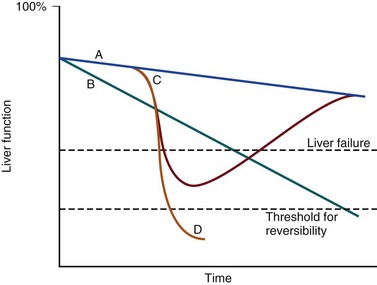
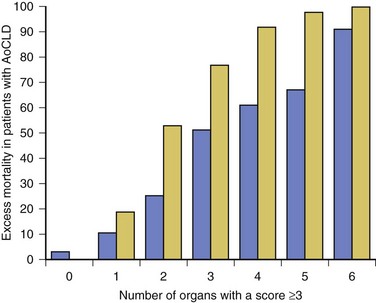
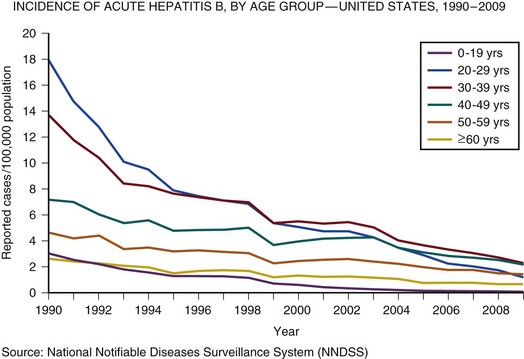
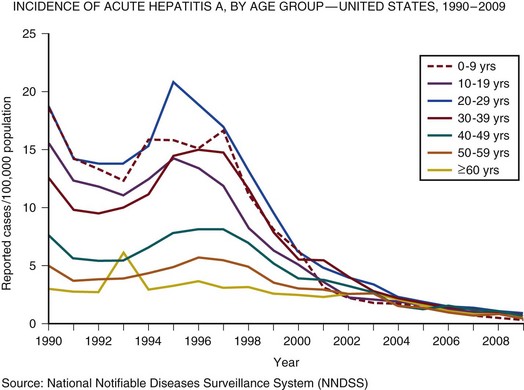
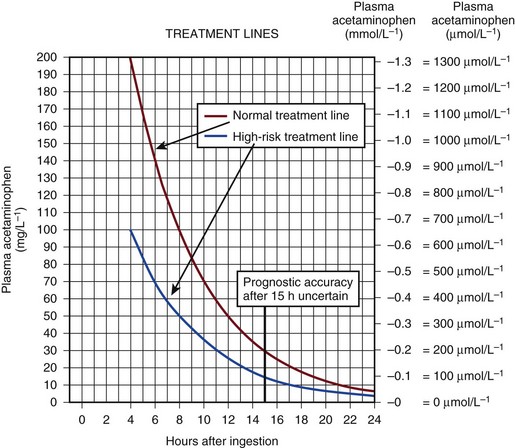
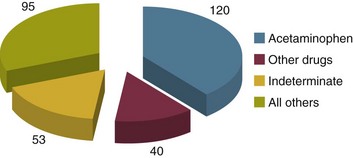
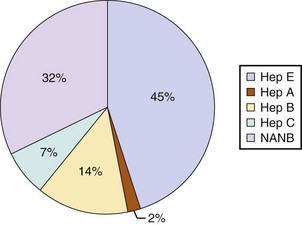
75 The pathologic basis of acute decompensation in chronic liver disease or acute on chronic liver failure (AoCLF) is incompletely understood but is thought to be precipitated by systemic inflammation, and if looked for hard enough, some signs or symptoms of infection will be seen in most patients.1,2 Patients with well-compensated cirrhosis often decompensate following a defined event. The precipitants can be split into two types: (1) those due to direct liver insult, such as ischemia, a toxic insult such as alcohol, or a superimposed viral infection and (2) those in which the liver is affected as a bystander in a systemic inflammatory process, such as following an episode of sepsis or a gastrointestinal bleed. This can be contrasted to the progressive liver failure of end-stage cirrhosis. Both present with similar clinical pictures but in AoCLF there remains the possibility of improvement and reversibility, and if these patients do recover they end up back on the previous mortality trajectory.1,2 Differentiating between the two is difficult but the presence of a precipitating factor and history can be useful. In patients with end-stage liver cirrhosis there is often a history of gradual deterioration in biochemical parameters, clinical status, and other organ function whereas in AoCLF there is often an acute deterioration. Organ support in the former setting is of dubious utility as it is almost always futile (Fig. 75.1). One group has proposed a working definition for AoCLF as “acute deterioration in liver function over a period of 2-4 weeks, usually associated with a precipitating event, leading to severe deterioration in clinical status, with jaundice and hepatic encephalopathy and or hepatorenal syndrome (HRS), with a high SOFA/APACHE II score.”1 AoCLF often occurs with multiple organ dysfunction. This may be because of the underlying precipitating event such as pneumonia or bacterial peritonitis or as a result of the severe deterioration in liver function. Often it is difficult to distinguish which came first. Renal failure is the most common organ failure associated with AoCLF and it is the severity of organ dysfunction that dictates the outcome.3 Alcoholic hepatitis is a common manifestation of alcohol abuse. Between 20% and 30% of heavy drinkers will present with it at some time. The clinical picture is variable from a relatively mild syndrome associated with loss of appetite, nausea, and vomiting with right upper quadrant pain to severe life-threatening liver decompensation, but the most obvious sign is the sudden onset of jaundice, and this can be the first indication of liver disease in a heavy drinker. Overall, following first presentation, 35% to 50% of patients will die within the first month of diagnosis.4 It usually presents following a bout of heavy drinking and often occurs on a background of cirrhosis. Patients frequently deteriorate after stopping alcohol. This may be due to the immunosuppressive effects of alcohol. In patients without cirrhosis abstinence and nutritional support can lead to a marked improvement in liver function over time. In alcoholic hepatitis liver enzymes are moderately raised with an aspartate aminotransferase (AST) rarely greater than 300 IU. The ratio of AST to alanine aminotransferase (ALT) is usually raised to greater than 2.6 A high white blood cell count and bilirubin are also typical. The liver is usually enlarged and fatty on ultrasound. Portal hypertension often complicates the clinical picture, presenting as worsening of ascites. Assessment of filling pressures can be difficult if ascites is tense. Further hemodynamic monitoring is recommended if there is any doubt regarding filling status or if cardiac output is thought to be compromised. The prognosis of patients with alcoholic hepatitis depends on the severity of the disease. Risk stratification is important, both in defining the indications for the use of specific pharmacologic agents aimed at the interruption of progression and in clinical research. Serum markers and liver histologic findings provide the best means for risk stratification, the presence of cirrhosis being a poor marker.7 In many cases, however, biopsy is precluded because of the risks of bleeding. Serum bilirubin, coagulation parameters, and creatinine have been shown to predict outcome and have been combined into several scoring systems; the discriminative function (DF); Child, Turcotte, Pugh (CTP); the Mayo end-stage liver disease (MELD) score; and the Glasgow Alcoholic Hepatitis (GAH) score have been used to evaluate outcome. Of these, the MELD score appears to offer the best prediction of mortality risk because of the inclusion of renal function (creatinine) as well as liver function; renal deterioration in this setting has a particularly poor prognosis.8 Standard critical care prognostic scores have also been used to assess the prognosis of patients with decompensated chronic liver disease of any cause. The sequential organ failure (SOFA) score has been shown to provide useful prognostic information.1,9 In early disease, abstinence and good nutrition, including supplementation with B vitamins, is the mainstay of management. In severe disease, progression to hepatic encephalopathy and organ failure can be rapid. In addition to general supportive care, specific therapies have been used in an attempt to halt and reverse the hepatic inflammation and prevent the fall into multiple organ failure and ultimately death. Of these, and pentoxifylline have been the most extensively studied.10 Control of bleeding episodes is via direct endoscopic therapy, usually in the form of band ligation in the case of esophageal varices.14 Terlipressin, a synthetic analog of vasopressin, can be used as an adjunct to band ligation. Terlipressin induces splanchnic vasoconstriction and reduces portal pressure and has been shown to reduce mortality rate.15 There is some evidence that somatostatin may reduce the amount of blood transfused but the evidence is weak.16 Uncontrolled bleeding due to either failed endoscopic or pharmacologic therapy or when resources do not allow immediate endoscopic therapy may be managed with balloon tamponade of the stomach or esophageal varices. Primary control of esophageal varices is usually successful but balloons should not be inflated for more than 12 hours because of the risks of mucosal necrosis. Care should be exercised when inserting and inflating a Sensgtaken-Blakemore or Minnesota tube because perforation of the esophagus is usually fatal in this setting. Following control with the insertion of a balloon tamponade device, further