THE TECHNIQUE OF EPIDURAL LYSIS OF ADHESIONS
Our discussion is limited mostly to the procedure that involves accessing the epidural space via the sacral hiatus. This is similar to the approach used for caudal epidural anesthesia. Radiography is used to guide needle and catheter placement and to perform epidurograms. When epiduroscopy is performed, radiography is also used to guide movement of the epiduroscope. For the catheter technique, the sacral hiatus is approached with a 15- to 16-gauge epidural needle with a curved tip (RX-Coude needle, Epimed International, Johnstown, NY; Fig. 33-1) about 2 to 3 cm off midline and 4 to 5 cm inferior to the sacral hiatus. To avoid penetration of the dural sac, the needle tip is advanced no further than to the S3 neural foramen. Epidurography is performed using 10 mL of nonionic radiographic contrast material to detect the presence of any filling voids (Fig. 33-2). Next, a steerable catheter is advanced into the void and 2 to 5 mL of contrast is injected as a marker, followed by 10 mL of 0.9% saline and hyaluronidase injection to remove the defect. Contrast is injected again to confirm filling of the void. Then, corticosteroid and local anesthetic are injected in small aliquots to a total volume of 10 mL. Thirty minutes later, 10% (hypertonic) saline is infused through the catheter and may be repeated two more times at 12- to 24-hour intervals. Then the catheter is removed. Precautions to prevent infection include aseptic technique, sterile dressing of the catheter entry site, and systemic antibiotics. A bacterial filter is attached to catheters after steroid injection as long as the catheter is in place. All fluids except steroid are injected through the filter.
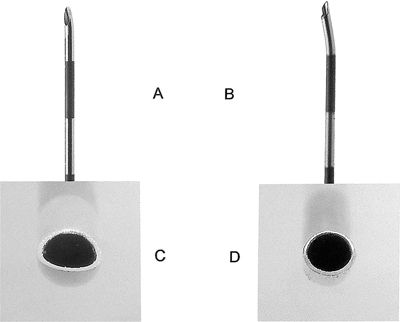
FIGURE 33-1. A: Tuohy needle tip. Note curved sharp tip. B: RX Coude needle tip. Note open tip-curve moved more proximally. C: Tuohy needle tip, end-on view. Note oval-shaped opening. D: RX Coude needle tip end-on view. Note round-shaped opening.
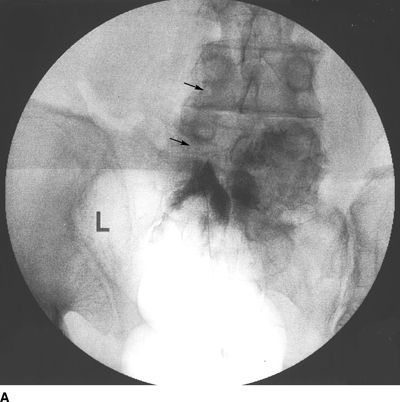
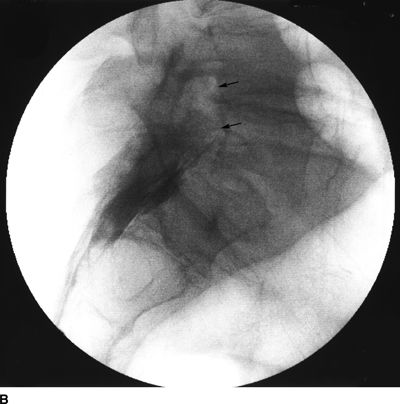
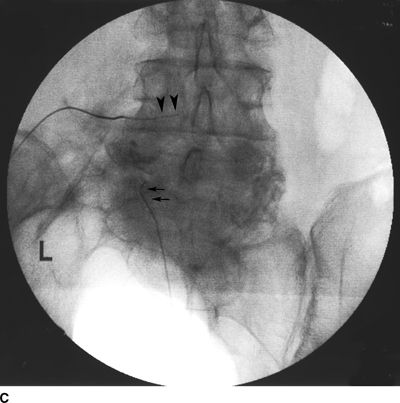
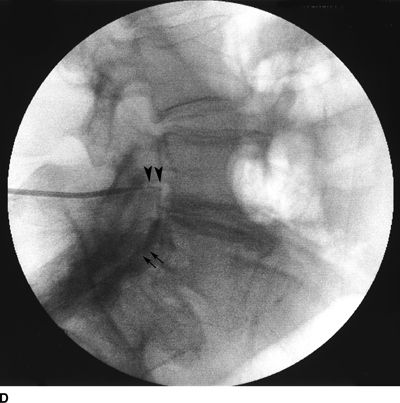
