
DAMAGE CONTROL MANAGEMENT/OPEN ABDOMEN
HISTORY AND EVOLUTION OF DAMAGE CONTROL
While historically the management of the injured patient was done by the general surgeon, treating these patients like an elective surgery patient, with attempts at definitive repair at onset, failed to obtain satisfactory results, mainly due to fundamental differences in physiology and anatomic issues. The multisystem trauma patient has the potential for severely destructive injuries across multiple organs and/or body spaces usually not seen with the elective surgery patient. In addition, a prolonged time from injury to definitive care allows for ongoing bleeding and visceral contamination, resulting in altered physiology on presentation. The elective surgery patient presents with stable physiology, and does not suffer from uncontrolled bleeding or visceral contamination generally. Finally, the initial evaluation between these patients is necessarily different. The multisystem trauma patient may not have the physiologic reserve to undergo extensive evaluation and imaging commonly utilized with elective surgical patients. Furthermore, the shock state common in emergent trauma patients often prevents the patient from being able to provide a basic medical history. It is these essential differences of altered physiology, delay of presentation, complications from ongoing bleeding and contamination, and limited or absent preoperative medical history and evaluation that make the traditional attempt of definitive repair at presentation often futile. Ongoing bleeding from coagulopathy, initial physiologic failure from unresuscitatable shock, and subsequent multiple organ system failure later in the patient’s hospital course present specific challenges that defy conventional therapy.–13
While this problem was known and discussed in the first half of the 20th century, it was not until the early 1980s that the foundations for a solution were placed. H. Harlan Stone presented work in aborting laparotomy for coagulopathic bleeding using abdominal packing and Burch with hepatic injuries demonstrated improvement in outcomes over traditional approaches.4,5 However, the term “damage control” and its sequence was not defined and refined until the 1990s by Rotondo and Schwab.–69
While initially developed for abdominal injury with uncontrolled hemorrhage, damage control concepts have expanded into other areas, including vascular surgery and orthopedics.10,11 These concepts have even altered the way the military organizes its treatment of severely injured soldiers.–1215 In emergent general surgery, damage control techniques have been applied to those patients who develop similar physiologic instability and intolerance of the shock state.–1620 However, with the aggressive resuscitation employed along with damage control, the incidence of abdominal compartment syndrome (ACS) and open abdomens has increased.21,22 The increased exposure to open abdomens has allowed surgeons to gain experience in their management with development of multiple methods of obtaining temporary abdominal closure. Additionally, recognition of ACS, both in trauma and with intra-abdominal catastrophe, and its treatment has probably been a huge source of the improved outcomes in both patient populations.16,22,23
New strategies have been devised to reduce the volume of resuscitation as well as deal with the metabolic alterations the shock state and the resuscitation itself creates. Permissive hypotension and “damage control resuscitation” using fresh frozen plasma in a 1:1 ratio with packed red blood cell have been recently debated in the literature.–2428 Many novel strategies involving transfusion ratios, alternative fluids, and hemostatic dressings are currently being evaluated.29 As resuscitative technique and our understanding of control of physiology prior to operative intervention improve, the need for damage control and its adjuncts may decrease.
DAMAGE CONTROL INDICATIONS
In the traditional approach by general surgeons, control of bleeding and contamination were achieved and followed immediately with definitive repair and closure of the abdomen at the first surgery. The core concepts of damage control surgery evolved out of the failure of traditional approaches to exsanguinating hemorrhage from traumatic injury. Damage control changed the traditional sequence via termination of the initial laparotomy after cessation of hemorrhage and control of contamination, preferably before the development of physiologic exhaustion as noted by the development of acidosis, coagulopathy, and hypothermia (“the bloody vicious cycle”).30 Instead, definitive repair is delayed until after reestablishment of the patient’s physiology in the ICU. Abdominal closure may even be delayed further if the resuscitation required to treat the patient would lead to ACS (Table 6.1).