endoscopic therapy, surgical shunting, or transjugular intrahepatic portosystemic stent shunts (TIPS) may be attempted. Of the shunt procedures used TIPS is the most commonly attempted, where technical skills are available. The procedure involves decompressing the portal circulation into the hepatic vein via a stent inserted percutaneously through the liver via the internal jugular vein. The procedure is a very successful salvage therapy for controlling bleeding but can induce certain complications, including (a) volume overload as a result of portal shunting into the right atrium, which can induce heart failure; (b) bypassing the portal circulation resulting in ischemic liver injury and, rarely, liver failure; and (c) worsening of encephalopathy grade (the most common complication) resulting in failure to wake from the procedure, the need for extended ventilation, and the risk of hospital-acquired infection. There have not yet been any controlled trials of TIPS as salvage therapy in this setting and it is unclear what role TIPS has in the management of acute bleeding varices as it may just result in prolongation of the dying process. Despite better prevention of rebleeding when compared to endoscopic therapy in patients with bleeding varices, a mortality benefit cannot be shown with TIPS.17 Recently, Garcia-Pagan and colleagues have shown that TIPS, not done as salvage therapy, but used as a primary form of therapy once the initial bleed has been controlled, results in a dramatic reduction in rebleeding rates and mortality rates.18 This study has huge resource implications and needs to be repeated. Coagulation abnormalities are commonly present in patients with acutely bleeding varices, and abnormalities in clotting factors have been shown to be independent prognostic factors in patients with esophageal varices. Attempts to treat coagulation abnormalities with clotting components should be undertaken during an acute bleed to include fresh frozen plasma (FFP) and platelets targeted to correct abnormal partial thromboplastin time (PTT) and platelet count. Activated factor VII has also been investigated in this setting. The authors showed that in patients with worse cirrhosis, as defined by Child-Pugh grades B and C, recombinant activated factor VII (rFVIIa) increased the chances of controlling the bleeding, although a more recent study by the same group shows no difference between groups.19 Bleeding from varices is associated with infection. Between 35% and 60% of cirrhotic patients with bleeding varices have documented infection over the following 2 weeks.20 The use of antibiotic prophylaxis has been shown to reduce the incidence of infection in this group of patients and to improve short-term mortality rate.20,21 It has been proposed that infection is the precipitant to an acute increase in portal pressure that may trigger bleeding.22 Patients with chronic liver disease are at increased risk of infection. The immunosuppression associated with chronic liver disease is incompletely understood but relates to a range of factors including an impaired innate and adaptive immune response.23,24 In cirrhotic patients with ascites, decompensation to liver failure is often precipitated by infection. Bacterial peritonitis is a common and severe complication. It can be completely asymptomatic but can present with a range of symptoms and signs including local signs of peritonitis, abdominal pain, diarrhea, and signs of systemic inflammation such as fever, rigors, raised white blood cell count, hypotension, and tachycardia.25 Bacterial peritonitis is often spontaneous, without any obvious source, although it can be secondary to other intra-abdominal disease. It is generally caused by aerobic gram-negative bacteria, although gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA) should be suspected if there is treatment failure, especially if the patient has been taking prophylactic antibiotics.26 In any patient with ascites and decompensation of liver disease, spontaneous bacterial peritonitis (SBP) should be suspected. Diagnosis is demonstrated by sampling of the ascitic fluid. An absolute leukocyte count in ascitic fluid of greater than 250/µL is diagnostic. Antibiotics should be started as soon as SBP is suspected, following blood culture and a diagnostic ascitic tap. Empiric broad-spectrum antibiotic coverage for gram-negative and MRSA organisms (depending on local prevalence) should be started until cultures are available. Renal failure is a significant complication of SBP in patients with cirrhosis and is discussed later. The use of human albumin solution (HAS) in SBP has been shown to reduce the incidence of progression to renal failure and should be considered in this setting.25 The presentation of AoCLD will depend on the precipitating event or events but the clinical picture and pattern of organ failure will on the whole look very similar. Hyperbilirubinemia and the resulting jaundice are almost universal, as are other biochemical manifestations of poor liver function. Plasma protein production is deranged, leading to a prolongation of prothrombin time and hypoalbuminemia. Thrombocytopenia due to hypersplenism and sepsis is also characteristic. The effects on the coagulation system are complex, however, as there is a concurrent reduction in the production of endogenous anticoagulant proteins in the liver resulting a relative rebalancing. Infection leads to consumption of factors and platelets and so the use of thromboelastography is recommended in bleeding patients. Lower levels of factors can result in difficulties maintaining coagulation during times of stress, particularly during infections, and evidence of a heparin-like effect has been shown.27 It is also interesting to note that the incidence of thromboembolism is not lower in patients with cirrhosis even in the setting of a prolonged international normalization ratio (INR), suggesting that standard measures of coagulation are unreliable.28 The pattern of circulatory changes associated with cirrhosis is distinctive. Hypotension and an increase in cardiac output are typical, resulting in a hyperdynamic circulation. Peripheral vasodilatation, however, is not distributed evenly and occurs mainly in the splanchnic circulation as a result of sinusoidal portal hypertension. Splanchnic vasodilatation results in effective arterial underfilling with the resulting activation of compensatory mechanisms.29 During decompensation circulatory changes become more pronounced with an increase in portal pressure; systemic vasodilatation worsens and blood pressure drops further and becomes less responsive to vasopressor support. Recent work shows that cardiac output may fall in those who develop hepatorenal failure, as do cardiac filling pressures and pulmonary artery pressure. These changes point toward some form of cardiac depressant factor associated with decompensation.29 