
CARDIOVASCULAR FAILURE AND CIRCULATORY SUPPORT—MONITORING AND ESSENTIAL ADJUNCTS
The acute care surgery patient is at increased risk of cardiovascular compromise. Whether the trauma patient with severe hemorrhage, or the emergency general surgery patient with peritonitis, these patients encounter fluid “shifts” and a systemic inflammatory response unseen in other areas of surgery or medicine. To optimize patient care, the acute care surgeon must have a firm grasp of the physiology, diagnosis, and management of cardiovascular failure.
Cardiovascular compromise leads to shock, the inadequate delivery of oxygen to supply cellular metabolic needs. In the acute care setting, the patient in cardiovascular failure will present with unique, but identifiable causes of shock that follow a predictable pattern of illness.
- Hypovolemic shock related to direct intravascular volume loss from hemorrhage or intravascular volume depletion from sequestration of fluid into the “third space”.
- Septic shock related to organ dysfunction or hypoperfusion as a result of infection (Discussed in Chapter 12).
- Cardiogenic shock related to intrinsic cardiac disease or as a component of multiple organ dysfunction syndrome (MDS), resulting in decreased myocardial contractility that impairs oxygen delivery.
- Cardiogenic shock related to extrinsic causes that inhibit the heart from delivering oxygen by affecting preload, afterload, contractility, or a combination of the three.
Treatment of cardiovascular failure is focused on identification of the etiology of shock, and restoration of adequate oxygen delivery. This chapter discusses the determinants of cardiac output and oxygen delivery, techniques to diagnose and monitor the patient in shock, the clinical situations most frequently observed in the acute care setting, and the treatment and support of the patient in cardiovascular failure.
CARDIOVASCULAR PHYSIOLOGY
Oxygen must be uploaded to the blood at the pulmonary capillary level, adequately transported by the heart, and offloaded at the tissue level. Whereas factors such as pH, temperature, and alveolar and capillary membrane permeability will influence these processes, this chapter focuses specifically on the heart’s ability to transport oxygen to the tissue (see Box 50.1).
BOX 50.1
The determinants of oxygen delivery are the amount of oxygen in the blood and how much blood is being pumped to the tissue:
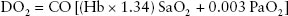

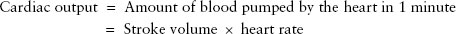
Stroke volume is determined by:
• Preload
• The initial length of myocardial muscle fibers is proportional to the left ventricular end-diastolic volume.
• As these fibers stretch, the energy of contraction increases proportionally until an optimal tension develops (i.e., ideal preload = ideal cardiac contraction)
• Afterload
• Resistance to ventricular ejection
• Measured clinically by blood pressure or SVR.
• Contractility
• Ability of the heart to alter its contractile strength independent of fiber length (preload)
• Decreased in intrinsic cardiac disease, contractility initially increased in sepsis but decreases in the later stages of sepsis, myocardial ischemia
Evaluation of Cardiovascular Performance
Hypotension is the harbinger of cardiovascular collapse. Although vasopressors may be appropriate in certain circumstances, the shock state should be diagnosed and categorized as a problem of preload, afterload, or contractility. Basic monitoring of oxygen saturation, continuous electrocardiography (ECG), and blood pressure measurements are vital, but other adjuncts help address this clinical scenario.
Determination of Preload
Controversy exists over the best method to determine preload.1–8 In general, each of the devices measures a pressure in an attempt to estimate or correlate with intravascular volume.