TABLE 6.1
INDICATIONS FOR DAMAGE CONTROL
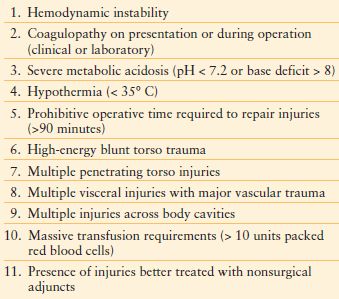
The decision to carry out a damage control procedure should be made early in the operation, before the development of hypothermia, acidosis, and coagulopathy. Waiting for the development of this triad to abbreviate the operation reduces the success of damage control surgery, as the components of this triad interact to worsen ongoing hemorrhage, ultimately leading to the patient’s demise.31 While temperature below 35°C, pH below 7.2 or base deficit exceeding 8, and evidence of coagulopathy clinically or via laboratory are often discussed as indications for abbreviating the operation in favor of damage control, no definitive values exist for consideration of a damage control procedure.–3234
In addition to physiology parameters, another indication for damage control is the presence of multiple injuries, both within and without the operative field, which would exceed the patient’s physiologic reserve to definitively repair. Complex injuries, such as those combined to the pancreas and duodenum, may require exceedingly long operative times. Preferably, the damage control laparotomy should be <90 minutes in duration.35
Injuries outside of the operative field are easily forgotten, especially those across multiple body cavities. Continuing or recurrent hemorrhage from these sites can hasten a patient’s advance toward physiologic collapse and may not be immediately identifiable by the operative team. Hemorrhage control should be treated as a continuum across body cavities and regions, with the surgeon starting at the perceived most compelling source of hemorrhage and expeditiously moving to other sites as the situation develops, keeping in mind sites that are outside the operative field.
Injuries that may be better treated with adjuncts, such as angiographic embolization of hepatic or pelvic injuries, are another potential indication for damage control surgery.36 When identified intraoperatively, such an injury can be temporized with packing followed by angioembolization. The variation in physiologic reserve across different patient populations must also be considered. The elderly, young, and those with multiple medical comorbidities have less tolerance for prolonged surgical procedures.
Thus, constant reevaluation of the patient is needed to identify those patients who would benefit from an abbreviated operative approach and subsequent aggressive secondary resuscitation. The evolving physiology, time for repair, concurrent injuries and their effects on the patient, and the patient themselves all factor into the decision to abbreviate the operation to prevent collapse of the patient’s physiology.
DAMAGE CONTROL SEQUENCE
When first conceptualized for exsanguinating truncal trauma, damage control focused upon management of ongoing hemorrhage and visceral contamination from abdominal injuries (part 1). This was followed by reestablishment of the patient’s physiology in the intensive care unit (part 2) before definitive repair in the operation room (part 3) and closure of the abdominal wall (part 4). With further process improvement, the significance of the prehospital setting (ground zero) and expansion of damage control to extra-abdominal injury began to be evaluated (Algorithm. 6.1). A description of the process in the exsanguinating abdominal trauma patient follows, though many parallels can be noted in other emergent surgery patients, such as those with abdominal sepsis.
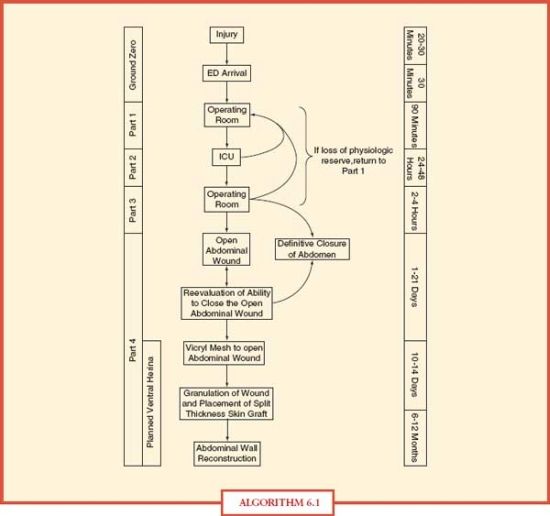
ALGORITHM 6.1 Damage control timeline.
GROUND ZERO: PREHOSPITAL CARE/INITIAL RESUSCITATION
The prehospital care received by the trauma patient can have profound implications on their outcomes. The need for rapid transport or transfer to definitive care cannot be overemphasized.37,38 Theories on resuscitation have arisen recognizing the potential of traditional methods of focusing on increasing hydrostatic pressure to normal levels to restore tissue perfusion to destabilize clots and increase bleeding, which may have a deleterious effect upon the patient.39 A moderate resuscitation goal to systolics of 80-90 mm Hg with concomitant signs of end-organ perfusion during transport may be more prudent for patients with prolonged transfer times.24,40 Some militaries are resuscitating only to the presence of a radial pulse and cleared sensorium in austere environs.–4143 Prehospital hypotension, despite resolution before arrival at the hospital, should be considered a warning sign for a more severely injured patient with the potential for a prolonged operation.44 Communication between the transporting crew and hospital personnel before arrival allows for mobilization of resources to prevent delays in the care of the unstable trauma patient.