There is a reduction in intrarenal blood flow, due to the activation of compensatory mechanisms designed to maintain arterial volume, such as the renin-angiotensin system and sympathetic nervous system. This results in a further reduction in glomerular filtration rate (GFR) and urine output. Cardiac function may be further compromised by liver failure–associated cardiomyopathy, which is manifested by a low or normal cardiac output in the setting of reduced afterload.30 There may also be a relative hypovolemia in the setting of a normal or raised central venous pressure because of raised intra-abdominal pressure due to ascites. Echocardiography is very helpful in the delineation of any cardiac dysfunction and can help assess pulmonary artery pressure to assess if the patient has portopulmonary hypertension.31 A pulmonary artery (PA) catheter should be used if there is any doubt. Acute kidney injury (AKI) is the most common form of organ dysfunction seen in patients with AoCLF and has significant attributable mortality risk.32 There are four main causes: those associated with bacterial infection are the most common, followed by hypovolemia and parenchymal disease, with hepatorenal failure the least common.3 However, hepatorenal failure has a particularly poor outcome. The diagnosis of AKI in the setting of liver disease is problematic, as conventional measures of renal function are less indicative of renal function. Hepatorenal syndrome is a severe and progressive reduction in renal function in patients with severe liver disease and presents in two clinical patterns, type 1 and type 2. Hepatorenal syndrome represents the renovascular response to the profound circulatory changes associated with cirrhosis. Type 2 HRS is characterized by a less severe and more gradual reduction in renal function associated with diuretic-resistant ascites and hyponatremia. It is usually seen in association with end-stage cirrhosis. Type 1 HRS is characterized by the rapid decline in renal function defined as a 100% increase in serum creatinine to a level greater than 221 mmol/L or a 50% reduction in 24-hour creatinine clearance to a level less than 20 mL/minute in less than 2 weeks.33 Initially HRS is potentially reversible as the kidneys are functionally normal; however, the longer the circulatory changes last without reestablishing the GFR, the less likely are the kidneys to recover, and the distinction between HRS and acute tubular necrosis becomes less clear. In most patients there is a precipitating event, while in others it occurs in close proximity to an event such as the resolution of SBP. The diagnosis is made by the exclusion of other causes and is based on criteria developed by the International Ascites Club.33 Until recently there was no effective treatment for patients with HRS other than liver transplantation, but over the past 15 years observational and interventional studies have shown that countering the compensatory circulatory changes that promote intrarenal vasoconstriction with use of systemic and selective vasoconstrictors plus plasma volume expansion with albumin can lead to significant improvement in renal function.34 The aim of therapy is to focus vasoconstriction on the dilated splanchnic vessels, resulting in a redistribution of the blood volume back into the systemic arterial circulation. Over time this results in suppression of the compensatory mechanisms; a reduction in plasma renin activity, sympathetic activity, and circulating catecholamines; and an associated increase in GFR, sodium excretion, and urine output.35 Many different vasoconstrictors have been tried in this setting. The drug most commonly studied is terlipressin and it is often given with 20% human albumin solution (HAS). This approach has been subjected to a randomized controlled clinical trial in which the addition of albumin was associated with an improvement in outcome.36 Evidence suggests that vasoconstrictor therapy, with or without plasma volume expansion, results in an improvement in renal function in about a third of patients with type I HRS. The best predictors of response to therapy is the baseline serum creatinine, and those who do respond have a significant rise in their arterial pressure.34,37 Ammonia production in the gut is important in the pathogenesis. Ammonia is usually cleared from the portal circulation in the liver. The mainstays of treatment are colonic cleansing with enemas and enteral disaccharides such as lactulose. Both of these procedures have been shown to improve encephalopathy grades. The restriction of protein, once fashionable in patients with liver disease, is contraindicated. The majority of these patients are malnourished and adequate enteral nutrition is essential.38 Large volume paracentesis should be considered in any patient with tense ascites. There is evidence that large volume paracentesis improves lung mechanics and oxygenation in nonventilated patients but data in ventilated patients are lacking.39–41 Hepatic hydrothorax is a persistent pleural effusion, almost always associated with ascites, usually on the right side of the chest. It can be massive in size, containing many liters of fluid, and is thought to be due to communication from the peritoneum. Management can be difficult. Direct drainage with thoracocentesis is usually inadvisable as the fluid accumulation is persistent, leading to continued need for chest tube placement, which carries the risk of infection. Management should be directed at the ascites and includes diuretics, salt restriction, and paracentesis. TIPS can be used to control both ascites and hydrothorax but is associated with worsening encephalopathy grade in some patients.42 Adrenal dysfunction in patients with critical illness has been documented extensively, particularly in patients with septic shock.43 Studies have suggested an improvement in outcome with steroid use but subsequent investigations have been less clear. Currently recommendations suggest steroid treatment should not be based on adrenal stimulation and should be reserved for those with vasopressor-resistant septic shock or early acute respiratory distress syndrome (ARDS) within 14 days of onset.43 Patients with liver failure have been shown to have a high incidence of adrenal suppression as well, although it is not clear if this has a different etiology to other critically ill patients. Patients with adrenal suppression have worse hepatic and renal function, more organ failure, and a higher intensive care unit (ICU) and hospital mortality rate.44 There remains, however, a lack of consensus regarding definitions of adrenal dysfunction and appropriate testing in patients with liver disease and in which patients require treatment.45 However, in patients with cirrhosis and septic shock admitted to the ICU with adrenal insufficiency as defined by a suboptimal response to ACTH stimulation, replacement of