Central Venous Pressure. Central venous catheters have the dual benefit of access to large veins for (IV) fluid resuscitation and drug delivery, but also allow the surgeon to estimate intravascular volume. The central venous pressure (CVP) is a measure of the right atrial pressure that is proportional to the right ventricular end-diastolic volume (EDV). This measurement, however, is influenced by a multitude of variables in the critically ill patient. Patients on mechanical ventilation with positive end-expiratory pressure (PEEP) demonstrate decreased venous return from increased intrathoracic pressure. These patients require higher filling pressures to maintain preload. Changes in venous capacitance, intra-abdominal pressure, or heart failure can also affect the accuracy of the CVP. Although CVP is not an exact measurement of intravascular volume, it provides a trend of the intravascular filling pressures, which is a useful guide to resuscitation6,7,9,10
Pulmonary Artery Catheter. Despite controversy, the use of the pulmonary artery catheter (PAC) remains widespread in intensive care units2–6,11–17 One area where the use of the PAC has been demonstrated to improve outcomes is in the trauma population.18 In a retrospective database analysis examining over 50,000 patients, the use of PAC was shown to improve mortality in three specific groups: (1) patients with Injury Severity Score > 25, (2) patients with initial base deficit >11, and (3) patients older than 61 years of age.18 Given the similarities between the trauma population and the emergency general surgery population (huge volume shifts over a short period of time, the presence of a systemic inflammatory response, frequent comorbidities, and large number of elderly patients), the use of a PAC to determine adequate perfusion warrants consideration. Moreover, the PAC also provides other hemodynamic data that can potentially assist patient care, and are discussed below.
PULMONARY ARTERY OCCLUSION PRESSURE
Also known as the pulmonary artery “wedge” pressure, the pulmonary artery occlusion pressure (PAOP) approximates the left ventricular EDV. However, in the setting of increased intrathoracic pressure from positive-pressure ventilation, structural heart defects, or certain tachyarrhythmias the PAOP becomes less reliable.19,20,21–22
END-DIASTOLIC VOLUME INDEX
End-diastolic volume index (EDVI) can be calculated based on the right ventricular ejection fraction and cardiac output, and provides an accurate assessment of preload.23 Cheatham et al.24 showed in a series of 64 critically ill patients that EDVI correlates more closely to cardiac index than PAOP, when variable levels of PEEP were applied to patients on mechanical ventilation.24
TRANSTHORACIC AND TRANSESOPHAGEAL ECHOCARDIOGRAPHY
The use of echocardiography has been investigated as a tool to guide resuscitation in the critically ill patient.22,25–29 Doppler data on blood velocity and measurements of the vessel diameter of the vena cava can be used to estimate preload. Echocardiography has the added benefit of examination of the heart chambers and cardiac wall motion to estimate cardiac index. It has also becomes a useful tool for the initial evaluation of the trauma patient as part of the Focused Assessment by Sonography in Trauma (FAST) exam to demonstrate cardiac tamponade30–35 Handheld ultrasound devices allow measurements of vessel diameter and may help guide resuscitation in critical illness.28,36,37 Transesophageal echocardiogram has shown promise in initial studies and may replace transthoracic echo as a guide for adequate resuscitation. However, widespread use of echocardiography has been limited by availability, cost, and the skill required for interpretation.
DETERMINATION OF AFTERLOAD
Afterload can be approximated by calculating the systemic vascular resistance (SVR). SVR = (80) (MAP – CVP)/CO (where MAP is mean arterial pressure and CO is cardiac output). This can be directly calculated with the use of a PAC. High afterload leads to increased work on the heart and decreased cardiac output. In contrast, cardiovascular failure secondary to a profound decrease in afterload is the classic clinical scenario of distributive shock (e.g., septic shock).
DETERMINING CONTRACTILITY
Exact measurements of contractility are difficult to ascertain. However, the acute care surgeon can use the other above-described tools such as echocardiography or a PAC to estimate contractility. In the hypotensive patient, if values determined for preload and afterload are within normal range, it is assumed that contractility is impaired.
Oxygen Delivery. Perhaps the most important question to answer is, “is enough oxygen being provided to the tissues?” Oxygen delivery can be determined indirectly by measuring products of metabolism and monitoring acid base status, or directly by measuring the oxygen content in the blood returning to the heart. Thus, oxygen delivery is the product of cardiac output and arterial oxygen content.