Advanced Trauma Life Support is the foundation for care at this stage.45 Airway management and correction of breathing disorders using adjuncts like endotracheal intubation and tube thoracostomy are well described. Circulation assessment with point control of hemorrhage and alignment of fractures should be performed. Resuscitation is begun via large bore intravenous and occasionally intraosseous catheters using a combination of isotonic crystalloid and blood product. A rapid evaluation using adjuncts, such as focused abdominal sonography in trauma, diagnostic peritoneal lavage, tube thoracostomy, and radiographic imaging of the chest and pelvis, to identify sites of ongoing hemorrhage is performed. The rapidity and ease of the mentioned adjuncts makes them particularly useful, but are not absolute. Diaphragm injuries can allow for transmission of blood between cavities. The areas prone to unobserved bleeding in an unstable patient are intrathoracic, intraperitoneal, pelvic/retroperitoneal, or long bone fracture sites. One must not forget the potential for bleeding prior to arrival from external wounds, especially those associated with the scalp. Generally, fluid in the peritoneal cavity with hypotension will lead to a celiotomy, while large volume loss from tube thoracostomy (>1,500 mL) or ongoing drainage (>200 mL per hour over 3-4 hours) indicates the need for a thoracotomy. Localization of ongoing hemorrhage allows for better planning of operative intervention and can be performed in <20 minutes.
PART 1: CONTROL OF HEMORRHAGE AND CONTAMINATION (INITIAL LAPAROTOMY)
The initial operative intervention is the focus of the next phase. The goal is an abbreviated operation for control of mechanical hemorrhage and visceral contamination. The operation is abbreviated to prevent depletion of the patient’s physiologic reserve and initiation of nonmechanical (coagulopathic) hemorrhage. After evacuation of the peritoneal cavity, hemorrhage control is established using abdominal packing, especially with hepatic, retroperitoneal, and pelvic injuries. Shed blood can be collected using a variety of techniques for return to the patient; however, they remove the clotting factors, leading to a coagulopathy without the concomitant use of plasma and cryoprecipitate replacement.
While specifics of operative intervention are described elsewhere, some underlying themes can be established.46,47 Central is full exposure of the injuries to identify ongoing bleeding and contamination. Numerous maneuvers, including Kocher, Mattox, Aird, and Cattell-Braasch, allow for exposure of the retroperitoneal structures as needed. Suture ligation and control of the vascular pedicle of solid organs is commonly used to gain rapid control of ongoing hemorrhage. Some solid organs, such as the spleen or isolated kidney with normal contralateral kidney, may be sacrificed if repair times are prohibitively prolonged. Those vessels that provide end-organ supply or outflow to vital organs/regions, such as the suprarenal vena cava and portal vein, however, require repair. Shunting techniques are available to allow temporary restoration of flow during the resuscitation (part 2) phase before definitive repair (part 3) while reducing ischemic injury.–4853 A large variety of hemostatic agents have entered the market over the last few years.54 While human data is limited to case series, animal data does exist showing improved outcomes in hemorrhage control. Some early generation agents released large amounts of thermal energy, leading to burns of uninjured tissues.55,56 Adjuncts, such as angiography with embolization, may be needed to obtain hemorrhage control in inaccessible areas, such as the pelvis.
Control of intestinal injuries causing contamination of the peritoneal cavity is the second important objective. Closure of the visceral wound with suture or stapling techniques can easily and quickly control ongoing contamination. Formal repairs are avoided in the unstable patient with intestines left in discontinuity until definitive repair is performed in part 3 of damage control.
Positioning in the operating room is important, especially with multiple compartment injuries. The combined thoracic/abdominal compartment injuries are not suited by either a supine or decubitus position used in abdominal or thoracic trauma. The taxicab hailing position will often allow for practical exploration of both compartments (Fig. 6.1). The patient is placed in a supine position with the chest laterally rotated 30 degrees anterior to the coronal plane using folded blankets to one side based upon preoperative evaluation. The arm can be included into the operative field if manipulation is required. The position also allows for sternotomy if needed.