FIGURE 33-2. A patient with chronic back pain and radiculopathy. AP (A) and lateral (B) radiographs after contrast injection. Marked scarring is evidenced by lack of anterior spread (L, left; arrows indicate filling defects caused by scarring). AP (C) and lateral (D) radiographs after epidural lysis of adhesions demonstrating contrast spread to the anterior epidural space. Two catheters have been advanced to the left lateral epidural space at the L5/S1 level (L, Left; arrowheads indicate the transforaminal catheter; arrows indicate the tip of the caudal catheter).
Skill in threading the catheter to the target site improves with learning and experience. It is helpful for steering to make a 15-degree bend in the catheter about 1 inch from the tip. Thread the catheter near the midline to just above S3. Then rotate the tip so it dives to the ventrolateral target near the involved nerve root. Steer with both the needle and catheter
When epiduroscopy is performed, the same approach used for the catheter technique is employed with two notable modifications: (i) the method used to access the epidural space and (ii) the use of 0.9% saline to open the epidural space to aid visualization and to wash away material that limits visualization. Access to the space is attained using the Seldinger technique. First, an epidural needle is placed on midline, a guidewire inserted, and a stab wound made with a scalpel blade. A 10-F dilator and sheath are then inserted. The guidewire and dilator are removed, and the epiduroscope is inserted through the sheath. We use a 20-mL syringe to manually inject 0.9% saline as needed to aid visualization.
 DEFINITION AND SCOPE
DEFINITION AND SCOPE
As experience with the lysis procedure increased and the technique was refined, complications were recognized. The likelihood of complications was found to be greater if pathologic processes are present, particularly arachnoiditis and extensive epidural scarring (as often found in patients with chronic pain following prior spine surgery; i.e., failed back surgery syndrome). Types of complications related to lysis of epidural adhesions are listed in Table 33-1, and the causes of complications are listed in Table 33-2. As is the case with many technically demanding techniques, the incidence of problems declines with increased operator experience. In our training program, fellows must be supervised closely for about 5 to 7 months.
TABLE 33.1 Complications Related to Lysis of Epidural Adhesions
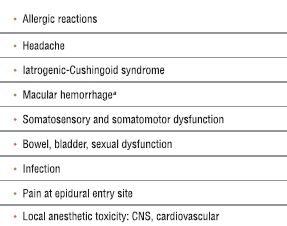
aHas been reported after an epiduroscope is used but not following use of a steerable catheter.
