The right dose differentiates a poison and a remedy.
—Paracelsus (1493–1541)
GLUCOCORTICOSTEROID PHYSIOLOGY
HYPOTHALAMIC-PITUITARY-ADRENAL AXIS SUPRESSION
GLUCOCORTICOSTEROID-INDUCED AVASCULAR NECROSIS
GLUCOCORTICOID-INDUCED OSTEOPOROSIS
Definition of the Complication
Endogenous steroids are involved in a large number of physiologic processes, including modulating immune function, behavior, cardiovascular function, growth, metabolism, and inflammatory responses.1 In regional anesthesia and pain medicine, exogenous glucocorticosteroids (GCS) are some of the most common pharmacologic agents in clinical use. Building on the initial work of Hench et al.,2 steroids were first injected into arthritic joints by Thorn (unpublished) and then by Hollander et al.3 These potent intermediate to long-acting GCS are now used to promote predominantly anti-inflammatory effects on target tissues. Routes of administration include oral, injectable, and transdermal. Injections are by far the most commonly used approach in pain medicine. Specifically, intermediate to long-acting corticosteroids administered for spinal/epidural use, intra-articular joint injections, periarticular joint injections, tendon sheath or ligamentous injections, trigger-point injections, neuroma or scar injections, and muscle injections are some of many potential targets.4–9
In spite of many known targets and the frequent use of these GCS, there is little consensus on the dose, frequency of use, or methodology appropriate to monitor activity, management, or prevention of long-term toxicity. Many toxicities known to be related to GCS use are delayed (often by months or even years), and thus many practitioners are unaware of the potential for serious complications related to the steroids or prevalence of these complications.10
 OVERALL SCOPE
OVERALL SCOPE
Exogenous administration of GCS leads to suppression of the hypothalamic-pituitary axis (decreased plasma cortisol, decreased plasma adrenocorticotropic hormone [ACTH], and adrenal atrophy), and a number of other potential toxic effects (Table 35-1). In addition, other side effects may be specific to the site of injection (e.g., spinal arachnoiditis after intrathecal injection11 and tendon rupture after tendon sheath injection12).
TABLE 35.1 Potential Toxic Effects of Exogenous Corticosteroids
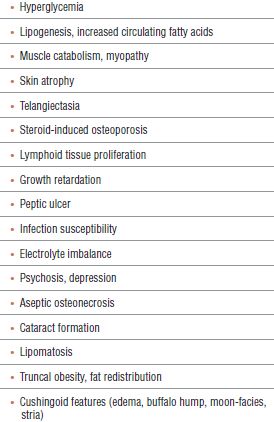
This chapter focuses on complications related to chronic steroid use. Transient elevations of blood glucose and impaired immune function are known and expected. Many possible complications are of little clinical significance for the average patient. Those complications, which may have prolonged clinical consequences, include steroid-induced osteoporosis, myopathy, collagen atrophy, avascular osteonecrosis, and spinal arachnoiditis. Because up to 82% of patients treated with long-term glucocorticoids (GC) will report some adverse event, this topic is pertinent to providers involved in their care.13
 GLUCOCORTICOSTEROID PHYSIOLOGY
GLUCOCORTICOSTEROID PHYSIOLOGY
GCS exert their effects by binding to a cytoplasmic GC receptor within target cells (Fig. 35-1). The GC receptor is expressed by part of a gene family, which includes cytosolic receptors for other hormones such as progesterone, estrogen, thyroid hormone, retinoic acid, and vitamin D. GC receptors are expressed in almost every type of cell, although density varies.
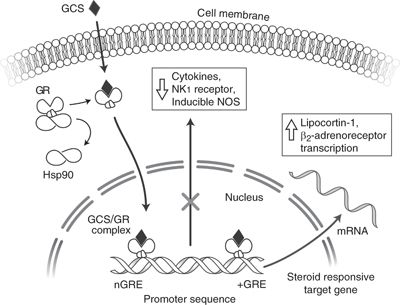
FIGURE 35-1. Glucocorticosteroids (GCS) pass through the cell membrane into the cytosol, where they interact with glucocorticoid receptors (GRs). A 90-kDa heat shock protein normally associated with the GR is split off, and the GCS/GR complex interacts with nuclear DNA glucocorticoid response elements (GRE and nGRE), which either promote or inhibit gene transcription. Cytokines: TNFa (tumor necrosis factor alpha), IL-1, IL-3, IL-4, IL-5, IL-6, and IL-8 (interleukins) transcription may be inhibited. Cytokine-induced NOS (nitric oxide synthase) is potently inhibited, as are effects on NK1 (neurokinin-1) transcription and other proinflammatory substances. Increased synthesis of lipocortin-1 may decrease lipid mediators of inflammation (leukotrienes, prostaglandins, and platelet-activating factor). Increased β2-adrenoceptor transcription may also occur.
