sleeping periods without adverse consequences. Therefore, bradycardia should become a concern when patients develop symptoms as a result of inappropriately slow heart rates or experience symptomatic pauses in rhythm, provoking significant activity limitations, dizziness, or syncope.
In some cases, inappropriately slow generation of P waves may combine with atrial tachyarrhythmias (such as paroxysmal atrial fibrillation) to create brady-tachy syndrome, also known as sick sinus syndrome. In such patients paroxysms of rapid supraventricular tachycardia may be separated by interludes of excessively slow atrial rates or pauses in sinus rhythm. Permanent pacing is frequently required to allow for pharmacologic control of rapid supraventricular rhythms. Pacing provides a suitable lower heart rate that might otherwise be dangerously suppressed by such rate- or rhythm-controlling agents.
In the acute setting, treatment can include atropine, dopamine, epinephirine and/or temporary pacing for an inappropriately slow heart rate due to impaired automaticity in any of its forms. Attention should be directed to potential causes, such as use of drugs with sinus-slowing properties (e.g., calcium channel blockers, beta-blockers, digoxin, antiarrhythmic agents) or metabolic conditions such as hypothyroidism. Acute hypoxia can also provoke slowing or pauses in heart rate.
Disorders of Impulse Conduction
Disorders of impulse conduction are defined as the failure of a generated impulse to reach its ventricular destination successfully or in timely fashion.1 Typically, these are broken down into disorders that result in slowing of conduction between the atria and ventricles or within the ventricles (first-degree AV block and intraventricular delay or block, respectively); disorders in which most but not all atrial impulses are conducted to the ventricles (second-degree AV block at the nodal level, and second-degree AV block at the infranodal level); and disorders in which only occasional atrial impulses are conducted to the ventricles (advanced AV block) and in which no impulses are conducted to the ventricles (third-degree or complete AV block).
First-Degree AV Block and Intraventricular Conduction Block
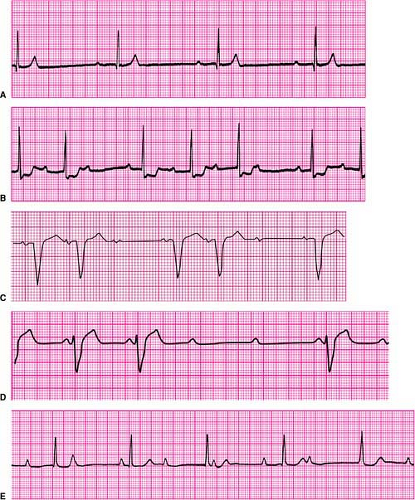
First-degree AV block is defined as prolongation of the PR interval (the onset of the surface P wave to the onset of the QRS complex) to >0.20 second (Fig. 23-2A). The most common location of such slowing is in the AV node. The less common cause is due to slowing of conduction in the bundle of His. Similarly, a slowing or partial block of conduction in the His–Purkinje system (as exemplified by bundle-branch
block) results in a lengthening of the time required to completely depolarize the ventricles. This is because conduction via the His–Purkinje system is much faster than conduction of an electrical impulse from muscle to muscle. When a portion of the His–Purkinje system fails to conduct (as in bundle-branch block) or conducts too slowly, the portion of the heart to which it extends is excited instead by muscle-to-muscle conduction. This results in longer time required to completely depolarize the ventricles and is expressed in a prolonged (wider) QRS. This is sometimes called aberrant
conduction or intraventricular conduction delay, or it may be described by the characteristic appearance of a right or left bundle-branch block. First degree AV block and intraventricular conduction block can occur independently of one another, or together. Each represents conduction that though slowed, is still ongoing through the AV node, and His-Purkinje system, respectively.
block) results in a lengthening of the time required to completely depolarize the ventricles. This is because conduction via the His–Purkinje system is much faster than conduction of an electrical impulse from muscle to muscle. When a portion of the His–Purkinje system fails to conduct (as in bundle-branch block) or conducts too slowly, the portion of the heart to which it extends is excited instead by muscle-to-muscle conduction. This results in longer time required to completely depolarize the ventricles and is expressed in a prolonged (wider) QRS. This is sometimes called aberrant
conduction or intraventricular conduction delay, or it may be described by the characteristic appearance of a right or left bundle-branch block. First degree AV block and intraventricular conduction block can occur independently of one another, or together. Each represents conduction that though slowed, is still ongoing through the AV node, and His-Purkinje system, respectively.