TABLE 33.2 Causes of Complications Related to Lysis of Epidural Adhesions
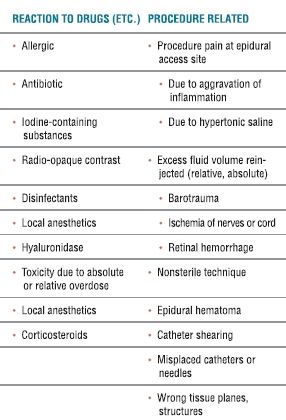
 COMPLICATIONS INDUCED BY MEDICATIONS
COMPLICATIONS INDUCED BY MEDICATIONS
Allergic Reactions
Classes of diagnostic and therapeutic agents used during epidural lysis that have allergenic potential include iodine-containing radiographic contrast agents, surgical disinfectants, antibiotics, local anesthetics, and hyaluronidase. It is important to obtain an accurate history to identify patients predisposed to allergic reactions. In certain cases, prophylactic use of corticosteroid and antihistamine may allow the use of allergens in susceptible patients. Documented anaphylactic reactions to local anesthetics and hyaluronidase are rare.4
Hyaluronidase has been documented to be effective in reducing failure from the lysis procedure from 18% to 6%.5 Use of hyaluronidase appears to facilitate the spread of injected solutions and opening of the lateral recesses. This helps to prevent loculation of the injectate and compression of nerves, spinal canal structures, and the blood supply to the spinal cord. Based on outcomes of a large number of epidural hyaluronidase injections, Moore4 suggested that the incidence of anaphylactic or sensitivity reactions may be 3%. Hyaluronidase is a proteinaceous substance, and, therefore, repeat administration could theoretically lead to sensitization. However, we have not seen a single serious sensitivity reaction following administration. Hyaluronidase is commonly injected with local anesthetic to produce retrobulbar nerve block, and allergic reactions in this setting are also rare. Nevertheless, caution must be advised because of the potential for an anaphylactic reaction. Appropriate treatment, including injectable epinephrine, must be readily available. A purified form of hyaluronidase, rHuPH20, synthesized in vitro, was introduced in 2005. The purified form does not contain extraneous allergenic material as do animal-derived crude extracts.6
Hypertonic Sodium Chloride
Hypertonic saline was originally injected intrathecally into patients under general anesthesia in efforts to treat pain associated with cancer.7 However, evidence from laboratory studies indicated that epidural administration might be safer and more effective. Thus, we began to use epidural hypertonic saline in our practice.8
Hypertonic sodium chloride injection is usually safe if at least 20 to 30 minutes have lapsed between local anesthetic and steroid injection and the subsequent injection of the hypertonic solution. In patients suffering from a demyelinating disease such as multiple sclerosis, we do not use hypertonic saline. We fear that the demyelination found in those with multiple sclerosis could well result in exaggerated effects, perhaps leading to direct neuronal injury. Indeed, King et al.9 demonstrated that hypertonic saline produces persistent block of unmyelinated nerves (C-fibers) in dorsal rootlets of cats in vitro.
During epidural lysis, intrathecal injection can occur if the needle or catheter unknowingly traverses the dura. Undesirable effects observed following intrathecal injection of hypertonic saline include severe pain and muscle cramps in affected segments, hypertension, cardiac arrhythmias, pulmonary edema, and cerebral infarction. Localized paresis lasting many hours, paresthesias sometimes persisting for weeks, transient hemiplegia, and permanent loss of sphincter control with sacral anesthesia have been reported as complications following intrathecal injection of hypertonic saline.10 Depending on the injection volume, these neurologic sequelae are often temporary and may be treated symptomatically. Subarachnoid injection is avoided only by strict adherence to technique. Injection of hypertonic saline through sharp needles where the needle tip may migrate through tissue planes should be avoided. The use of a spring-tip catheter reduces intrathecal migration and makes exact catheter localization with site-specific injection possible. Waiting 20 to 30 minutes after injecting local anesthetic (0.2% ropivacaine or 0.25% bupivacaine) to be sure motor block due to subdural spread does not develop also promotes site specific injection.