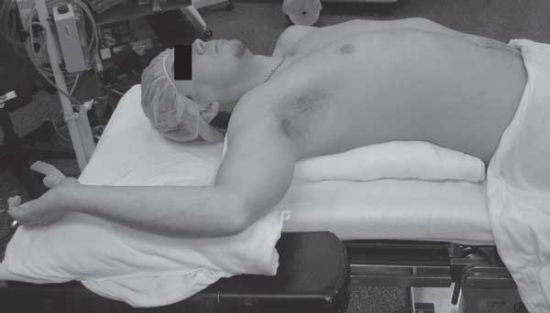
FIGURE 6.1. Taxi cab hailing position: note torso rotation to 30 degrees anterior to coronal plane with blankets. Arm abducted to 90 degrees for exposure of chest wall.
Temporary abdominal closures can further decrease the time in the operating room. Use of the abdominal pack or vacuum-assisted abdominal dressing allows for rapid reentry while preserving fascial integrity for latter closure.–5761 In addition, effluent from the abdominal cavity can be managed and quantified to facilitate the ongoing resuscitation.
PART 2: RESUSCITATION IN INTENSIVE CARE UNIT
After the initial operation, the patient is transferred to the intensive care unit to reestablish the patient’s physiology by aggressive resuscitation with intravenous fluids and blood products in conjunction with correction of hypothermia, acidosis, and coagulopathy. The fluid choice for resuscitation can have major effects. The use of normal saline in large volumes leads to a hyperchloremic (nongap) metabolic acidosis.62 Animal models suggest the potential for proinflammatory mediator stimulation with lactated Ringer’s solution.63,64 Colloids have been suggested as an substitute resuscitative fluid; however, evidence of superiority to crystalloid does not exist, and maybe even increases mortality in trauma patients.–6567 Hypertonic saline reduces inflammatory mediator activation in animal models, but has failed to show superior improvements in mortality or morbidity in clinical trials.–6871 Isotonic crystalloids (normal saline/lactated Ringer’s solution) are used for the majority of resuscitations.
A debate over transfusion policies in patients requiring massive transfusion protocols (>10 units packed red blood cell in first 24 hours) rages.–2528,72–83 A host of articles on transfusion ratios have been published since 2007 with the military’s report on improved survival with a 1:1:1 platelet:plasma:pRBC transfusion ratio compared to the traditional 1:4 to 1:5 plasma:pRBC ratio. While most of the civilian literature supports this aggressive transfusion policy in a small group of patients (1%-3% of civilian trauma patients), not all studies have shown survival benefits. In addition, the optimal ratio of plasma to red blood cells has yet to be fully determined. Massive transfusion is not without complications, including the potential for acute transfusion reactions, electrolyte disorders (hypocalcemia and hyperkalemia), acidosis, hypothermia, dilutional coagulopathy, and alteration of red blood cell morphology and oxygen-binding affinity.54
Recombinant factor VIIa has also been evaluated for its potential in hemorrhage control due to the acquired coagulopathy of trauma. Multiple case reports and one single randomized controlled trial have been done with variable results.–8498 On the whole, it appears to reduce the transfusion requirements, especially if given early in the course of acquired coagulopathy, and correct the coagulation profile, but mortality does not appear to be improved. The recent multicenter CONTROL trial was discontinued for futility due to low mortality rates.99 Also, the potential for thromboembolic events has been argued given its mechanism of action.100 Other compounds, such as prothrombin complex concentrates (PCCs), are also now being evaluated. While having a history of clinical use in hemorrhage from vitamin K antagonist coagulopathy and hemophilia with inhibitors, animal models are being developed for trauma with PCCs.–101109
While induced hypothermia has shown benefit in some elective surgical interventions, hypothermia on arrival to definitive care (spontaneous hypothermia) has shown almost uniform negative consequences in trauma patients.–110117 A depletion of energy levels in the hypothermic trauma patient compared to the elective surgical patient exists.–118121 Some of this depletion may be due to metabolic exhaustion and inability to restore the adenosine triphosphate supply, while some may be due to uncontrolled shivering in the prehospital setting and the energy consumption associated with it.122 Recent studies on hypothermia in trauma patients have used a temperature of 36°C to define hypothermia compared to the cooler 35°C in older studies, with identification that the effects of hypothermia occur at warmer temperatures than previously thought.–115117,123,124 While hypothermia has some potential positive effects on cellular preservation, especially in the central nervous system, a multitude of negative effects are noted, including alterations in metabolism and drug clearance, altered renal function and diuresis, electrolyte disturbances due to ion shift and renal loss, and inhibition of the coagulation pathways and platelet dysfunction.125 Given the increased risk of death associated with hypothermia in trauma, expedient rewarming of the patient is recommended. A multitude of noninvasive and invasive measures, including intravascular heat exchange catheters and body cavity lavage, exist.126 However, whether prevention of hypothermia in the prehospital setting translates into improved outcomes has yet to be shown.