hydrocortisone (50 mg every 6 hours) results in a higher incidence of shock resolution and hospital survival when compared to historical control subjects.46 Despite ICU care, the outcome in patients with decompensated cirrhosis is poor. In one study overall cumulative mortality rates were 36% in the critical care unit, 46% in the hospital, and 56% at 6-month follow-up.47 In patients who require organ support within the critical care environment a number of observations can be made. Derangement of acute physiology at admission is a predictor of outcome, as it is for unselected patients admitted to the ICU.48 In addition, the number of organs requiring support also is predictive of outcome, as it is in unselected patients.47,49 If you compare the number of organs failing (defined as an organ-specific SOFA score greater than 3) there is an excess mortality rate associated with cirrhosis when compared to an unselected group (Fig. 75.2).50 Patients with cirrhosis and three organ systems requiring support have a mortality rate in excess of 90% in some studies.49 Severity of liver disease at admission has a significant bearing on outcome irrespective of indication for admission to the ICU.49,51 Acute liver failure (ALF) is a syndrome manifested by the rapid cessation of normal function in individuals with previously normal livers. The rate of decline in function dictates the manner in which the syndrome manifests and influences the outcome. The cause is the main influence on the rate of progression and the likelihood of spontaneous recovery.52 The pathologic basis of the massive hepatic necrosis was described in detail by Lucké and Mallory following the Second World War in 1946.53 The presence of the American army in East Asia and Africa resulted in exposure to both epidemic and serum hepatitis, and data regarding the clinical course of the syndrome and its pathologic features were collated via the army medical services. In 1970 Trey and Davidson introduced the term fulminant hepatic failure (FHF) to encompass the current clinicopathologic understanding of the syndrome.54 This definition was an attempt to encapsulate the clinical course and to differentiate it from decompensation of chronic liver disease. They described a syndrome of rapidly progressing liver failure (within 8 weeks) in which the defining point was the onset of hepatic encephalopathy following the onset of symptoms in someone without previous liver disease. They make the point that the syndrome is potentially reversible in some patients. This definition is still used today; however, it has become clear that this definition is too narrow and that subgroups exist. This point is important as these subgroups predict the likely prognosis and potential for survival without a liver transplant.52 The rate of progression from the onset of jaundice or other initial symptoms (such as fatigue or acute viral illness) to development of encephalopathy is used to define subgroups. This in part relates to the cause of liver failure and to the way the pathologic expression of the pattern of organ failure presents. For example, patients with significant acetaminophen-induced hepatotoxicity will generally present with liver failure within 7 days of ingestion, unless ingestion was staggered over a period of time when the timing of liver insult is difficult to define. Patients often present with cardiovascular collapse and renal failure before they become encephalopathic. In contrast, patients presenting with seronegative hepatitis (unknown cause) can have a very variable presentation, some with a prolonged illness over a period of months, resulting in a patient who is deeply jaundiced with evidence of portal hypertension, such as ascites, at the onset of encephalopathy, and others with a relatively short presentation period. These two extreme ends of the syndrome split the group into hyperacute and subacute, with ALF in the middle. Interestingly, it is the hyperacute group that has the best chance for spontaneous recovery, although this group has the highest risk of cerebral edema. The subacute group has the worse prognosis with medical management alone.52 ALF is rare, with about 1 to 6 cases per 1 million of the population in the developed world.55 The incidence in the rest of the world is less clear because of the paucity of data (Box 75.1). Worldwide, and in the developing world in particular, approximately 95% to 100% of patients presenting with ALF will have viral hepatitis.56 Within the United Kingdom and as recently reported in the United States, paracetamol (acetaminophen) hepatotoxicity is the leading cause of ALF. This is followed by liver failure of unknown cause or seronegative hepatitis.57,58 The pattern of ALF within the United Kingdom and United States has been changing over the past 30 years.57,58 Up until the late 1990s, the rate of hospital admission due to paracetamol ingestion had risen year by year. In the United Kingdom paracetamol overdose (POD) is usually due to deliberate self-harm. In contrast, the U.S. data suggest that over half of all patients with ALF due to POD were due to therapeutic misadventure. Some doubts have been expressed regarding this interpretation as misadventure in some cases that appear to be occult suicide attempts.58,59 In 1998 legislation was introduced in the United Kingdom to restrict the over-the-counter sale of paracetamol to 16 tablets from most retail outlets and 32 from pharmacies in the form of blister packs. Interpretation of the effects of the legislation have proved to be complex, in a large part because there were no prospective audits initiated at the time to study it. Early interpretation suggested that admissions to hospital, severe liver toxicity, and transplantation for POD fell.60 The picture is more complex, though. Death rates have fallen; however, much of this reduction can be related to the withdrawal of co-proxamol during the mid-2000s. Co-proxamol, a combination of paracetamol and dextropropoxyphene (a mild opioid), was a prescription-only medication associated with a high degree of fatality if taken in overdose, most of this out of hospital and attributed to the effects of dextropropoxyphene on respiratory depression.61 Admission to hospital because of POD has continued to rise year by year but the number of pills taken on average has fallen and so the case fatality rate is lower. The numbers of patients being referred to transplant centers is also lower than before the legislation, but the number of patients transplanted has remained about the same, indicating the small number of determined overdoses and misadventure cases has remained relatively stable. In the United Kingdom and the United States, the incidence of acute hepatitis A virus (HAV) and hepatitis B virus (HBV) infection has fallen dramatically since the 1980s57,58 (Figs. 75.3 and 75.4). Less than 1% of acute hepatitis A or B progress