MIXED VENOUS OXYGEN SATURATION
In the nonstressed setting, only 25%–30% of oxygen delivered to the tissues is actually extracted from the blood. Therefore, the blood returning to the heart from both the upper and lower parts of the body, or the mixed venous oxygen saturation (SVO2), is normally 70%.38 In the setting of shock, a low SVO2 reflects inadequate delivery or increased extraction of oxygen by the tissue. The SVO2 is most accurately measured at the coronary sinus where there is mixing of blood from the IVC and SVC, but for practical purposes it can be measured from an upper extremity central line or a PAC. SVO2 can now be monitored in a continuous fashion with the help of continuous cardiac output PACs. Measuring SVO2 and normalizing SVO2 during resuscitation has been shown to improve survival.10
Lactate and Base Deficit
In the setting of insufficient oxygen delivery, lactate is generated as a result of altered metabolism in the pyruvate kinase pathway. Serum lactate concentration is an indirect measurement of oxygen debt. The admission lactate, highest lactate concentration, and time required to normalize lactate have all been shown to predict survival.10,39–43 Base deficit is a measurement calculated, usually from an arterial blood gas, of the amount of alkali required to correct a pH to 7.4 with a PaCO2 of 40. Base deficit has also been shown to correlate with survival in the trauma patient, and both lactate and base deficit can be used as guides to resuscitation.10,39,–43
Several modalities have also shown promise as alternative measurements to assess cardiac performance:
- Oxygen extraction index—A potential variable to measure oxygen delivery44
- Heart rate variability—A biomarker that potentially can be followed to predict and monitor physiologic reserve in the critically ill patient45–49
In summary, the first step in treatment of the acute care surgery patient in cardiovascular failure is to determine why the patient is in failure. Elucidation of the etiology of heart failure as a problem of preload, afterload, or contractility, using the tools above, provides the necessary information to support the patient.
SUPPORT FOR CARDIOVASCULAR DYSFUNCTION
The key to treatment of the patient in cardiovascular failure is restoration of adequate oxygen delivery; think of this as “source control” first. In the case of hypovolemic shock in the trauma patient, the source is bleeding. In the case of infection, the source may be an abscess. In the case of intrinsic cardiogenic shock, the source may be the heart itself. In extrinsic cardiogenic shock, the source may be due to failure of another organ system; an adverse side effect of a medication; or a disease process that negatively affects preload, afterload, or contractility. The earlier the treatment to restore cardiovascular function is begun, the better the patient outcome.
Fluid Resuscitation
After 30 years of active investigation, controversy still exists over the use of crystalloid versus colloid for fluid replacement. Not only does the acute care surgeon need to choose what type of fluid to give, but how much. The danger of too much fluid compartment syndrome, acute respiratory distress syndrome [ARDS], versus the danger of too little fluid, (acute kidney injury tissue ischemia) must be balanced. In general, in the face of hypovolemia and shock, fluid resuscitation should be performed aggressively in the acute setting. On the other hand, resuscitation should be to specific endpoints, rather than undirected fluid infusion. Some data exist that permissive hypotension and minimal fluid resuscitation in the prehospital setting may improve outcomes in trauma patients,50,51 the theory being that if bleeding has not been controlled, fluid resuscitation and relative hypertension will lead to more bleeding, hemodilution, and coagulopathy. Permissive hypotension assumes rapid transfer from the scene to definitive treatment, and is most applicable to the penetrating trauma population. Further study is necessary before this method can be applied outside of this every specific penetrating trauma population.
