 RV clot
RV clot
 McConnell’s sign
McConnell’s sign
I. OVERVIEW: Venous thromboembolic event (VTE) refers to both deep venous thrombosis and pulmonary embolism. Episodic thrombosis and/or embolism may appear to be discrete events, but increasingly they are considered to be manifestations of the same process.
A. Deep Venous Thrombosis (DVT) refers to clot in the deep vessels of the venous circulation. While local discomfort with limb swelling and postthrombotic syndrome can occur, the majority of morbidity and mortality results from embolization to the pulmonary circulation. While the proximal veins of the lower extremity are the most common site of involvement and have a higher risk of embolization, upper extremity DVT can also lead to pulmonary embolism. Without DVT prophylaxis, the incidence of DVT in the intensive care unit (ICU) is quite high with ranges of 10% to 30% in medical patients and up to 60% in trauma patients. This risk can be significantly reduced, but not eliminated, with prophylaxis.
B. Pulmonary Embolism (PE) occurs when the pulmonary artery or one of its branches becomes obstructed by material. While thrombus is the most common, air, fat, or tumor can also cause embolism. The clinical significance of PE is broad and ranges from an incidental finding with no signs or symptoms to catastrophic circulatory collapse leading to death. The incidence of PE is approximately 112 per 100,000 person-years. The mortality from PE approaches 20% with higher mortality rates in those who are unstable at the time of presentation.
C. Risk Factors. DVT and PE have similar risk factors. Some of the most common risk factors in ICU patients include the following:
1. Recent surgical procedures: The risk of VTE is highest in patients who have had major orthopedic or general surgery procedures. Specific procedures associated with a high risk of VTE include hip or knee replacement and coronary artery bypass. Major trauma also has a high risk of VTE.
2. Spinal cord injury: Risk is highest immediately following injury and decreases with time.
3. Malignancy: Risk is highest in patients with advanced solid organ malignancies. Certain chemotherapy regimens may also increase risk.
4. Central venous lines: can lead to line-associated DVT with potential for embolization to pulmonary vasculature
5. Oral contraceptive pills: also increased with hormone replacement therapy
6. Pregnancy: Greatest risk occurs in postpartum period.
7. Immobility: due to injury, travel, or hospitalization
8. Thrombophilias: including antiphospholipid antibody syndrome, heparin-induced thrombocytopenia, as well as hereditary causes, including antithrombin deficiency, Protein C and S deficiencies, Factor V Leiden mutations, and prothrombin gene mutation
9. Previous VTE
D. VTE in the ICU. The incidence of VTE in the ICU is difficult to determine as the diagnosis requires a high degree of suspicion in patients with other ongoing pulmonary processes, and obtaining confirmatory testing is not always feasible in a critically ill patient. Regardless of the reason for ICU admission, critically ill patients are at increased risk for VTE as most patients are immobile and many receive central venous catheters. The incidence in postmortem studies of ICU patients is 7% to 27%.
E. Prophylaxis in ICU patients. The use of pharmacologic prophylaxis with either low-dose heparin or low-molecular-weight heparin (LMWH) decreases but does not eliminate the risk of DVT in patients admitted to the ICU and thus should be used in patients without contraindications, such as active bleeding. Patients who cannot receive pharmacologic prophylaxis should receive mechanical prophylaxis. Intermittent pneumatic compression of the lower limbs is the mechanical intervention with the highest quality data. Regardless of the strategy used for prophylaxis, it is important to remember that no strategy provides complete protection against VTE.
II. DIAGNOSIS
A. DVT
1. Exam: Limb pain, edema, and palpable cord are suggestive of DVT. Sudden severe pain associated with cyanosis or evidence of compartment syndrome or impaired arterial circulation is suggestive of massive proximal thrombosis (phlegmasia cerluea dolens). Exam alone is not sufficient to rule in or rule out DVT.
2. Ultrasound utilizes vein compressibility and/or color flow imaging. It has high sensitivity and specificity for DVT although may miss clots in pelvic veins. Ultrasound is ideal in ICU patients as it can be done at the bedside without radiation exposure.
3. Computed tomography (CT) venogram can be done at the same time as a CT for evaluation of PE, eliminating the need for an additional imaging study; however, this test leads to increased radiation exposure and many protocols have higher doses of contrast material. CT-venogram, unlike ultrasound, can evaluate for pelvic thrombosis.
4. Magnetic resonance (MR) venogram can image the lower extremity veins, including pelvic veins, without use of contrast material or radiation. Disadvantages include availability and time required to obtain study.
