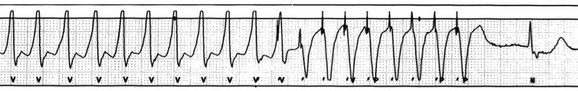
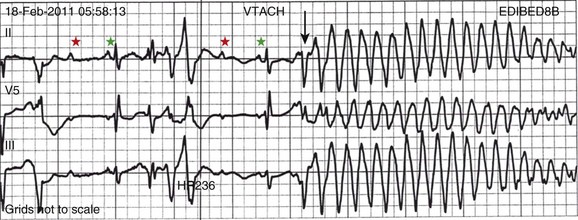
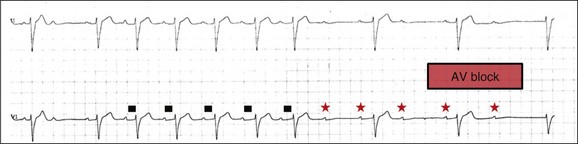
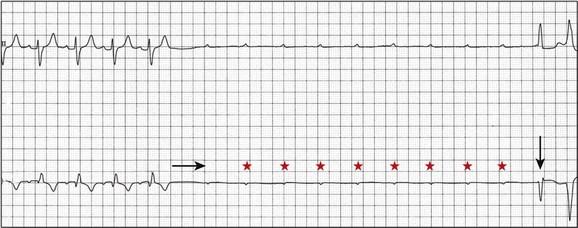
5 INDICATIONS FOR PERMANENT PACEMAKER IMPLANTATION Pacing Indications for Sinus Node Dysfunction Pacing Indications for Acquired Atrioventricular Block INDICATIONS FOR TEMPORARY PACING Atrioventricular Nodal Dysfunction High-Grade and Paroxysmal Atrioventricular Block Electrolyte and Metabolic Derangement CONDITIONS THAT DO NOT NORMALLY REQUIRE PACING ESOPHAGEAL AND TRANSTHORACIC PACING Prevention of Atrial Arrhythmias Treatment of Atrial Arrhythmias Diagnosis of Wide Complex Arrhythmias SPECIAL CONSIDERATIONS IN TEMPORARY PACING COMPLICATIONS OF PERMANENT PACEMAKER IMPLANTATION SPECIAL CONSIDERATIONS IN PERMANENT PACING The American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), and the Heart Rhythm Society (HRS) have jointly engaged in the production of guidelines for cardiac implantable electronic device (CIED) implantation, with publication of updated Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities in 2008.1 These recommendations are primarily evidence-based, and this publication includes an extensive review of the literature on the topic. As with other guideline documents, recommendations are classified as class I, II, or III as follows: • Class I: Benefit • Class II: Conditions for which there is conflicting evidence or divergence of opinion about the usefulness/efficacy of the procedure. • Class IIa: Benefit • Class IIb: Benefit ≥ Risk; the procedure/treatment may be considered, although additional studies with broad objectives are needed or additional registry data would be helpful. • Class III: Risk ≥ Benefit; the procedure/treatment should not be performed/administered because it is not helpful and may be harmful. The level of evidence or weight of evidence to support these recommendations is ranked as follows: • Level of evidence A: Data derived from multiple randomized clinical trials or meta-analyses. • Level of evidence B: Data derived from a single randomized trial or nonrandomized studies. • Level of evidence C: Consensus opinion of experts, case studies, or standard of care. SND is the most common cause of bradyarrhythmias in clinical practice. The typical age at the time of diagnosis of SND appears to be in the seventh or eighth decade of life, with a mean or median age of 71 to 74 years in randomized clinical trials evaluating pacemaker therapy.2–4 However, clinical manifestations of SND may occur at any age and may be secondary to any one of several potential causes, including destruction of the SN, ischemia, infarction, infiltrative disease, surgical trauma, autonomic dysfunction, or endocrinologic abnormalities.1,5 Clinical manifestations of SND are diverse, and symptoms may include fatigue, reduced exercise tolerance, dyspnea on exertion, presyncope, lightheadedness, dizziness, or syncope. In the absence of any clearly reversible cause of bradycardia, the only effective treatment for symptomatic bradycardia in patients with SND is permanent pacing. Box 5.1 outlines recommendations for permanent pacing in patients with SND.1 Third-degree AV block refers to absence of impulse conduction from the atria to the ventricles, and this may be congenital or acquired. Permanent pacing is often indicated for acquired complete block without reversible causes. AV block may also occur in patients with SND, and 20% of patients with SND will have some degree of AV block.4 In addition, following permanent pacemaker implantation for SND, the risk of developing AV block within 5 years of follow-up is 3% to 35%.6–9 Patients with AV block may be asymptomatic, or may have symptoms that vary from mild lightheadedness, dizziness, shortness of breath, or fatigue to presyncope and loss of consciousness. The decision regarding permanent pacemaker implantation should take into account whether or not symptoms are attributable to bradycardia, as well as the cause and “level” of AV block. Completely reversible causes of AV block, such as electrolyte disturbances or Lyme disease, should be excluded. Permanent pacing indications for acquired AV block are summarized in Box 5.2. Pacing indications for chronic bifascicular block and pacing for AV block associated with acute myocardial infarction are outlined in Boxes 5.3 and 5.4. In specific situations, permanent pacing may also be clinically indicated in some patients with carotid sinus hypersensitivity, neurocardiogenic syncope, or obstructive hypertrophic cardiomyopathy, and following cardiac transplantation.1 Historically, antitachycardia pacemakers were occasionally utilized to treat recurrent supraventricular arrhythmias, but they are rarely used in contemporary practice with the availability of catheter ablation therapy. Pace termination of ventricular tachycardia is frequently utilized for the treatment of monomorphic ventricular tachycardia as part of implantable cardioverter-defibrillator (ICD) therapy, and can also be used to terminate frequent arrhythmia episodes using a temporary transvenous pacing system in the