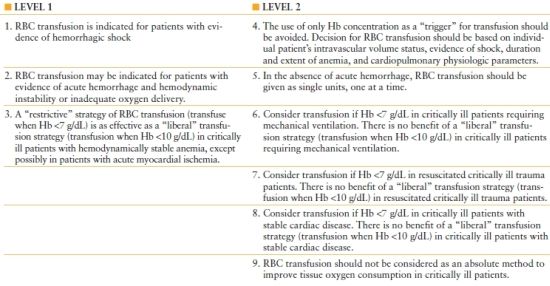
In some instances, the choice of fluid must also include the use of blood products. Blood product transfusion is potentially deleterious52–61 As a simplified rule, when bleeding is the cause of hypotension, blood should be used as the resuscitative fluid. When bleeding is not the cause, it should not be used. Recently, the Eastern Association for the Surgery of Trauma and the Society of Critical Care Medicine published practice management guidelines for red blood cell transfusion in the critically ill62 (Box 50.2). Another topic, which has received attention, is the ratio of blood products being transfused. Experience from the military suggests that red blood cell concentrates (packed red blood cells, PRBCs) FfP, and platelets should be given in a 1:1:1 ratio in the traumatically injured, exsanguinating patient.63–68 This research has been confirmed in the civilian population as well by the improved survival demonstrated by the initiation of institutional massive transfusion protocols.69,70 The ideal ratio among packed red blood cells, fresh frozen plasma, and platelets is still to be determined, but it seems clear that the transfusion of PRBCs alone in the face of bleeding is not as effective as adding the additional clotting factor products; that is, replace what the patient has lost.
BOX 50.2
CLINICAL PRACTICE GUIDELINES: RED BLOOD CELL (RBC) TRANSFUSION IN ADULT TRAUMA AND CRITICAL CARE
Pharmacologic Support
Multiple medications are available to assist the patient in cardiovascular failure, depending on the specific cause (Table 50.1). It is important to remember that these medications have multiple effects on the cardiovascular system. Catecholamine agents (dopamine, dobutamine, epinephrine, norepinephrine) exert their effects through binding to alpha and beta receptors. Activation of alpha-1 receptors increases SVR. Activation of beta-1 receptors leads to inotropy. Activation of beta-2 receptors leads to peripheral vasodilation. Phosphodiesterase inhibitors such as milrinone cause an increase in intracellular cyclic adenosine monophosphate (cAMP), and subsequent inotropy. In general, when the cause of failure is reduced afterload, a vasopressor should be chosen. When the etiology is related to contractility, an inotrope should be used. An algorithm for the management of the patient in cardiovascular failure is presented in Algorithm 50.1. Make sure “the tank is full” (the patient is volumerepleted), and that mean arterial blood pressure, oxygen delivery, and cardiac output are adequate.
TABLE 50.1
EFFECTS OF COMMONLY USED VASOACTIVE MEDICATIONS
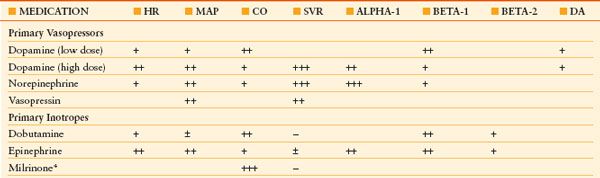
Number of plus signs reflects strength of attribute.
CO, cardiac output; HR, heart rate; MAP, mean arterial blood pressure; SVR, systemic vascular resistance; ALPHA-1, alpha-1 adrenoceptor agonist; BETA-1, beta-1 adrenoceptor agonist; DA, dopaminergic receptor agonist
*Milrinone exerts inotropic effects, but is a direct arteriolar smooth muscle relaxant and thus a direct vasodilator
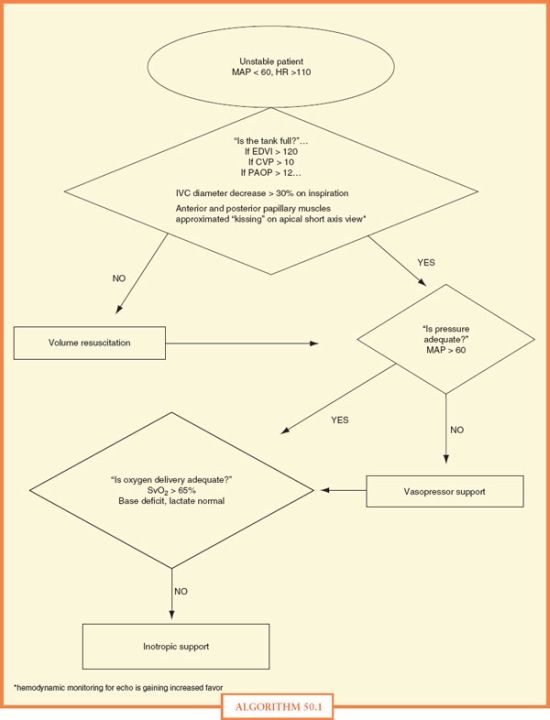
ALGORITHM 50.1 Unstable patient algorithm.
