Fig. 44.1
Sacrum and lumbosacral nerves. (a) Posterior view displaying the terminal filum in the cauda equina (1), the spinal ganglia in the hemilaterally opened sacral canal (2), and the sacral hiatus (3) (Images kindly supplied by Danilo Jankovic). (b) Anterior view displaying the lumbosacral trunk (1), the anterior pelvic sacral foramina (2), and the sacral plexus (3) (Images kindly supplied by Danilo Jankovic)
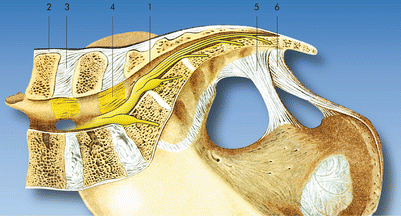
Fig. 44.2
Sacral canal cross section. (1) Sacral canal, (2) supraspinous ligament, (3) interspinous ligament, (4) ligamentum flavum, (5) sacrospinous ligament, (6) sacrotuberous ligament (Images kindly supplied by Danilo Jankovic)
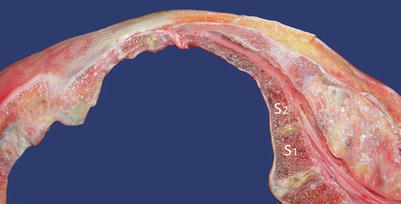
Fig. 44.3
Sacral canal cross section. The sacral canal has a diameter of 2–10 mm in the anteroposterior direction, with a variable capacity of 12–65 mL. The dura normally ends at the level of the second sacral foramina. S1 and S2 referred to the first and second sacral vertebra (Images kindly supplied by Danilo Jankovic)
Of note, the dura normally ends at the level of the second sacral foramina. However, due to anatomic variants of dura termination and sacral hiatus size, older studies have suggested the distance from the dura to the hiatus can range from 1.6 to 7.5 cm (average 4.5 cm) [26]. It has now been shown that the minimum distance between S2 and hiatus apex is as little as 0.75 cm [1]. The epidural venous plexus which is concentrated in the anterior space of the caudal canal generally terminates at the level of S4, although it can terminate even lower in elderly patients [15].
Indications and Contraindications
Caudal epidural steroids are most commonly considered for patients with chronic low back pain with radicular pain secondary to disc herniation or radiculitis who have not responded to conservative modalities of treatment. The caudal approach is especially appealing in postlaminectomy patients with post-lumbar laminectomy syndrome or sacral involvement or those who have bilateral and multilevel pathology. These injections are also performed for patients with chronic axial or discogenic pain and spinal stenosis [13, 23].
Contraindications for caudal epidural injections can be divided into absolute and relative. Accordingly, the known risks of epidural steroids must be weighed against any potential benefit. Ideally, patients should be actively involved in the decision-making process.
Absolute
Patient refusal
Coagulation disorders, anticoagulant therapy
Sepsis
Local infections (skin diseases) at the puncture site
Arachnoiditis
Immune deficiency
First trimester pregnancy
Severe decompensated hypovolemia, shock
Specific cardiovascular diseases of myocardial, ischemic, or valvular origin, if the planned procedure requires higher sensory spread
Acute diseases of the brain and spinal cord
Increased intracranial pressure
A history of hypersensitivity to local anesthetics, without a prior intradermal test dose
Relative
Pilonidal cyst
Congenital anomalies of the dural sac and its contents
Technique: General Considerations
A written medical note with detailed neurologic history, physical examination, and radiographic reports including a working diagnosis should be in the chart and reviewed immediately prior to the procedure.
Written informed consent should be completed documenting a discussion of the options, potential benefits, and potential risks.
Intravenous access should be established and appropriate monitoring should be applied and maintained throughout. Accordingly the presence of all necessary equipment including resuscitation equipment should be confirmed.
The patient is then placed in a prone position, with one pillow under their head and one under their lower abdomen to reduce spinal lordosis. They are instructed to abduct their heels while keeping their toes together; this internal rotation ensures relaxation of the gluteal musculature. In obese patients, the gluteal cleft may be separated by attaching a broad band of sticky plaster between the skin of the buttocks and the operating table. Strict sterile technique must be employed and a large area should be prepped with an antiseptic solution that is approved for neuraxial procedures. It is also important to keep accurate records of the procedure and any unexpected events or complications.
For the procedure, some clinicians recommend a 22-g spinal needle to minimize procedural pain, whereas the authors suggest a Tuohy needle and catheter in order to deliver the injectate more cephalad in the sacral canal. Care should be taken not to introduce free skin particles with epidermal cells into the spinal canal, due to the potential risk of epidermoid tumors [6]. There are wide variations in the volume of the sacral canal; we recommend a preparation of corticosteroids with local anesthetic agents and/or normal saline in volumes ranging from 10 to 15 mL.
Caudal epidural injections can be performed using fluoroscopy, ultrasonography, or a landmark-based technique. These techniques are discussed below. It should be noted that in adults, the rate of inaccurate placement with landmark-based technique can be as high as 35 % in experienced hands [14, 30]. In addition, intravascular placement rates of 14 % and extra-epidural placement rates of 9 % have been reported. There are controlled studies that suggest “blind” caudal injections are not more effective than epidural injections of normal saline either for short-term outcomes or in the long term [5].
X-Ray-Guided Technique
Fluoroscopically guided caudal epidural anesthesia is considered the gold standard by most clinicians. Despite the lack of strong evidence that fluoroscopically guided injections are more effective, they are undoubtedly safer than a blind injection, and as such the use of C-Arm fluoroscopy makes rational sense [5].