Steroids
Epidural steroid injection has been used to treat radiculopathy since the 1950s.11 The issues are how much and which type of steroid one should use and whether the preservative may be cytotoxic and produce adhesive arachnoiditis. The fact that steroids will precipitate and form a sludge-like material is also a concern (see Chapter 27 for a discussion of epidural steroid injection). There is growing concern that intra-arterial injection of particulate steroid may produce vascular occlusion with subsequent devastating neurologic damage (see Chapter 26 for a discussion of transforaminal injection of steroids). In our current practice, we typically use 4 mg of dexamethasone because it is not particulate and 10 mL of 0.2% ropivacaine or 0.25% bupivacaine administered in 2- to 3-mL increments. During prior years, we have used triamcinolone acetate as well as methylprednisolone acetate. Betamethasone is preferred by some clinicians based on the belief that its aqueous vehicle is safer than the organic vehicle and other additives to formulations of triamcinolone and methylprednisolone. Steroid-related adverse effects are rare in the setting of epidural lysis of adhesions. Adverse effects of longer-term use of corticosteroids are discussed in Chapter 32. In a review of complications associated with epidural steroid injections, Abram and O’Connor12 concluded that all reports of neurologic sequelae following neuraxial steroid injections involved subarachnoid injections or attempted epidural injections in which subarachnoid placement could not be ruled out. These complications included arachnoiditis and aseptic meningitis. Increased susceptibility to epidural infection (epidural abscess) has also been associated with epidural steroid injection especially if chronic distal site infection is not recognized.
Radiographic Contrast
Before the advent of water-soluble agents, oil-based agents were used and produced numerous cases of arachnoiditis when administered intrathecally. Thereafter, ionic agents became widely used, but generalized seizures and a number of fatal outcomes were reported after injection of ionic contrast media with inadvertent spread from the epidural space to the subarachnoid space.13 These observations have led to the near-universal use of nonionic contrast agents for spinal injections of all types, the most common agent being iohexol. Common ionic agents that should be avoided in the epidural space are meglumine diatrizoate and sodium diatrizoate. Other frequently used ionic agents are diatrizoate, iothalamate, and metrizoate. Nonionic agents, such as iohexol, are the agents of choice for diagnostic myelography and are safe and effective for both epidural and intrathecal use. The most common nonionic agents in clinical use include iohexol, iopamidol, and ioversol. The most common reactions to these are anaphylactoid, and there are some high-risk groups that should be recognized (e.g., those with previous contrast reactions). Less common are osmotically-mediated toxic reactions, including acute renal failure, and rare are reactions such as arachnoiditis. If the wrong contrast is injected, for example, an ionic contrast agent like diatrizoate, after 20 to 30 minutes, blood pressure, and heart rate increase and the patient may report a sensation of heat associated with tonic-clonic, seizure-like twitching down the torso, and extremities. Upon verification that wrong agent was injected, aspiration of cerebrospinal fluid (CSF) from the subarachnoid space and irrigation and barbotage with copious preservative-free saline has been recommended by some experts. Administration of systemic steroids and treatment of seizures as needed with anticonvulsant medication is warranted.
Local Anesthetics
We typically inject 10 mL of 0.25% bupivacaine or 0.2% ropivacaine during the procedure for lysis of epidural adhesions. Before doing so, fluoroscopic imaging with contrast injection is done to confirm epidural location of the catheter (not intravascular, subdural, substance, or subarachnoid). If a vein is entered, the catheter is moved to a different site. Intravenous injection of bupivacaine can lead to cardiac toxicity, but the use of contrast should detect intravascular injection before local anesthetic is administered. The small dose of local anesthetic used during this procedure is unlikely to produce cardiac toxicity even in the event it is all given intravascularly. A detailed description of the presentation of local anesthetic-related complications appears in Chapter 7.
 COMPLICATIONS INDUCED BY FLUID INJECTION AND NEEDLE AND CATHETER PLACEMENT
COMPLICATIONS INDUCED BY FLUID INJECTION AND NEEDLE AND CATHETER PLACEMENT
Fluid Injection into a Confined Space: Barotrauma and Ischemia
Epidural scarring can lead to confined compartments within the epidural space. Injection of fluid into one of these compartments or into the subdural space can have the net effect of creating a space-occupying lesion that causes direct barotrauma to underlying tissue and/or blocks blood flow to the spinal cord or cauda equina—producing ischemic injury. The term loculation is used for this phenomenon. In 1997, Rocco et al.14 elucidated the concept of compartmental filling in the diseased epidural space. They described the presence of epidural compartments that are sequentially filled during fluid injection. The fluid first fills one compartment and depending on the pattern and degree of epidural scarring fluid then overflows into adjacent compartments.