CVP, central venous pressure; EDVI, end-diastolic volume index; HR, heart rate; MAP, mean arterial blood pressure; PAOP, pulmonary artery occlusion pressure; SVO2, mixed venous oxygen saturation
In summary:
- A thorough understanding of cardiovascular physiology is a necessity for the acute care surgeon.
- When treating the patient in cardiovascular failure, first determine whether the problem is one of preload, afterload, or contractility, and their effect on oxygen delivery.
- By using adjuncts such as a PAC, measuring pressures such as CVP or PAOP and measuring markers of oxygen delivery such as SVO2, lactate, and base deficit, the acute care surgeon can guide the resuscitation of the patient in cardiovascular failure.
- Once the cause of the cardiovascular failure is recognized, “source control” is the key to treatment. Through the use of fluid resuscitation, vasopressor, and inotropic support, the acute care surgeon may return the critically ill patient to his/her normal physiologic state.
CLASSIFICATION OF CARDIOVASCULAR FAILURE
The acutely ill surgical patient may develop cardiovascular failure from a variety of pathologic causes. These disease processes can be simply classified into either intrinsic or extrinsic causes of heart failure. Intrinsic causes of cardiovascular failure stem from a disease of the myocardium, the heart valves, or the conducting system of the heart. Extrinsic causes of cardiovascular failure occur in the presence of a structurally normal heart; however, an external source impedes contractility or negatively affects afterload or preload. Although their etiologies differ, both extrinsic and intrinsic causes prevent adequate oxygen delivery in light of increased metabolic demand.
Intrinsic Cause of Cardiovascular Failure
There are four basic causes of intrinsic cardiovascular failure in the postoperative patient. These include myocardial ischemia, congestive heart failure (CHF), valvular heart defects, and cardiac arrhythmias. Patients with intrinsic cardiovascular failure suffer from a substantial increase in perioperative morbidity and mortality in the setting of acute surgical illness (see Table 50.2).
TABLE 50.2
RISK STRATIFICATION OF NONCARDIAC SURGICAL PROCEDURES

Myocardial Ischemia and Infarction. Myocardial infarction (MI) occurs in 5%–7% of postoperative elective general surgery patients, but is associated with a mortality as high as 40–70% in the symptomatic patient.71 The acute care general surgery patient is two to five times more likely to suffer from cardiovascular complications when compared to age-matched controls undergoing similar procedures on an elective basis.71 The risk of postoperative MI is highest from the time of surgery through the first 3 postoperative days. This may correlate with increased perioperative fluid shifts accompanied by an increase in circulating catecholamines, leading to a supply/demand mismatch of the myocardium. In addition, the hypercoagulable state induced by recent surgery increases the risk of coronary artery thrombosis.71–73
DIAGNOSIS OF POSTOPERATIVE MI
Insidious in the postoperative period, MI can be misdiagnosed without a heightened level of suspicion. There are four risk factors for perioperative MI: (1) the presence of preoperative cardiac risk factors, (2) perioperative hypotension, (3) new onset ST or T wave changes, and (4) large intraoperative blood loss necessitating transfusion.71,72,74 Unlike the elective general surgery patient, diagnostic testing and risk stratification in the acutely ill surgical patient often occurs after the operation. It is imperative that patients with a history of MI, diabetes mellitus, hypertension, or stroke be monitored with postoperative 12-lead ECG’s and cardiac enzyme determination. Only 50% of postoperative MI patients present with classic anginal symptoms. ECG and cardiac enzymes are useful screening tests. The 99th percentile of the normal value range for both troponin and CK-MB is the cut off value for the diagnosis of acute MI. Transthoracic echocardiography (TTE) provides additional information such as preservation of left ventricular function, the presence of a ventricular aneurysm, or wall motion abnormalities, all of which affect postoperative management. Other signs of acute MI include a fixed defect on echocardiogram or radionuclide perfusion scan, new pathologic Q waves, or perioperative arrhythmia or hypotension.