First-degree AV block and intraventricular conduction block do not require treatment. However, their presence can confound rhythm diagnosis, as, for example, when supraventricular tachycardia occurs along with a bundle-branch block, resulting in wide-complex tachycardia, or when the P wave blends into the preceding T wave of a sinus tachycardia, resulting in what appears to be a supraventricular tachycardia without apparent P waves.
Second- and Third-Degree AV Block
Second-degree AV block is divided into two forms, depending upon where the block occurs, either in the AV node or in the bundle of His (infranodal). Intermittent block in the AV node is referred to as Mobitz type I (or Wenckebach-type) second-degree AV block. It is characterized by successive prolongation of the PR interval prior to a P wave that fails to conduct to the ventricle, which results in a pause. This is followed by a shorter PR interval associated with the next conducted P wave, after which the process repeats itself (Fig. 23-2B).
In contrast, Mobitz type II (or infranodal) second-degree AV block is not heralded by PR prolongation before a P wave fails to conduct to the ventricle. This form of block is characteristic of the “all or nothing” conduction properties of the His–Purkinje system, suggesting the block’s anatomic location in the conduction system distal (ventricular) side of the AV node (Fig. 23-2C). Whereas Mobitz type I (Wenckebach-type) AV block is frequently benign and often attributable to transient effects of increased parasympathetic tone upon the AV node, Mobitz type II block is always regarded as pathologic and indicative of the need for a permanent pacemaker.
Advanced AV block is a more serious form of second-degree AV block in which multiple (two or more) successive P waves fail to conduct to the ventricle, although occasional P waves may still conduct (Fig. 23-2D). It indicates a more advanced stage of deranged conduction that is leading to complete AV block. Advanced AV block can occur at the level of the AV node, or be infranodal.
Third-degree (or complete) AV block is the complete failure of atrial impulses to conduct to the ventricles (Fig. 23-2E). Such block can occur in the AV node and in or distal to the bundle of His infranodal. Subsidiary pacemaker activity is what maintains a cardiac rhythm in such circumstances, the absence of which would be lethal.
In general, unless they are attributable to an identifiable and reversible cause—such as excessive effects from calcium channel blockers, beta-blockers, antiarrhythmic drugs, or severe hyperkalemia—acquired Mobitz type II second-degree AV block advanced AV block, and complete AV block (with or without symptoms) usually require permanent pacing. In contrast, Mobitz type 1 second-degree AV block does not usually require pacing, unless it provokes serious symptoms in the absence of a readily reversible cause. The need for temporary pacing in all such patients is determined by their hemodynamic stability and the effectiveness of their escape rhythm.
Evaluation and Treatment of Bradycardia
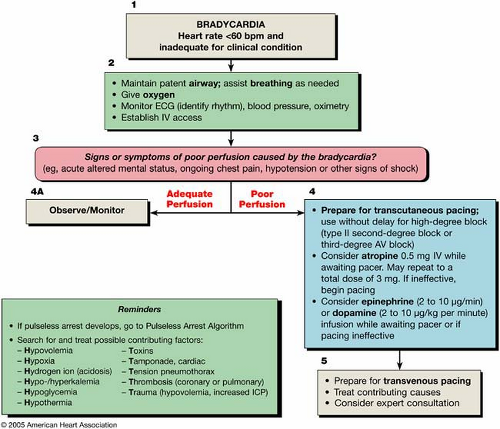
The bradycardia algorithm (Fig. 23-3) provides an overview of the evaluation and treatment of a bradycardia. Box numbers in the text refer to the numbered boxes in the algorithm.
Evaluation
Bradycardia is conventionally defined as a heart rate of <60 bpm (Fig. 23-3, Box 1). A slow heart rate may be physiologically normal for some patients and not require treatment. Conversely, while it is highly unlikely that a heart rate in the normal range would itself cause hemodynamic compromise, a normal resting heart rate might be inadequate for some clinical situations (such as sepsis or with significant volume depletion), and heart rates >60 bpm may be inadequate for others. The bradycardia algorithm focuses on the management of clinically significant bradycardia (that is, a heart rate that is inadequate under the clinical circumstances).