to ALF. In the United States and United Kingdom, as a proportion of the total, the number of admissions with ALF due to viral hepatitis has fallen steadily and is currently responsible for less than 5% of all admissions in the United Kingdom and 11% in the United States.57,58 Indeterminate hepatitis (non-A to E hepatitis, seronegative ALF, non-A/non-B hepatitis) is often presumed to be viral in origin and is the most common presentation excluding POD in the United Kingdom and United States along with viral hepatitis in the developing world. It is a diagnosis of exclusion and, as diagnostic capabilities improve, is falling in incidence in some centers.57 Acetaminophen-induced liver failure is the cause of the vast majority of hyperacute liver failure. Acetaminophen poisoning is a common cause of presentation to acute and emergency departments in the United Kingdom and United States; however, the case progression to ALF following paracetamol ingestion is rare at just 0.6% of all presentations in the United Kingdom.62 Following poisoning the half-life of acetaminophen is greatly prolonged because of the saturation of glucuronidation and sulfate conjugation. As a result, there is an increase in the quantity of NAPQI produced. NAPQI is extremely reactive in biologic systems and has a short half-life. Following poisoning, reaction of NAPQI occurs within the centrilobular portions of the liver and leads to necrosis in experimental models. It reacts with cellular constituents in a covalent and noncovalent manner. The exact mechanisms by which NAPQI induces cell death are incompletely understood but include the deactivation of critical cellular proteins, the induction of reactive oxygen species, and the activation of Kupffer cells.63 The loss of regulatory protein function results in abnormal calcium homeostasis and resultant energy failure within the cell and mitochondria.63 Other events such as noncovalent interaction with intracellular signaling and lipid peroxidation also contribute to the toxicity of this molecule. Following this primary toxic phase there is a secondary or extrinsic phase. This extrinsic phase is equated with the recruitment of immune cells to the liver. The liver is one of the major immune organs of the body. Up to 35% of the liver is made up of nonparenchymal cells, including endothelium, Kupffer cells, and resident lymphocytes. These cells, together with macrophages within the liver, perform a major role in immune regulation and in the filtering of antigens from the gut contents via the portal circulation. They are also implicated in the pathologic processes that occur following liver insult. Massive activation of immune cells in response to the intrinsic cellular damage induces the release of cytokines and chemokines both locally and into the systemic circulation.64,65 The minimum dose that can induce hepatic damage appears to be about 125 mg/kg. This represents 15500-mg tablets in a 60-kg individual, although hepatic necrosis has been recorded at much lower doses, especially if associated with hepatic enzyme induction. Doses above 250 mg/kg (30500-mg tablets in a 60-kg individual) will often produce damage, and doses in excess of 350 mg/kg invariably produce significant damage.66 The antidote for acetaminophen poisoning is N-acetylcysteine (NAC). It provides complete protection against hepatotoxicity if given within 12 hours of nonstaggered ingestion.67 Within 12 hours, if the time from ingestion is known with certainty and a plasma acetaminophen level is obtained, reference can be made to the nomogram to see if the potential for hepatotoxicity is present. The nomograms are unreliable if the time from ingestion is uncertain or if there was staggered ingestion over a period of time, as often occurs with therapeutic misadventure or repeated overdose. The use of alcohol often accompanies ingestion, making timing unreliable. Situations that alter normal cytochrome P-450 function such as drug induction, (chronic ethanol use, phenytoin, and isoniazid) again render the information unreliable.68 The use of the nomogram as the only basis for the decision to withhold NAC therapy is to be discouraged because of the uncertainty associated with this timing and the catastrophic potential if NAC is erroneously withheld (Fig. 75.5). The main effect of NAC is to increase hepatic glutathione production. This promotes the conjugation of NAPQI and its subsequent excretion. In addition, NAC may act as an antioxidant within and outside the liver. It is most effective if given within the first 8 hours following overdose but is still effective following this, although less so. There is some evidence that NAC is effective when administered to the patient up to 72 hours following poisoning, although the mechanism of action is unclear and probably relates to antioxidant effects rather than to any effect on acetaminophen metabolism.69 The role of NAC in established ALF from any cause is more controversial despite widespread use, but recent data have lent support to its use certainly at lower coma grades. However, the mechanism of action is unclear.70,71 Both epidemic and serum hepatitis were recognized well before the viral form was discovered. In the seminal work of Lucké and Mallory in 1946, they describe 196 patients who died of ALF following both epidemic hepatitis and serum hepatitis, related to the administration of blood products during World War II.53 ALF following acute viral hepatitis is uncommon, with a reported incidence of 0.2% to 4% depending on the underlying cause.72 Liver failure following viral hepatitis tends to run an acute or hyperacute course with the onset of encephalopathy occurring within days or weeks of the first symptoms.73 Hepatitis A is now rare in the United States and Western Europe but is still a common form of acute enterally transmitted hepatitis in the underdeveloped world where it is mainly a mild and self-limiting illness of children.73,74 Infection with hepatitis A carries the lowest risk of conversion to acute hepatic failure of all the hepatotropic viruses. In the West the incidence of ALF following hepatitis A appears to be higher than in the endemic areas. It occurs more commonly in adults and is more severe. Persistent infection with hepatitis A has also been reported75 and even recurring following liver transplantation.76 Diagnosis is made on the basis of IgM antibodies at the time of hospitalization although false-negative results can occur.77 Hepatitis B may lead to ALF is several settings. It occurs most commonly following acute infection but can occur following an acute increase in viral replication following immunosuppressive therapy such as cancer chemotherapy or steroids as well as with coinfection with other viral agents such as delta virus. The host immune response is thought to be responsible for the severity of reaction to the virus, subsequent clearance, and