Metabolic issues must also be addressed during this phase. In addition to electrolyte disturbances that need to be expediently corrected, hyperglycemia is commonly present in these critically ill patients. Strict glycemic control with aggressive insulin therapy has become commonplace since Van den Berghe’s 2001 study; yet, more recent studies have given variable results.–127133 A recent meta-analysis found that intensive insulin therapy increased the risk of hypoglycemia without a survival benefit in critically ill patients; though a survival benefit was noted in the surgical population when stratified by patient type (mixed, medical, or surgical).134 Given these recent studies, some have concluded that targeting age-normal glucose levels in units not equipped or accustomed to aggressive insulin therapy should be avoided and instead to target “to maintain blood glucose levels as close to normal as possible without evoking unacceptable fluctuations, hypoglycemia, and hypokalemia”.135
While multiple resuscitation endpoints have been described, no single test is ideal.–136138 Even once vital signs are normalized, the majority of patients exhibit evidence of inadequate tissue perfusion and oxygenation.139 Given the poor response of traditional vital signs, such as heart rate and blood pressure, to detect shock, alternative measures are being developed, such as shock index and heart rate variability.–140150 Resuscitation should continue until the shock state has resolved as determined by multiple methods of evaluation. While the medical critical care literature general shows a lack of efficacy of pulmonary artery catheter-based resuscitation, surgical literature tends to support its use, especially with right ventricular end-diastolic volume index (RVEDVI) pulmonary catheters.–151153 This discrepancy probably exists due to the differences in the patients themselves and volumes used to resuscitate between medical and surgical disease processes. A RVEDVI pulmonary catheter can help guide the resuscitation volume and pressure use in the difficult resuscitation.
Generally, once hemorrhage is controlled, the patient can be resuscitated and prepared for return to the operating room in the following 24-48 hours. While normalization of vitals usually occurs rapidly, a short period of time to regain true physiologic reserve is needed. In hypothermic trauma patients, the energy stores lag behind normalization of vitals, even beyond 24 hours from injury.119 Failure to regain a stable physiology in a short time is an indication that ongoing bleeding is present or the patient is developing an ACS. This should hasten the return to the operating room for reexploration before recovery of the patient’s physiology.
Care should be taken to prevent iatrogenic injuries during this and subsequent phases. Inappropriate ventilator strategies can produce or extend injury. The ARDSNet studies demonstrate the ventilator as a major inducer of pulmonary injury and acute respiratory distress syndrome (ARDS).–154157 A lung protective strategy of 4-6 mL/kg of lean body weight should be used. Sedation and analgesia are commonly needed to promote synchrony between the patient and ventilator with these strategies.158 A small group of patients may even require neuromuscular blockade to achieve synchrony; however, their use has not shown improvement in either mortality or oxygen consumption in ARDS. Their use has been associated with higher prevalence of myopathy and neuropathy, especially with renal or hepatic dysfunction or asthma.–159163
ACS is a common complication seen in the severely injured patient. While normal intra-abdominal pressure is 5-7 mm Hg, pressures above 12 mm Hg are designated intra-abdominal hypertension.–164166 In some patients, the increased visceral edema is noted at the initial surgery manifested by the inability to return the visceral block to the peritoneal cavity; while in others, a delayed presentation with development during the resuscitation takes place.22 ACS, the clinical entity hallmarked by hypotension, increased ventilatory pressures, and oliguria in the presence of intra-abdominal hypertension, should be routinely monitored for by checking bladder pressure measurements.167,168 Clinical examination and abdominal circumference measurements correlate poorly with intra-abdominal pressure as the elasticity of the abdominal wall is not a linear function of distention.–169171 While intra-abdominal pressures exceeding 30 mm Hg are most associated with ACS, patients have been noted to develop this syndrome with pressures below this level.172 In 2006, the World Society of Abdominal Compartment Syndrome (WSACS) defined ACS as sustained intra-abdominal pressures above 20 mm Hg associated with new organ dysfunction or failure.166
The physiologic effects of ACS are global. The cardiovascular system undergoes an increase in peripheral vascular resistance from compression of the vascular beds and increased sympathetic signaling while venous return to the heart via the inferior vena cava is decreased due to the increased abdominal and thoracic pressures.–173175 Additionally, cardiac compression causes atrial natriuretic peptide secretion to decrease, resulting in further fluid sequestration. Eventually, the patient becomes unresponsive to volume loading.176 Pressure measurements (central venous pressure, pulmonary capillary wedge pressure) from pulmonary artery catheters become uncoupled to volume status.177 In this situation, RVEDVI, which does not depend upon pressure measurements, becomes the best measure of volume status.136,152 Ultimately, a cycle of hypotension treated with volume resuscitation leads to further edema and increases in intra-abdominal pressures that worsen the clinical syndrome.