5. D-dimer: The main utility of the D-dimer assay is in ruling out DVT in the emergency department setting. Due to a high degree of false positives in the setting of critical illness, this test has minimal utility in the ICU.
B. Pulmonary Embolism
1. Clinical exam: Although findings such as tachycardia, hypoxia, tachypnea, fever, and hypotension may raise clinical suspicion for PE, exam alone is not enough to rule in or rule out PE.
2. Lab tests: While brain natriuretic peptide (BNP) and troponin may be elevated, these tests lack the necessary specificity to be useful in diagnosis. Additionally, as in DVT, the D-dimer is often elevated even in ICU patients without PE, limiting its utility in diagnosis.
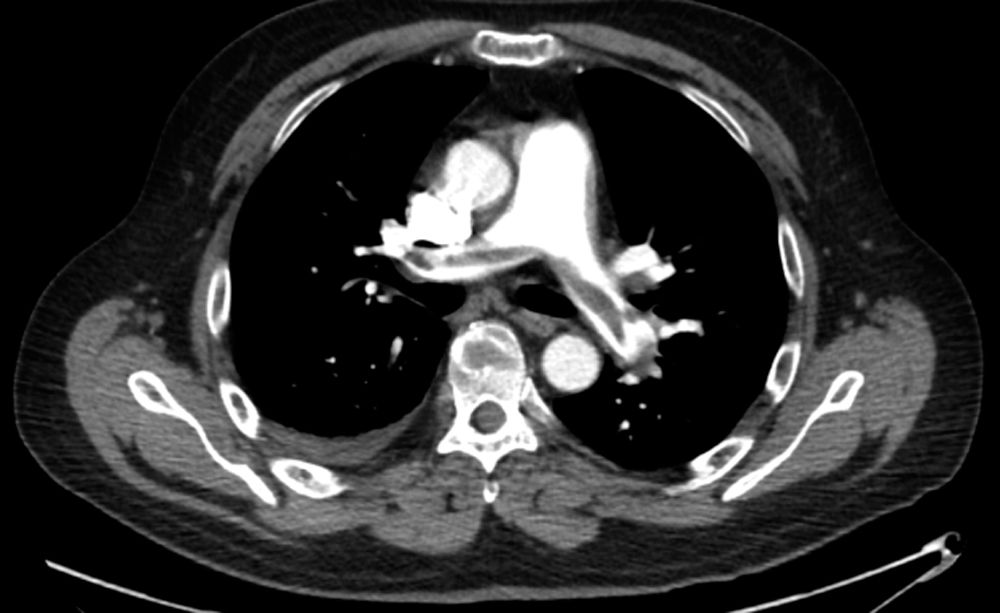
FIGURE 21.1 A CTA from a patient with a saddle PE. Clot can be seen extending into both the left and right main pulmonary arteries.
3. EKG: While EKG abnormalities, such as sinus tachycardia and evidence of right ventricular strain, are common, they lack both the specificity and sensitivity to make a diagnosis of PE.
4. Chest x-ray (CXR): The majority of patients have nonspecific abnormalities on CXR. If infarction has occurred, this may appear as a wedge-shaped opacity at the periphery.
5. CT angiography (CTA) (Fig. 21.1) has become the diagnostic imaging study of choice, as it can be performed in minutes and additionally allows for simultaneous evaluation of other pulmonary processes. Addition of venous imaging improves sensitivity. Disadvantages include the requirement that the patient be able to comply with a breath hold, radiation exposure, and need for iodinated contrast. Poor-quality imaging due to either motion artifact or poor timing of contrast bolus may decrease sensitivity.
6. Ventilation/perfusion ( /
/ ) scan is useful in patients who cannot tolerate CTA as it does not require iodinated contrast. However, its interpretation requires clinical correlation as a low-probability scan does not entirely rule out PE and a high-probability scan does not guarantee presence of PE. In addition, in patients with significant underlying pulmonary disease, perfusion scans are difficult to interpret and have a high false-positive rate.
) scan is useful in patients who cannot tolerate CTA as it does not require iodinated contrast. However, its interpretation requires clinical correlation as a low-probability scan does not entirely rule out PE and a high-probability scan does not guarantee presence of PE. In addition, in patients with significant underlying pulmonary disease, perfusion scans are difficult to interpret and have a high false-positive rate.
7. Pulmonary angiography: As the quality of CTA has improved, the use of pulmonary angiography has decreased; however, it remains the gold standard for diagnosis of PE. Angiography is generally well tolerated with a low complication rate and has the additional benefit of allowing for direct assessment of pulmonary hemodynamics.