the induction of ALF. This can be seen following the withdrawal of immunosuppressive therapy when there is a very active immune response to the increased viral load. In acute infection surface antigen (HBsAg) is often negative but IgM antibodies to the viral core (HBcAb) will usually be positive. Mutations to the precore stop codon or the core promotor region of the viral genome may be associated with a higher incidence of ALF.78 These particular genes code for HBeAg, and lack of this antigen is associated with a more profound immune response. There have been reports of a very high incidence of ALF associated with outbreaks of acute hepatitis B in the setting of intravenous drug use and chronic hepatitis C infection.79 Lamivudine antiviral therapy for acute HBV-induced hepatitis has been tried, but because of the lack of controlled trials, it is difficult to know if it or other drugs like it help.80 Hepatitis C, as a cause of ALF, is rare in northern Europe and the United States but has been described.81 There is a wide spectrum of clinical presentation associated with acute infection with the more florid presentation associated with a more rapid clearance rate, suggesting that the magnitude of the initial immune response is important.82 Liver failure associated with acute infection appears to be more common in India and the Far East.83 Acute infection may contribute to decompensation in patients with preexisting liver disease, and hepatitis C seropositivity may predispose to liver failure when coinfection with another hepatotropic virus is present.83 Hepatitis E is likely the most common cause of ALF worldwide and certainly for the Indian subcontinent.83 In the Far East acute HBV infection is the most common cause of ALF due to the high levels of endemicity.84 The existence of hepatitis E was inferred before serologic evidence was available by a process of exclusion. It was long assumed that most if not all epidemic enteric hepatitis was due to the A virus. When serologic markers for hepatitis A became available in the early 1980s it was apparent that the majority of waterborne epidemic hepatitis were due to other agents, producing a syndrome similar clinically to hepatitis A.85 Hepatitis E does not produce a chronic infection and in the vast majority is a self-limiting infection that occurs most commonly in young adults, in contrast to hepatitis A, which is primarily an infection of children. The incidence of hepatitis E associated with ALF is low, with a case-related mortality rate reported at about 0.5% to 4% in the general population but with a much higher mortality rate in pregnancy, as high as 20% in the third trimester. Pregnancy itself appears to be a risk factor for ALF, with a quarter of all infected female patients reported as pregnant in one series. However, this may not be particular to hepatitis E but rather due to the high incidence of epidemic hepatitis E in a relatively immunosuppressed state and pregnant patients do not have a worse prognosis compared to nonpregnant patients with ALF due to hepatitis E.83,86 Five genotypes have been described with 1 to 4 infecting humans and genotype 5 infecting only birds. Genotype 1 is the most common in Asia, with genotype 2 more common in Africa and South America. Genotype 3 can infect both human and animals, whereas genotype 1 and 2 have been described only in humans.87 In the West, travel to endemic areas is a risk factor, but sporadic cases are now being seen more commonly in the developed world. Some of these cases have been associated with contact with animals.87 Seronegative hepatitis is the second most common cause of ALF worldwide in most published series. In northern Europe and the United States it comes in behind paracetamol toxicity, and in the developing world it is second to acute viral hepatitis (Figs. 75.6 and 75.7). Seronegative hepatitis can be conveniently thought of as a single entity. In reality it is probably an amalgam of various causes that have defied definition or characterization, including acute presentations of autoimmune hepatitis, idiosyncratic drug reactions, and viruses.88,89 Seronegative hepatitis has a variable clinical presentation including a slow insidious onset of general malaise, jaundice, and ascites followed by progressive signs and symptoms of liver failure. At presentation the patient may be deeply jaundiced and may already have ascites and splenomegaly. It can also present with a hyperacute picture. The pattern of signs, symptoms, and organ failure is dictated by the rate of progression. In subacute seronegative hepatitis, the presenting clinical picture can be similar to that of decompensated chronic liver disease, causing occasional diagnostic difficulty. A liver biopsy is sometimes needed to differentiate between the two. Autoimmune hepatitis can occur at any time of life from childhood until old age. It is more common in women with a male/female ratio of 1:3. Presentation can be asymptomatic, discovered following routine laboratory testing, or more commonly, the infection may present with jaundice and general malaise. In rare cases autoimmune hepatitis can present as ALF.90 Unfortunately there are no serologic tests with sufficient sensitivity or specificity to make the diagnosis certain. Patterns of markers in the right clinical setting and in the absence of other causes may be useful.91 The diagnosis of autoimmune hepatitis in the setting of ALF is difficult and a degree of uncertainty often lasts. Classically there is a combination of elevated immunoglobulin levels, autoantibodies, and confirmatory histologic evidence of hepatitis in the absence of active viral markers. Elevation of autoantibodies is often seen in patients with ALF due to other causes such as drug-induced liver disease.90 Liver failure should be assessed in the same way as for other causes, and once signs of encephalopathy become apparent, standard prognostic criteria apply. The role of steroids is unproved but should be considered in patients prior to the onset of encephalopathy. Once the patient is listed for transplantation steroid use is controversial because of increased risk of infection. Drug-induced liver disease may be due to a known dose-dependent toxicity, as with paracetamol. Alternatively, unpredictable, rare, idiosyncratic reactions can occur with any drug with a frequency of about 1 in 1000 to 1 in 100,000 patient prescriptions.68 Drug-induced liver failure can mimic all forms of acute and chronic liver disease. However, the predominant clinical presentation consists of either acute hepatitis or cholestatic liver disease. The former has a reported mortality rate of 10% irrespective of the drug. The patterns of injury associated with these idiosyncratic reactions relate to the mechanism of damage and the cells involved.92 Many patterns have been described but it is massive hepatic necrosis that most often presents as