Increases in ventilatory pressures occur with ACS. While impediment of diaphragm movement from increased intra-abdominal pressure is present, resulting in 20%-80% of the intra-abdominal pressure being transmitted intrathoracic, other factors are involved.178 Typically in these patients, aggressive volume resuscitation has led to increased chest wall edema and extravascular lung water, resulting in decreased pulmonary compliance.179 Furthermore, increases in positive end-expiratory pressures (PEEP) are used to help in the recruitment of lung lost to a decline in negative pleural pressures that result in ventilation–perfusion mismatch, especially in the lower lung lobes. The combination of elevated PEEP along with transmission of intra-abdominal pressures results in increased intrathoracic pressures, which can further act to decrease cardiac return and output.180 The increase in intrathoracic pressures coupled with a decline in pulmonary compliance leads to the increase in ventilatory pressures needed for lung expansion.
Renal function impairment, noted by oliguria or anuria, is the third clinical component of ACS and is independent of volume expansion and maintenance of cardiac output.181,182 Outside of direct compression of the ureters from a pelvic hematoma, ureteral compression is not a cause of the renal failure as stenting fails to correct the oliguria. While mixed results have been seen in studies on direct renal parenchymal compression, compression of the renal vein creates ACS like failure, probably due to renal venous hypertension and decreased blood flow in the kidney.–183185 Additionally, a decline in the filtration gradient probably occurs as the increase in intra-abdominal pressure exceeds proximal tubular pressures, leading to a decrease in glomerular filtration rate. Activation of the renin-angiotensin-aldosterone pathway further worsens oliguria with increases in antidiuretic hormone and aldosterone.186,187 The combination of decreased cardiac output and renal venous compression along with parenchymal compression and endocrine alterations leads to the “dose-dependent” oliguria and anuria of ACS.
In addition to the aforementioned systems, ACS affects other body systems. Visceral blood flow is almost uniformly decreased and may be a component in intestinal anastomosis failure.–188191 Additionally, bacterial translocation appears to increase with increased intra-abdominal pressures.192,193 Transmission of intra-abdominal pressures is associated with increased intracranial pressures.–194196 Some have advocated decompressive laparotomy for patients with head injury and intracranial hypertension.–197199 Additionally, abdominal wall blood flow is altered with increasing intra-abdominal pressures.200 Closure with increased tension and relative tissue ischemia may result in tissue necrosis and fascial dehiscence to further complicate management of the abdominal wound.
Risk factors include severe hemorrhagic shock, damage control surgery (especially with fascial closure), and elevated penetrating abdominal trauma index. Elevated pulmonary peak pressures and low gastric mucosal pH also correlate with its development.201 Aggressive fluid resuscitation has been linked in several studies with volumes in excess of 10 L crystalloid, 10 units packed red blood cells (massive transfusion), 0.25 L/kg of crystalloid, or 6 L of crystalloid or units of packed cells in 6 hours associated with an incidence between 5% and 30%, depending on the patient population and definition used for ACS.22,202,203 Tighter abdominal closures, including primary fascial closure, have the highest incidence of ACS, death, ARDS, and multiple organ failure; however, use of the temporary abdominal closures does not guarantee its prevention.–204206 The development of ACS in patients with temporary abdominal closures has a higher mortality than in those patients with an open abdomen without development of ACS.207 It is through delays in identification and commencing therapy that increases in morbidity and mortality occur.208 Recognition and treatment with decompression of the abdominal cavity improves perfusion and organ function.209,210 In one prospective study, the mortality of severity-adjusted patients with an open abdomen approached that of patients without an open abdomen with aggressive monitoring and treatment of ACS based upon the WSACS guidelines.23,211
PART 3: DEFINITIVE INJURY REPAIR (SUBSEQUENT LAPAROTOMY)
Once the patient’s physiologic reserve has been restored, definitive repair can be undertaken (Table 6.2). Abdominal packing is removed and a complete examination with identification of all injuries and control of remaining errant bleeding points is performed. Uncontrolled hemorrhage, hemodynamic instability, or inability to undergo a prolonged operation would prompt an abbreviated laparotomy for control of hemorrhage and visceral contamination followed by return to the ICU for resuscitation.