Injection of hypertonic saline solution into a closed loculated compartment within the epidural space can produce direct trauma by pressing on adjacent neural structures. Hypertonic saline is hyperosmolar and will also cause fluid shifts that will further increase the volume and pressure within loculated compartments as osmosis draws fluid into the space. To reach equilibrium, the 10% sodium chloride must reach 0.9% sodium chloride, and in the process the volume expands 11 times over the injected volume. Normally, fluid placed within the lateral recesses of the epidural space flows freely through the neuroforamen to the paraspinous region outside the spinal canal. To avoid barotrauma, it is important to document free flow of contrast from the epidural space to prevent excess pressure buildup that may compress the nerves, the spinal cord, or the nerve roots through which blood supply is carried to the spinal cord. Severe neurologic sequelae ranging from temporary sensory or motor deficits to complete paraplegia can follow barotrauma. The manifestations are variable and are dependent on the exact structure or structures compromised. The only means of preventing such injury is by ensuring that any visible areas of loculation are opened during the lysis procedure in order to establish drainage during the lysis procedure, before hypertonic saline is administered.
Figure 33-3 illustrates the appearance of loculation in the cervical area of a patient and subsequent relief by additional fluid injection and flexion-rotation or chin to shoulder movement of the patient’s head. The patient had a prior C5-6, C6-7 cervical fusion for chronic neck pain following a whiplash-type injury. Subsequent to fusion, the patient was involved in another motor vehicle accident that caused hyperextension of the neck and developed left-sided severe facial pain. The patient had severe allodynia in the temporal area, the ear, and just in front of the ear spreading down toward the ramus of the mandible. The differential diagnosis was atypical facial pain versus C3 radiculopathy, and the patient was scheduled for cervical epidural neurolysis. Contrast was injected and a Racz Stim Cath (Epimed International, Johnstown, NY) was threaded through the left side of the C4 area laterally. Considerable difficulty was encountered in advancing the catheter. Following multiple attempts, there was a clear pop, and the catheter could then be threaded cephalad to the C3 nerve root. Injected contrast remained primarily on the left side and tracked across to the right side at the C2-3 area (Figs 33-3A, B). This tracking has been recognized recently as perivenous counter spread (PVCS), that is, spread of contrast within the epidural space preferentially along the course adjacent to venous structures suggesting extensive scarring and restriction of contrast spread. PVCS is a danger sign indicating pressure build up.15 To open a runoff pathway, flex the neck, which enlarges the neural foramen. Rotation of the head further facilitates the sliding of the inferior articulating process over the superior articulating process making the neural foramen larger.16 The patient immediately reported bilateral arm and neck pain with the injection of only 2 mL of iohexol (240 mg/mL). The absence of lateral spread was recognized, and hyaluronidase (1,500 U in 10 mL) was slowly injected through the catheter. The patient was asked to rotate the head left to right. During the slow injection, the contrast was suddenly seen to be displaced through the neuroforamina to the outside of the spinal canal, releasing pressure on the spinal cord (Fig. 33-3C, D). The patient’s pain rapidly subsided. Direct stimulation of the C3 nerve root reproduced the patient’s pain, suggesting the diagnosis of posttraumatic C3 neuropathic pain. Toward the conclusion of the lysis procedure, the C2 through C4 area had clear runoff on fluoroscopy (Fig. 33-3E). At this point, local anesthetic and steroids (0.2% ropivacaine-containing 4 mg dexamethasone diluted in a total volume of 6 mL was injected in l-mL increments to a total of 4 mL). The facial pain stopped and the catheter was fixed in place and connected to a bacterial filter. Thirty minutes later, 3 mL of 10% sodium chloride was infused with the patient in the left lateral dependent position. On the 2nd day, the catheter was injected with 5 mL of 0.2% ropivacaine through the bacterial filter, followed by 4 mL of 10% sodium chloride. This was repeated the same day a second time, and the catheter was removed. At the time of discharge, the facial pain was not present. The patient remains pain free 6½ years later without additional interventions.