CLASSIFICATION OF ST SEGMENT ELEVATION VERSUS NON–ST SEGMENT ELEVATION MI
In addition to the patient’s hemodynamic status, treatment of an acute MI is directed by the presence or absence of ST segment elevation on ECG.72,74–76 A non–ST segment elevation MI (NSTEMI) is initially managed with medical therapy. If there is not an adequate response to medical therapy, coronary angiography may be necessary. In addition to medical therapy, an ST segment elevation MI (STEMI) will require emergent coronary angiography to determine need for coronary revascularization. The absence of ST elevation (NSTEMI) indicates reversible subendocardial ischemia from partial occlusion of the coronary artery. The presence of acute ST elevation (STEMI) indicates complete occlusion of the coronary artery with transmural infarction of the myocardium. The patient with acute ST elevation is at risk to develop irreversible myocardial damage if reperfusion does not occur quickly. These patients suffer from ventricular aneurysm, papillary muscle rupture, or ventricular arrhythmias and are at increased risk for sudden cardiac death.
THE DILEMMA OF POSTOPERATIVE BLEEDING IN ACUTE TREATMENT OF MI
Due to concern of postoperative bleeding, there is a paucity of class 1 data on the treatment of postoperative MI in the acute care surgery patient population. We advocate postoperative antiplatelet therapy with or without anticoagulation in the treatment of acute MI for the following reasons. The mortality of postoperative MI in the acute care surgery population is 40%–70%.72–76 With the exclusion of neurosurgical procedures, the mortality of postoperative anticoagulation in the noncardiac surgical population is low. However, the morbidity is not inconsequential, leading to major increase in postoperative hemorrhage and reoperation rates.
MEDICAL MANAGEMENT OF ACUTE MYOCARDIAL INFARCTION
Multiple medical treatments should be initiated once the diagnosis of postoperative MI has been made. These include antiplatelet therapy, systemic anticoagulation, beta-adrenergic blockade, adequate analgesia, statin therapy, and mechanical adjuncts for treatment of cardiogenic shock.72–74,76
Antiplatelet therapy is the gold standard for the treatment of acute coronary syndrome and has been shown in randomized clinical trials to decrease mortality when administered early in the course of MI in the general population.74,77,78 Aspirin and clopidogrel are currently the most commonly used antiplatelet agents. Aspirin directly inhibits cyclooxygenase and is an inhibitor of thromboxane synthesis. Clopidogrel inhibits platelet aggregation via the adenosine diphosphate (ADP) pathway and offers greater survival benefit than the use of aspirin alone. The risk of postoperative bleeding with the use of both drugs is substantial. There are no randomized trials that clearly delineate the risk:benefit ratio of postoperative antiplatelet therapy in the acute care surgery patient. There are, however, class II data that support the therapeutic benefit of aspirin therapy in the treatment of acute MI in the noncardiac surgery patient.72–74 Aspirin therapy may increase the risk of re-operation for postoperative bleeding but there has been no demonstrated increase in mortality from this. Antiplatelet therapy must also be considered postendovascular intervention. Recommendations for antiplatelet therapy after percutaneous coronary intervention are described elsewhere in this chapter (Table 50.3).