ALF. Liver failure associated with severe cholestasis and veno-occlusive disease is also seen.92 Liver injury usually is seen within 6 months following the initiation of therapy. Even if the diagnosis of drug-induced liver failure is considered, a search for other possible causes should be performed. Although any drug is capable of inducing liver injury, more common causes include herbal remedies and recreationally used drugs such as “ecstasy” and cocaine. If suspected, a comprehensive history with a timetable of drug initiation should be constructed. Management includes stopping the offending drug and supportive care. Transplantation should be considered once liver failure occurs as the outcome from drug-induced liver failure, other than that induced by paracetamol, can be poor.93 Presentation profiles for various drugs causing ALF are shown in Table 75.1.68 Table 75.1 Idiosyncratic Drug Reactions and Effects on Cells From Lee WM: Drug-induced hepatotoxicity. N Engl J Med 2003;349(5):474-485. In general, pregnancy-associated liver failure has the best prognosis when compared to all other causes of ALF, and prompt recovery can be expected with delivery of the fetus in most cases, if recognized early enough. Pregnancy-associated liver failure can present in several ways including the syndromes of preeclampsia and the HELLP syndrome (hemolysis, elevated liver function tests, and low platelets), liver rupture, and acute fatty liver of pregnancy.94 Any cause of ALF can occur during pregnancy, and in particular viral hepatitis can be particularly fulminant in its course. This is especially true for hepatitis E and herpes simplex virus infection. Although originally thought to be a variant of preeclampsia there is evidence that HELLP syndrome may be a separate entity and in fact more related to acute fatty liver of pregnancy.95 Both HELLP and preeclampsia appear to be related to an endothelial injury, possibly immunologically initiated with activation of the coagulation and complement cascades and an imbalance of prostaglandin and thromboxane resulting in increased vascular tone, microangiopathic hemolytic anemia, and vascular thrombosis.96 About 2% to 12% of severe preeclampsia is complicated by HELLP.94 HELLP is thought to occur in approximately 1 in every 1000 live deliveries and patients usually present in the third trimester with nonspecific signs often seen in preeclampsia such as weight gain due to edema and hypertension. In addition, right upper quadrant pain accompanied by nausea and vomiting is commonly seen. Laboratory abnormalities include hyperbilirubinemia due to liver dysfunction and evidence of hemolysis. Transaminases are modestly raised and the platelet count is usually less than 100,000/µL. Liver biopsy, while commonly normal in preeclampsia, shows specific changes of periportal necrosis and fibrin microthrombi. Microvascular steatosis may also be present.96 The liver failure associated with HELLP is manifest as a prolonged prothrombin time and ascites. Renal failure is common. Maternal mortality rate is low but fetal mortality rate has been reported to be between 20% and 60%, although some reports suggest this is lower at 7% and associated with twin pregnancies.96–98 The treatment of choice is delivery of the baby. Conservative therapy is associated with an increase in both maternal and fetal complications. Spontaneous rupture of the liver can occur in the setting of both preeclampsia and the HELLP syndrome, although it can occur de novo. It often presents with sudden onset of right upper quadrant pain accompanied by signs of hypovolemia or shock and is more common in multiparous women.96 Spontaneous rupture of the liver has high maternal and fetal mortality rates. Its pathogenesis is unclear but it appears that periportal hemorrhage associated with HELLP syndrome may occur close to the capsule, resulting in lifting and bleeding into the potential space. These areas of the capsule then coalesce and rupture. Management of this devastating complication includes prompt delivery of the fetus, local surgical control with packs, and aggressive management of the accompanying coagulopathy. Embolization of any feeding vessels in the liver may be of utility if such skills are available. Hepatectomy followed by liver transplantation can be lifesaving and has been performed. Acute fatty liver of pregnancy (AFLP) occurs during the third trimester of pregnancy and should be considered in any patient exhibiting signs of liver dysfunction. It is uncommon with an incidence of approximately 1 in 6659 live births.99 If left untreated, maternal and fetal mortality rates are high. The treatment of choice is delivery and prompt recovery can then be expected. There is usually a prodromal illness over a couple of weeks with nonspecific symptoms progressing to jaundice and encephalopathy. Symptoms and signs of preeclampsia or HELLP syndrome are seen in a third of cases and there is some evidence of a common origin due to a fetal fatty acid metabolism disorder.95 Diagnosis is critical and it should be differentiated from viral hepatitis or hepatic failure due to other causes. Liver biopsy can aid in the diagnosis and can be performed via the jugular route if coagulopathy precludes the conventional approach. Characteristic zone 3 microvesicular steatosis is seen. Delivery is the best treatment if diagnosed early. Characteristic features include normoblasts on blood smears and high serum urate. Bleeding can be a major problem during operative delivery. On occasion transplantation may be the only viable option. Triggers for transplantation in pregnancy-induced liver failure are not well defined and the Kings College Criteria perform poorly. Arterial lactate in the setting of encephalopathy appears to be the best predictor.98 Wilson’s disease is a rare autosomal recessive disorder resulting from copper toxicity with primarily brain and liver manifestation. It usually presents in the second or third decade of life, although it can present from early childhood until late middle age.100 The disease can present with predominantly liver or neurologic symptoms. Neurologic symptoms relate to the distribution of copper to the basal ganglia and result in movement disorders. Patients presenting with liver disease may presents with an active hepatitis, established cirrhosis, or ALF. Other signs such as Kayser-Fleischer rings are associated but not pathognomonic for Wilson’s disease. These greenish brown rings in the cornea result from the deposition of copper. ALF due to Wilson’s disease can present at any age but more commonly presents in the early 20s. High urinary copper excretion is possibly the most predictable laboratory finding, although the patient may be anuric on presentation. A low serum ceruloplasmin is an additional indicator but again is unreliable in ALF. A high serum bilirubin in combination with modest elevations of