8. Echocardiogram is useful in assessing right ventricular function. Findings suggestive of PE include increased right ventricular (RV) chamber size with decreased function, although this finding can be seen in a variety of cardiac and pulmonary diseases. More specific findings include presence of RV clot (Online Video 1) and McConnell’s sign (Online Video 2), in which there is akinesis of the mid-free RV wall with normal apical function. Although none of these findings are diagnostic of PE, as echocardiogram can be performed at the bedside, it can be used to help confirm a clinical diagnosis in a patient too unstable to travel for more definitive testing.
III. INITIAL EVALUATION OF THE PATIENT WITH CONFIRMED OR SUSPECTED PE.
A. Assess Severity: The initial bedside assessment of a patient with PE is focused on assessing severity. Patients with hypotension and shock without other cause are considered to have massive PE (defined as systolic blood pressure of less than 90 mmHg or a drop of at least 40 mmHg from baseline for at least 15 minutes) and require immediate intervention.
1. The pathophysiology of massive PE is complex. When obstruction occurs by embolism, the RV experiences an acute rise in pressure and volume, causing shift of the interventricular septum toward the left ventricle. In addition, as RV output decreases, the left ventricular preload also falls. This combination of septal shift and decreased RV output can ultimately lead to decreased cardiac output, shock, and hemodynamic collapse (Fig. 21.2).
2. In patients without massive PE, it is important to distinguish between those at higher risk for hemodynamic collapse versus those with lower-risk PE. This is primarily accomplished through either direct or indirect assessment of RV compromise or strain. Those patients with hemodynamic stability but evidence of RV strain are considered to have intermediate-risk PE.
B. Physical Exam should focus on signs of shock, including hypotension and signs of end organ hypoperfusion, indicating massive PE. In the hemodynamically stable patient, hypoxia and tachycardia, particularly if worsened with activity, may suggest a patient is at risk for decompensation.
C. Lab Testing: Troponin elevation is seen in roughly one-third of patients with PE and is likely due to increased stretch on the right ventricle due to pressure overload in massive or intermediate-risk PE. Troponin elevation is associated with both presence of RV strain on transthoracic echocardiogram (TTE) and increased mortality. BNP or NT-pro-BNP elevation is seen in approximately one-half of patients with PE, reflecting RV myocyte stretch. Like troponin, an elevation is associated with RV strain on TTE and increased mortality when compared with patients with normal BNP and NT-pro-BNP levels.
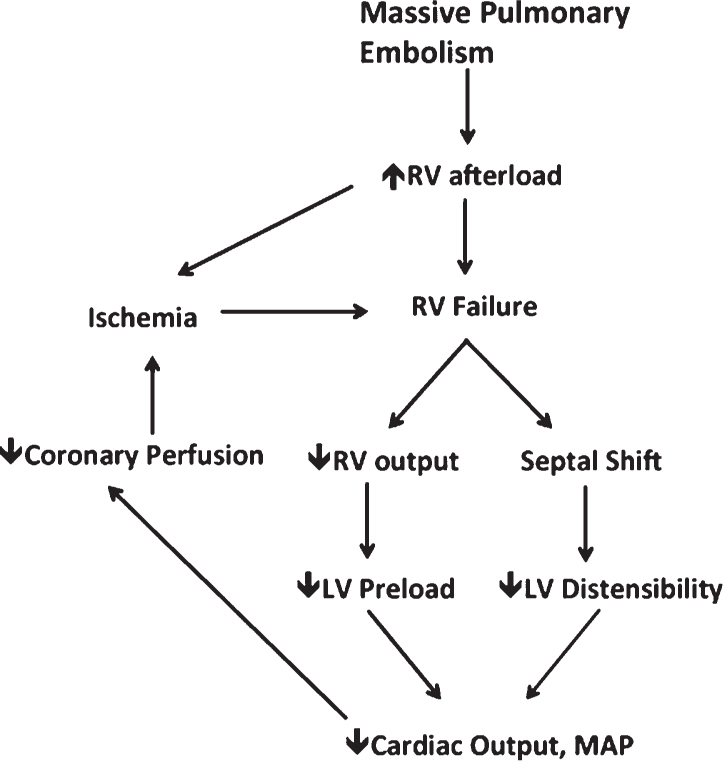
FIGURE 21.2 A schematic demonstrating the cycle by which massive PE leads to hemodynamic instability.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