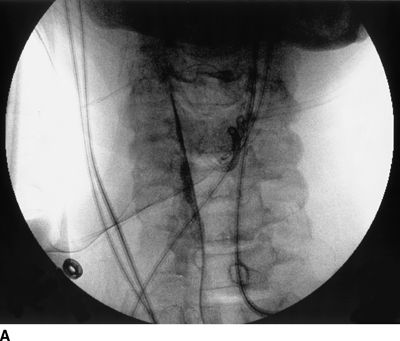
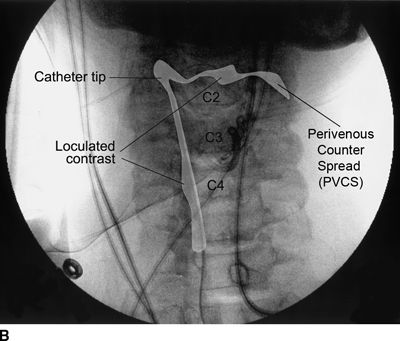
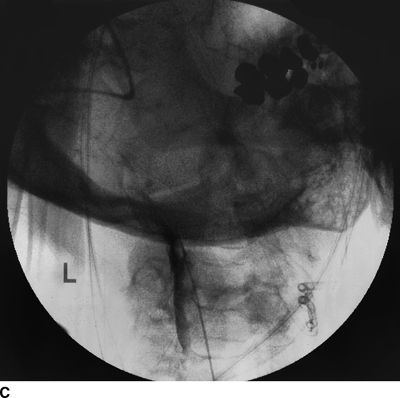
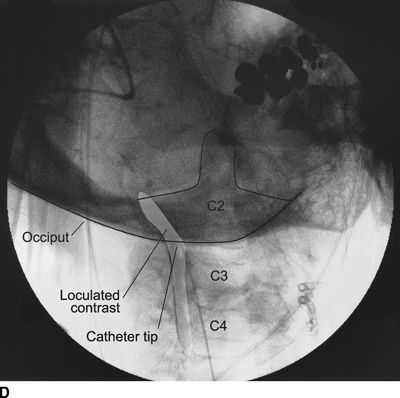
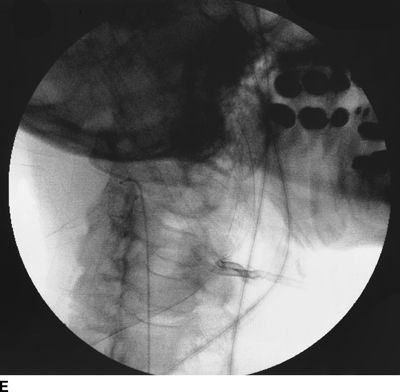
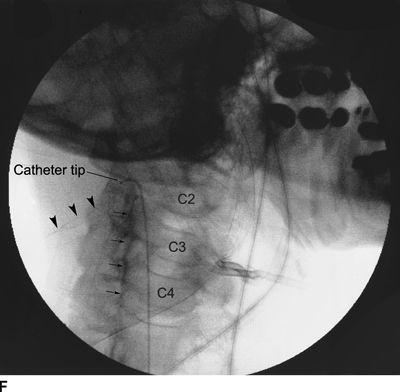
FIGURE 33-3. A: Cervical AP radiograph of patient following contrast injection demonstrates restricted spread (loculation); B: Labeled image. C: Following hyaluronidase and 0.9% saline injection and side-to-side flexion-rotation head movement contrast can be seen entering the neural foramen; D: Labeled image. E: Contrast runoff in the C2, 3, 4 area; F: Labeled image.