TABLE 6.2
SEQUENCE OF DEFINITIVE REPAIR
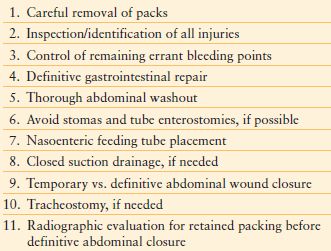
The definitive repairs required will depend upon the injury and organ system concerned; however, one should consider limiting the use of prosthetic material in a contaminated wound and the need to return to the operating room for further washouts prior to definitive closure. Some repairs can be considered that would not have been undertaken in a traditional, single operative setting. For example, isolated colon injuries have traditionally been treated with an ostomy; however, in a resuscitated patient, one might consider a primary anastomosis, especially if the patient would poorly tolerate an ostomy.212 The abdomen should be thoroughly irrigated upon completion of repairs. Closed suction drains should be used, when necessary. While definitive closure of the abdomen is preferred, a temporary abdominal closure may be required. One should attempt to ensure the intestines are located below the fascial level. The omentum is useful in providing coverage of the abdominal viscera to provide additional protection from injury.
Stomas and tube enterostomies should be avoided if possible owing to potential complications, as considerable changes in abdominal wall geometry can occur in the critically ill patient.–213215. However, nutritional support is incredibly essential in this patient population.216 Postpyloric nasoenteric feeding tubes provide continuous feeding, even during return trips to the operating room. Initiation of enteral feeds should be delayed in unstable patients because of the possibility for intestinal ischemia, but ought to be started after physiology is restored and proven stable. Even with an open abdomen, early enteral feeding is possible and associated with reduced septic complications.–217220 Earlier primary fascial closure, lower fistula rates, and decreased cost compared to patients with later (>4 days) enteral feeding was noted in one study of open abdomens.221
Since acute lung injury and ARDS manifest themselves during the 2nd and 3rd hospital days and can prolong ventilator requirements for several weeks, consideration of a tracheostomy should be made early.–222228 Ventilator circuit disruption after onset of pulmonary dysfunction can have devastating consequences; a tracheostomy provides for a more secure airway during this tenuous time. While early tracheostomy may decrease ventilator days and ICU stay, a high degree of misclassification may exist in this literature.229
PART 4: OPEN ABDOMINAL WOUNDS AND DEFINITIVE ABDOMINAL CLOSURE
Before definitive abdominal closure is obtained, a radiographic evaluation is made. Given the rapid nature of the initial operations and use of packing, closing counts cannot be trusted. Imaging allows for further evaluation that retained foreign bodies are not present and provides for a permanent record of complete pack removal.
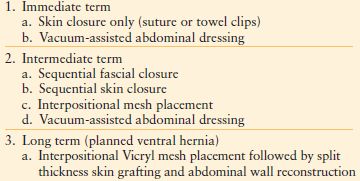
Primarily due to the aggressive resuscitation used with damage control at present, approximately 40%-70% of patients cannot initially have primary fascial closure.59,60,230 Temporary closures are desirable for patients undergoing repeated operations or have a distended visceral block preventing closure without ACS developing. While closing an abdomen, increases in airway pressure above 10 mm Hg from baseline indicate that fascial closure should be delayed.
Multiple temporary closure methods are available (Table 6.3).–5759,61,230–236 Optimally, a temporary closure should control the viscera while preventing additional peritoneal cavity contamination or visceral injury. The abdomen should be sealed and effluent controlled to preserve skin/soft tissue integrity. The closure should avoid unnecessary tension to prevent subsequent ACS. Finally, fascial integrity should be preserved for latter use during definitive closure.
TABLE 6.3
CLOSURE OF OPEN ABDOMINAL WOUNDS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