The inactive GC receptor is bound to a large protein complex that includes two subunits of the heat shock protein HSP90. HSP90 may facilitate a receptor conformation optimal for binding. Once the GC binds to the GC receptor, HSP90 dissociates to allow nuclear entry and binding to DNA. Steroids produce their effect by activating GC receptors to regulate the transcription of certain target genes. GC receptors form a dimer that binds to DNA at sites called GREs in the target genes, either inducing or suppressing gene function.13 Steroids inhibit transcription of several cytokines (e.g., IL-1, TNFα, and IL-6). Steroids are potent inhibitors of cytokine-mediated inflammation. Transcription factor activator protein (AP-1) binding, normally activated by cytokines such as TNFα, may be inhibited by steroids.14
Besides their effects on inflammatory mediators, steroids may provide analgesia through several other routes, including blockade of nociceptive C-fiber conduction, interacting with norepinephrine and 5-hydroxytryptamine (5-HT) neurons in the dorsal horn substantia gelatinosa, counteracting the substance P suppression of naturally produced steroids in the spinal cord, and promoting favorable functional alterations within the spinal cord.15
 HYPOTHALAMIC-PITUITARY-ADRENAL AXIS SUPRESSION
HYPOTHALAMIC-PITUITARY-ADRENAL AXIS SUPRESSION
Scope
The propensity of exogenous corticosteroids to suppress the hypothalamic-pituitary-adrenal (HPA) axis has been recognized for decades.
Pathophysiology
Normally, secretion of cortisol by the adrenal glands exerts a negative feedback on the hypothalamus and pituitary gland. Rising cortisol levels in the plasma cause a decrease in the secretion of corticotropin releasing factor from the hypothalamus and a decrease in secretion of ACTH from the pituitary, resulting in a reduction in cortisol secretion by the adrenal glands. Exogenous steroids are capable of causing the same suppression (Fig. 35-2).
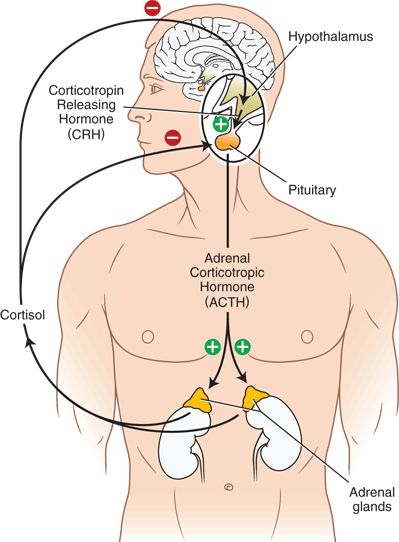
FIGURE 35-2. The normal Hypothalamic-Pituitary-Adrenal (HPA) axis.
Risk Factors
There is little known regarding what quantity of steroids is “safe” (or even effective) to inject. Early studies of epidural or intrathecal corticosteroids used one to four doses of 80 mg methylprednisolone over the course of days to weeks until symptomatic improvement occurred.4
Many pain practitioners have patients in their practice who achieve time-limited relief of pain, but repeat doses are arbitrarily limited due to concerns of steroid complications. Recommendations still persist to give no more than three doses in 6 months.16 Recommendations for a ceiling of 3 mg/kg methylprednisolone were based on a study of high-dose (280–600 mg) methylprednisolone used through an epidural catheter for 2 to 3 days.17 An animal study revealed the effects of a single 2-mg/kg dose of triamcinolone on serum cortisol. Adrenal suppression was evident for up to 4 weeks.18 In a large literature review of the safety of epidural steroids, Abrams and O’Connor19 noted the safety of using smaller doses and the lack of major complications overall.
Urine GC screening tests have revealed measurable levels of triamcinolone up to 9 months after the patients last received injectable steroid, with HPA suppression evident for up to 5 months after injection.20
Diagnosis
Patients may present with the stigmata of Cushing’s syndrome, including weight gain, fragile skin, easy bruising, lethargy, hirsutism, and skin striae. Cushing’s syndrome represents the presence of excessive systemic GC. Although these patients resemble those with hypercortisolism, they will have suppression of the HPA axis on testing.
Low plasma cortisol, decreased urine cortisol excretion, low ACTH levels, and diminished response to cosyntropin stimulation are indicative of HPA suppression. Furthermore, exogenous corticosteroid levels can be measured in urine or serum.
More frequently, patients will have HPA suppression that is clinically detectable without evidence of Cushing’s.21
Prevention
Currently, there is no consensus on the effective dose of steroids or the maximum cumulative allowable dose of steroids, although recommendation for doses not to exceed 3 mg/kg/y total has been published in the literature.22 Even this does not guarantee safety. HPA axis suppression has been reported after a single epidural steroid injection.23
Treatment
Patients exhibiting evidence of hypercortisolism or of HPA suppression need to be closely monitored until normal physiologic activity has returned. They may not require GC supplementation, but this should be considered. Referral to an endocrinologist for appropriate diagnosis and management should be considered. Certainly, further administration of exogenous GC by injection should be limited.