Initial Patient Stabilization
As with all potential life-threatening arrhythmias, initial management of any patient with bradycardia should focus on the support of airway and breathing in addition to their circulatory status, as necessary given the frequent association of bradycardia with hypoxia (Fig. 23-3). This includes provision of supplementary oxygen, initiation of cardiac rhythm monitoring, measurement of blood pressure and oxyhemoglobin saturation, and initiation of intravenous (IV) access for pharmacologic support of heart rate and blood pressure, if required. If the patient is stable, a 12-lead ECG should be performed to document and better define the cardiac rhythm. The ECG may also identify a potential cause for the arrhythmia, such as acute ischemia or signs suggestive of an electrolyte derangement.
Assessment of Serious Signs and Symptoms
Signs and symptoms of poor perfusion are assessed for whether they are causally related to the bradycardia (Fig. 23-3, Box 3). Signs and symptoms of bradycardia may be
mild; asymptomatic patients should be monitored expectantly for signs of deterioration (Box 4A) even if they do not require immediate treatment. Provide immediate therapy for patients with hypotension, acute altered mental status, chest pain, congestive heart failure, syncope, or other signs of shock related to the bradycardia (Box 4).
mild; asymptomatic patients should be monitored expectantly for signs of deterioration (Box 4A) even if they do not require immediate treatment. Provide immediate therapy for patients with hypotension, acute altered mental status, chest pain, congestive heart failure, syncope, or other signs of shock related to the bradycardia (Box 4).
Classification of AV Block and Possible Cause
In appropriate evalution of the patient with bradycardia includes a general focused assessment of their vital status and the identification of any comorbidities or potential reversible causes (e.g., ischemia, electrolyte abnormalities, or drugs). AV blocks are classified as first-, second-, and third-degree, as described in detail in the preceding section. They may be caused by medications or electrolyte disturbances as well as by structural problems resulting from acute myocardial infarction or ischemia.
Therapy
Once a symptomatic bradycardia is identified, preparations are made for transcutaneous pacing and/or for pharmacologic interventions, whichever is most expedient and appropriate for the circumstances (Fig. 23-3, Box 4). For severely symptomatic patients, especially when the block is at or below the His-Purkinje level or when an escape rhythm is inadequate, these measures should be seen as temporizing. Expert consultation should be quickly sought for placement of a temporary transvenous pacemaker (see Chapter 24 for further discussion of pacing therapies).
Atropine
In the absence of reversible causes, atropine remains the first-line drug for acute symptomatic bradycardia.2 Atropine works by reversing cholinergic-mediated decreases in heart
rate and thereby blocks the effects of vagal nerve discharges on the sinus and AV nodes. It is useful in treating symptomatic sinus bradycardia and AV block at the nodal level.4 In one randomized clinical trial in adults2 and additional lower-level studies,3,4 IV atropine improved heart rate and signs and symptoms associated with bradycardia. If the bradycardic event was acutely mediated by enhanced vagal tone, treatment with atropine may be sufficient to reverse the condition. Transcutaneous pacing is usually indicated if the patient fails to respond to atropine, although second-line drug therapy with drugs such as dopamine or epinephrine may be successful (see below).
rate and thereby blocks the effects of vagal nerve discharges on the sinus and AV nodes. It is useful in treating symptomatic sinus bradycardia and AV block at the nodal level.4 In one randomized clinical trial in adults2 and additional lower-level studies,3,4 IV atropine improved heart rate and signs and symptoms associated with bradycardia. If the bradycardic event was acutely mediated by enhanced vagal tone, treatment with atropine may be sufficient to reverse the condition. Transcutaneous pacing is usually indicated if the patient fails to respond to atropine, although second-line drug therapy with drugs such as dopamine or epinephrine may be successful (see below).
Atropine is not indicated in the treatment of bradycardia from infranodal AV block (Morbitz type II second-degree AV block and advanced or third-degree block with wide QRS ventricular escape complexes). Theoretically, atropine may increase the rate of sinus node discharge and accelerate AV conduction, thus worsening the degree of AV block in such circumstances.