It is recommended to start with the C-arm in an AP (anterior–posterior) orientation to ensure that the patient’s pelvis is not laterally tilted. This is especially true in obese patients. Subsequently, in the lateral view, the sacral hiatus will usually be visible as a translucent opening toward the base of the caudal canal. Identification of the coccyx immediately caudad can also help with localization of the hiatus. Similarly, the clinician may palpate the coccyx and sacral cornua to help identify the point of needle insertion.
After adequate local anesthesia infiltration, an 18-gauge needle may be used to perforate the skin. At this point, the desired needle (spinal or Tuohy) is pushed through this perforation and advanced toward the hiatus. AP and lateral fluoroscopic images are taken to confirm the depth and direction of needle advancement. Once the needle is visualized just inside the canal, the Tuohy needle can be rotated 180° as necessary to enable smooth advancement. It should not be advanced beyond the S3 foramen to avoid damaging the sacral nerve roots and to minimize the risk of subarachnoid or subdural placement.
After negative aspiration for blood and CSF, appropriate myelographic quality radiocontrast material should be injected under live fluoroscopy. If venous runoff is noted, the needle tip can be withdrawn and moved until the venous runoff is no longer seen. If CSF is aspirated or a subdural or subarachnoid pattern is seen, the procedure should be terminated and repeated at a later date. Under normal circumstances, when contrast media is injected into the epidural space, a classic “Christmas-tree” pattern will be visualized within the bony canal. It should be noted that adhesions will limit dye spread. Subarachnoid dye spread will be noted centrally and extend significantly cephalad with only a small volume. Subdural injections will tend to be more patchy, and the contrast spread will not be as wide or predictable as subarachnoid pattern [5, 25]. Images of stages of a fluoroscopically guided caudal injection are seen below in Fig. 44.4. Examples of a wide variety of contrast spread patterns have been published elsewhere [28].
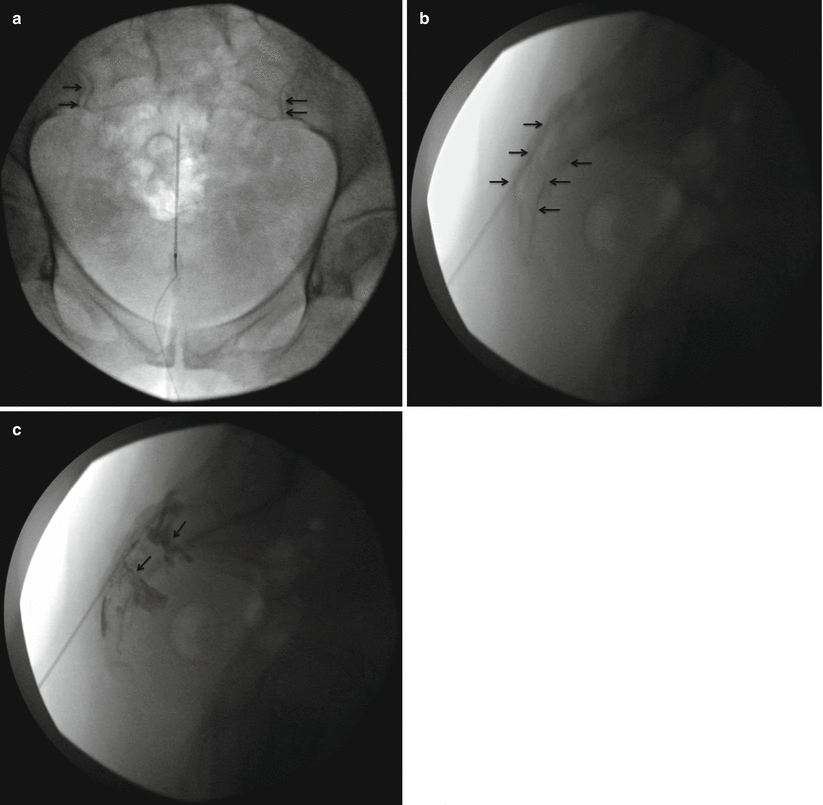

Fig. 44.4
Images of fluoroscopically guided caudal injection of steroids (Reproduced with permission from Philip Peng Educational Series). (a) Anteroposterior view of needle in the sacral canal. With the presence of bowel gas, it is a challenge to visualize the details of the sacrum and caudal canal. The sacroiliac joint is indicated by the arrows. (b) Lateral view showing the needle in the sacral canal which is indicated by the arrows. (c) Lateral view after injection of contrast medium. The contrast (indicated by arrows) is seen leaking out from the sacral foramen
After final placement of the needle or catheter and repeat negative aspiration, local anesthetic should be injected as a test dose prior to injection of the full dose of medication in aliquots. The patient may report pressure upon injection which should be halted until this pain has dissipated. Upon completion of the procedure, the stylet should be reinserted prior to needle removal. A sterile adhesive bandage should also be applied over the puncture site.
Ultrasound-Guided Technique
Ultrasonography in the field of pain medicine continues to grow as the technology advances. It is noninvasive, safe, simple to use, does not involve exposure to radiation, and has the potential to provide real-time images.
The potential benefit of ultrasound imaging for caudal epidural steroids was first described in 2003 [17]. The investigators found it to be especially helpful in moderately obese patients who were unable to assume the prone position. It is also useful when faced with decreased bone density, gas or feces in the rectum, or where fluoroscopy is not available or contraindicated [15, 20, 22].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