Summary
In summary, HPA axis suppression is a relatively common complication of GC administration. There is no established guideline for the effective dose or the maximal cumulative dose of steroids that should be allowed. Further, the lack of information sharing amongst practitioners can lead to patients receiving excessive cumulative doses secondary to use of GC for multiple medical and pain conditions.
 GLUCOCORTICOSTEROID-INDUCED AVASCULAR NECROSIS
GLUCOCORTICOSTEROID-INDUCED AVASCULAR NECROSIS
Scope
Avascular necrosis (AVN), also known as aseptic necrosis or osteonecrosis, is a condition in which the vascular supply to bone is compromised, leading to bone cell death. GC-induced AVN can occur at any bony location within the body. However, AVN of the hip produces particularly devastating functional consequences. GC-induced AVN can occur at any age and is often seen in individuals in their 30s and 40s. AVN typically occurs only after long-term GC administration, but may develop within as little as 2 to several weeks of initiating GC therapy.24
Pathophysiology
Hypotheses for the development of AVN include microvascular occlusion due to marrow lipid in the femoral head and resultant hypertension, fat emboli, and impaired repair of fatigue fractures. It is also thought that AVN may be caused by osteoblast or osteocyte apoptosis (programmed cell death).25 Weinstein et al. demonstrated that subchondral fracture sites in GC-induced AVN patients were rich in apoptotic osteocyte lining cells. Conversely, femoral heads from AVN patients with alcoholism or sickle cell disease were devoid of this feature. Therefore, they proposed that AVN is not the appropriate term for this GC-induced condition, as the bone is not truly necrotic. Instead, bone microfractures are not repaired, because the osteocytes do not properly sense the need to do so.25
Risk Factors
In a review of the literature, Clinkscales and Cleary26 found that for AVN the average cortisol equivalent doses were 17.5 g per patient, with an average time to symptom onset of 60 weeks. In one case, a 42-year-old patient received a total of 16 injections of either 40 mg of triamcinolone or 80 mg of methylprednisolone for treatment of hay fever. The patient subsequently developed AVN in both hips, which was related to his GC use.24 Socie et al.27 reviewed 4,388 patients treated with bone marrow transplant. Seventy-seven of these patients developed AVN, and only two did not receive GC therapy. Steroids were used to treat graft versus host disease (GvHD), and patients received them for a mean of 15 months. Older age increased the risk for AVN.27 In a similar fashion, Marsh et al.28 described a 21% incidence of AVN after doses of 5 mg/kg/d methylprednisolone to treat serum sickness over a 2- to 4-week period.
In contrast to chronic steroids, their utilization for acute injury has provided no conclusive evidence that short-term use (even in large doses) contributes to the development of AVN. In response to the National Acute Spinal Cord Injury Study-2 (NASCIS-2),29 Wing et al.30 studied 59 spinal cord injured patients who had received the high-dose spinal cord injury protocol dosing (methylprednisolone 30 mg/kg followed by 5.4 mg/kg/h for 23 hours) and compared them to 32 patients who had not received steroids. In spite of such large doses, there were no reported cases of AVN in these patients with a mean age of 32 years. Thus, it does not appear that short-term 23-hour very high-dose therapy has higher than a 5% risk of AVN.
Diagnosis
Diagnosis of AVN is made through the use of imaging studies. Magnetic resonance imaging (MRI) may show earlier changes than plain x-ray (Fig. 35-3). Large weight-bearing joints are the most likely to be involved. Clinical suspicion regarding the onset of new pain or functional aberration should be investigated as early as possible. Patients may present with an antalgic gait or frank limp, but may be asymptomatic early in the course of AVN. Diagnostically, it is known that up to two-thirds of patients can have bilateral involvement, and thus any asymmetry in exam should be noted. When the hip is involved, the patient may have significant pain if the examiner abducts and internally rotates the hip.
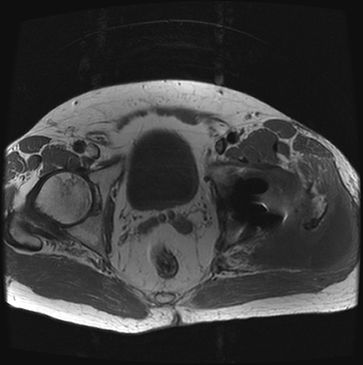
FIGURE 35-3. MRI illustrating avascular necrosis (AVN) in the left hip. Axial T1-weighted MRI demonstrating area of necrosis in the left femoral head.