TABLE 50.3
AMERICAN HEART ASSOCIATION/AMERICAN COLLEGE OF CARDIOLOGY RECOMMENDATIONS FOR ANTIPLATELET THERAPY AFTER PERCUTANEOUS CORONARY INTERVENTION (STENT PLACEMENT) IN THE PERIOPERATIVE PERIOD
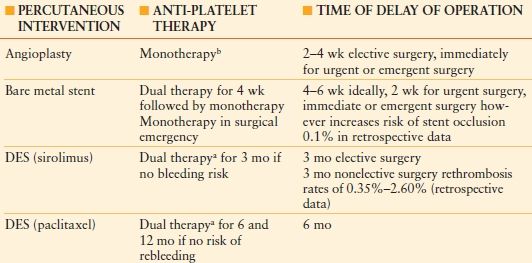
Recommendations for antiplatelet therapy poststent placement.
aDual therapy, aspirin plus thienopyridine (ticlopidine or clopidogrel).
bMonotherapy, aspirin.
Anticoagulation with unfractionated or low molecular weight heparin is considered an adjunct to antiplatelet therapy. These agents inhibit fibrinolysis, leading to thrombin generation in acute coronary syndrome. For this reason, the low molecular weight heparins may afford an advantage of being direct thrombin inhibitors. Their disadvantages may be their long half-life and accumulation in renal insufficiency, which may be problematic in the case of postoperative bleeding.
BETA-BLOCKER THERAPY
The benefits of beta-blockade, when appropriately used in the treatment of acute MI, have been detailed in the literature.71 Beta-blockers decrease tachycardia, ventricular arrhythmias, increase preload and have been proved to decrease the perioperative mortality by 28% in the first week, with the most benefit achieved in the first 48 hours. All patients previously placed on a beta blocker should continue beta blockade in the peri-operative period. Premature discontinuation of beta blockade in the perioperative period is associated with an increased risk of MI.
Morphine provides analgesia and has a cardioprotective effect in patients with NSTEMI.72,74 It decreases sympathetic tone, reduces heart rate, and lowers blood pressure. Morphine may also offer some benefit in treatment of acute pulmonary edema. Its vasodilator effects decrease afterload and left end-diastolic pressure, thus increasing cardiac output. Its vasodilatory effects, however, may induce hypotension. In addition, excessive use of narcotics in the postoperative setting has been associated with prolonged mechanical ventilation and increased length of ICU stay.72,73
Prophylactic use of nitroglycerin may provide symptomatic relief of anginal symptoms but does not provide a meaningful increase in survival benefit.72,74 Indiscriminate use of nitroglycerin in the postoperative period has been associated with increased risk of hypotension.
HMG-CoA reductase inhibitors or statins have been shown to decrease mortality at 6 months to a year in patients who are considered to be at high coronary risk.72,74 In addition, they have been shown to decrease the incidence of sudden cardiac death. Patients who have been on statin therapy previously should continue with it in the perioperative period. For patients undergoing vascular surgery with at least one coronary risk factor, the addition of statin therapy may afford meaningful survival benefit.72–74
Angiotensin concerting enzyme (ACE) inhibitors have also been shown to reduce morbidity in acute MI.72–74 The greatest therapeutic benefit is in patients with a decreased left ventricular ejection fraction (<45%) and symptoms of heart failure. In addition patients who have suffered an acute MI with preexisting hypertension or documented mitral regurgitation have an elevated risk of developing ischemic cardiomyopathy, and should be started on an ACE inhibitor within 48 hours of the acute ischemic event.
There are currently no randomized clinical trials that clearly define the therapeutic benefit of cardiac catheterization in the postoperative acute general surgery patient. Indications for catheterization are listed in Table 50.4 and a decision tree regarding catheterization of the postoperative patient is presented in Algorithm 50.2.72,74,76,77–78
TABLE 50.4
CLASS I DATA-INDICATIONS FOR CARDIAC CATHETERIZATION

LAD, left anterior descending coronary artery
LVEF, left ventricular ejection fraction
MI, myocardial infarction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