Atropine Dosing
The recommended atropine dose for asystole and slow pulseless electrical activity is 1 mg IV, repeated in 3 to 5 minutes if the arrhythmias persist. For bradycardia, the dose is 0.5 mg IV every 3 to 5 minutes to a total dose of 3 mg (0.04 mg/kg). A total dose of 3 mg (0.04 mg/kg) results in full vagal blockade in humans and is the maximal total dose recommended by current ACLS guidelines. Doses of atropine sulfate of <0.5 mg may be parasympathomimetic and paradoxically result in further slowing of the heart rate.6,9 Atropine can be administered intravenously or by intraosseous means and is also well absorbed through the tracheal route of administration. Its administration should not delay implementation of external pacing for patients with poor perfusion.
Atropine Use in Acute Coronary Syndromes
Atropine should be used cautiously in the presence of ACS or infarction because excessive increases in heart rate may worsen ischemia or increase the zone of infarction. In rare cases, ventricular fibrillation (VF) and ventricular tachycardia (VT) have followed IV administration of atropine.
Atropine Use Following Cardiac Transplantation
The use of atropine in the cardiac transplant patient should be avoided. It will likely be ineffective because the transplanted heart lacks vagal innervation. Paradoxical responses to atropine have also been reported after heart transplantation, including paradoxical slowing of heart rate and development of high-degree AV block, the mechanism of which is unclear.7,11 Notably, transplanted hearts have increased sensitivity to sympathetic stimulation; therefore, sympathomimetic drugs, as an alternative to atropine, should be used cautiously in such patients.10
Pacing
Use of atropine is discouraged in type II second- or third-degree AV block or in patients with third-degree AV block with a new wide-QRS complex (which implies that the location of the block lies in or below the bundle of His in the distal conduction system). These patients require immediate pacing if serious symptoms or hemodynamic instability is present. Even if such patients are hemodynamically stable, one should prepare them for pacing, as clinical deterioration can occur suddenly and unexpectedly.
Transcutaneous pacing is a class I intervention for life-threatening bradycardias. It should be started immediately for patients who are hemodynamically unstable, particularly those with high-degree (second-degree Mobitz type II, advanced, or third-degree infranodal) block.
Transcutaneous pacing is noninvasive and can be performed by ECC providers at the bedside. Initiate transcutaneous pacing immediately if there is no response to atropine, if atropine is unlikely to be effective, or if the patient is severely symptomatic. Verify mechanical capture by pulse or arterial pressure waveform (if such monitoring has been established). Some limitations apply. Transcutaneous pacing is painful, and it may be challenging to confirm effective mechanical capture (see Chapter 24). It is important to verify mechanical capture and reasses the patient’s condition after transcutaneous pacing has been established. Use analgesia and sedation for pain control, and continue efforts to identify the cause of the bradyarrhythmia.
If transcutaneous pacing is ineffective (e.g., inconsistent capture), prepare for emergent transvenous pacing and consider obtaining expert consultation. Even if effective, transcutaneous pacing should be regarded as only a temporizing measure, and expert consultation should be expeditiously obtained regarding placement of a transvenous pacemaker.
Alternative Drugs to Consider
Other drugs may be considered when the bradycardia is unresponsive to atropine or for which atropine may be contraindicated and as temporizing measures while awaiting the availability of a pacemaker or if transcutaneous pacing is ineffective. Epinephrine and dopamine are alternative drugs to consider. They are widely available and familiar to clinicians (Fig. 23-3, Box 4).
Epinephrine (2–10 mg/min)
If the patient has severe symptoms (e.g., severe bradycardia with hypotension), the drug of choice is a catecholamine infusion (either epinephrine or dopamine). An epinephrine infusion of 2 to 10 μg/min is titrated on the basis of heart rate, blood pressure, and systemic perfusion. Epinephrine infusion is also appropriate if the patient has symptomatic bradycardia unresponsive to dopamine.
Dopamine (2–10 mg/kg/min)
Although dopamine can be administered at doses of 2 to 20 μg/kg/min or higher, the vasoconstrictive effects of the drug tend to predominate over its chronotropic properties when doses exceed 10 μg/kg/min. Dopamine may be administered in doses of 2 to 10 μg/kg/min and titrated to desired hemodynamic targets. Conversely, this agent may cause splanchnic vasodilation and relative hypo-volemia owing to intravascular volume shifts when given in low doses. For this reason it is necessary to assess intravascular volume and provide fluid support, if necessary, whenever you give dopamine in low doses.
Glucagon