transaminases and alkaline phosphatase is often seen, as is intravascular hemolysis, contributing to the raised bilirubin level. Patients with severe liver failure due to Wilson’s disease have an almost 100% mortality rate without liver transplantation, which should be considered as soon as the diagnosis is made.100 There is debate as to whether liver failure secondary to Wilson’s disease is truly ALF, as cirrhosis is invariably present on liver biopsy at the time of presentation. Nevertheless, many patients present acutely and the presentation is often catastrophic. In this sense the timing of the onset and the lack of previous symptoms place fulminant Wilson’s disease in the ALF group. In an uncontrolled series the administration of D-penicillamine before the onset of encephalopathy was associated with survival.101 The syndrome can be considered as part of the hypoxic hepatitis group as zone 3 necrosis is often seen. The metabolic demand of the neoplastic cells and congestion within the liver sinusoids results in infarction of hepatocytes. Hepatosplenomegaly and a raised alkaline phosphatase are often present. Other stigmata including palpable lymphadenopathy and marrow or peripheral blood film changes may be present. Imaging may be diagnostic, especially with massive hepatomegaly, but biopsy may be required.102 ALF secondary to Budd-Chiari syndrome is usually fatal without transplantation. The syndrome is defined as outflow obstruction to the hepatic veins and the underlying pathogenesis is thrombosis in the majority but tumor invasion or vascular membrane obstruction may be the cause. This is a rare disorder that occurs predominantly in young adults and affects more women than men. Overall, the 5-year survival rate varies from 50% to 80% in different series.103 The vast majority of patients with the syndrome have at least one predisposing clotting abnormality, either congenital or acquired, such as a malignancy, myeloproliferative disorders, protein C or S deficiency, polycythemia rubra vera, lupus anticoagulant, antithrombin III deficiency, antiphospholipid syndrome, etc.104
Diagnosis and Management of Liver Failure in the Adult
Decompensation of Chronic Liver Disease
Precipitating Factors of Acute on Chronic Liver Failure
Alcoholic Hepatitis
![]() Features suggestive of malnutrition such as muscle wasting and vitamin deficiency are seen commonly. In severe decompensation other intercurrent illnesses such as pneumonia or urinary tract infection or a gastrointestinal bleed are often present. Systemic inflammation may be the precipitant of decompensation in these patients.5
Features suggestive of malnutrition such as muscle wasting and vitamin deficiency are seen commonly. In severe decompensation other intercurrent illnesses such as pneumonia or urinary tract infection or a gastrointestinal bleed are often present. Systemic inflammation may be the precipitant of decompensation in these patients.5
Portal Hypertensive Bleeding
![]() Portal hypertension is a significant complication of chronic liver disease leading to the formation of portosystemic collateral vessels. Of these vessels, the most significant clinically are those that occur in the wall of the stomach and esophagus. In patients with portal hypertension as a result of cirrhosis the development of gastrointestinal varices occurs in approximately 60% at the time of diagnosis.11 The incidence of the first acute bleed in an unselected patient is relatively low at about 5% per year but it can be catastrophic when it occurs.12 The mortality rate associated with the event has fallen over the past 20 years from approximately 30% to 50% to about 20% and control of the initial bleeding episode is achieved in about 90% of patients.13 Half of all fatalities occur in the first 5 days following an acute bleed and about half of them are due to uncontrolled bleeding and the rest are due to multiple organ failure. This pattern remains the same when mortality rate in the first 6 weeks following the first bleed is examined.13
Portal hypertension is a significant complication of chronic liver disease leading to the formation of portosystemic collateral vessels. Of these vessels, the most significant clinically are those that occur in the wall of the stomach and esophagus. In patients with portal hypertension as a result of cirrhosis the development of gastrointestinal varices occurs in approximately 60% at the time of diagnosis.11 The incidence of the first acute bleed in an unselected patient is relatively low at about 5% per year but it can be catastrophic when it occurs.12 The mortality rate associated with the event has fallen over the past 20 years from approximately 30% to 50% to about 20% and control of the initial bleeding episode is achieved in about 90% of patients.13 Half of all fatalities occur in the first 5 days following an acute bleed and about half of them are due to uncontrolled bleeding and the rest are due to multiple organ failure. This pattern remains the same when mortality rate in the first 6 weeks following the first bleed is examined.13
Bacterial Peritonitis
Supportive Management in Critical Care
Outcome and Data on ICU Use in Decompensated Cirrhosis
Acute (Fulminant) Liver Failure
Etiology
Acetaminophen (Paracetamol)
Viral Hepatitis
Acute Presentation of Autoimmune Hepatitis
Drug-Induced Liver Disease
Type of Reaction
Effect on Cells
Examples of Drugs
Hepatocellular
Direct effect or production by enzyme-drug adduct leads to cell dysfunction, membrane dysfunction, cytotoxic T-cell response
Isoniazid, trazodone, diclofenac, nefazodone, venlafaxine, lovastatin
Cholestasis
Injury to canalicular membrane and transporters
Chlorpromazine, estrogen, erythromycin and its derivatives
Immunoallergic
Enzyme-drug adducts on cell surface induce IgE response
Halothane, phenytoin, sulfamethoxazole
Granulomatous
Macrophages, lymphocytes infiltrate hepatic lobule
Diltiazem, sulfa drugs, quinidine
Microvesicular fat
Altered mitochondrial respiration, beta-oxidation leads to lactic acidosis and triglyceride accumulation
Didanosine, tetracycline, acetylsalicylic acid, valproic acid
Steatohepatitis
Multifactorial
Amiodarone, tamoxifen
Autoimmune
Cytotoxic lymphocyte response directed at hepatocyte membrane components
Nitrofurantoin, methyldopa, lovastatin, minocycline
Fibrosis
Activation of stellate cells
Methotrexate, excess vitamin A
Vascular collapse
Causes ischemic or hypoxic injury
Nicotinic acid, cocaine, methylenedioxymethamphetamine
Oncogenesis
Encourages tumor formation
Oral contraceptives, androgens
Mixed
Cytoplasmic and canalicular injury, direct damage to bile ducts
Amoxicillin-clavulanate, carbamazepine, herbs, cyclosporine, methimazole, troglitazone
Pregnancy-Induced Liver Disease
Wilson’s Disease
Neoplastic Infiltration
Budd-Chiari Syndrome
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Diagnosis and Management of Liver Failure in the Adult