intensive care setting (Fig. 5.1). Pacing may be useful in the prevention of pause-dependent, polymorphic ventricular tachycardia as well (Fig. 5.2). Permanent pacing is indicated for pause-dependent ventricular tachycardia, with or without QT prolongation (class I indication, level of evidence C), and is reasonable for high-risk patients with congenital long QT syndrome (class IIb indication, level of evidence B).1 Atrial-based pacing (“AAI” or “DDD” mode) is considered the preferred pacing mode for prevention of polymorphic ventricular tachycardia associated with the congenital long QT syndrome. Pacing the left ventricle can improve hemodynamics in patients with dilated cardiomyopathy and bundle branch block by altering the activation sequence and influencing regional contractility, and is particularly effective in patients with left bundle branch block. This is referred to as “biventricular pacing” or “cardiac resynchronization therapy” and is beyond the scope of this chapter. The “level” of AV block—whether at the level of the AV node or below the AV node in the His-Purkinje conduction system—is critical in determining the need for temporary pacing. AV nodal block improves with measures that accelerate AV nodal conduction, like atropine, dopamine, and isoproterenol. Infranodal block—that is, block below the level of the AV node at the bundle of His or bundle branches—may paradoxically worsen with these agents owing to downstream block in an already diseased His-Purkinje system. The origin of the escape rhythm—whether “proximal” or “distal” in the cardiac conduction system—predicts both its rate and stability. Complete heart block at the level of the AV node is associated with an escape rhythm arising from the AV junction, His bundle, or proximal fascicles; a narrow QRS morphologic pattern; and a heart rate in excess of 60 beats per minute. Infranodal block is associated with an escape rhythm arising from the bundle branches or even ventricular myocardium, a wide and often bizarre QRS morphologic pattern, and a heart rate in the range of 40 beats per minute.10 Complete heart block with a junctional escape rhythm does not typically require temporary pacing, unless accompanied by hypotension. In contrast, complete heart block with a ventricular escape rhythm is inherently unstable and usually requires temporary pacing, even if hemodynamically stable. History can be quite helpful in risk stratification. A history of syncope in a patient who presents with advanced second-degree AV block may portend a higher degree of AV block or pause-dependent torsades de pointes, and there should be a very low threshold for temporary pacing.11 In the critical care setting, it is crucial to distinguish between paroxysmal and vagally mediated AV block. Vagally mediated AV block due to extrinsic, parasympathetic input is characterized by progressive sinus slowing, progressive PR prolongation, Mobitz type I second-degree AV block immediately before the onset of complete heart block, and sinus slowing during the episode (Fig. 5.3). In contrast, high-grade AV block due to an intrinsic failure of a diseased His-Purkinje system—also termed “paroxysmal” AV block—is characterized by a constant sinus rate, or even sinus acceleration during the episode (Fig. 5.4). Patients with paroxysmal AV block usually have some sort of baseline conduction abnormality on their surface 12-lead electrocardiogram—most commonly, right bundle branch block—but this finding is not absolute. The hallmark of paroxysmal AV block is immediate transition from apparently normal conduction to complete AV block and ventricular asystole. This is usually triggered by a pause after a premature atrial or ventricular depolarization, but vagally mediated sinus slowing can have the same effect, complicating the interpretation of these events. Vagally mediated heart block is typically benign, is atropine responsive, and does not require temporary pacing. Paroxysmal AV block can be fatal and requires temporary transvenous pacing until a permanent pacemaker can be placed.12 Hyperkalemia can precipitate complete AV block and can also elevate pacing thresholds in permanent pacemaker systems.13–15 A progressive increase in extracellular potassium raises the resting membrane potential, inactivating voltage-gated sodium channels that depend upon a sufficiently negative resting membrane potential for normal function. The effect is more pronounced in the atrium and ventricle than the cells of the specialized conduction system, explaining why the characteristic changes in the P wave and QRS complex typically precede sinoatrial (SA) and AV nodal dysfunction: “peaked” T waves and QT shortening (potassium level 5.5 mEq/L); PR prolongation and QRS widening (potassium level 6.5 mEq/L); a “sinoventricular” rhythm, due to the apparent absence of atrial activity (potassium level 8-9 mEq/L); and lastly, a “sine wave” pattern due to merging of the QRS and T wave that predicts impending cardiac arrest (potassium level 10 mEq/L).16 Nevertheless, the 12-lead electrocardiogram may be entirely normal in cases of pronounced hyperkalemia, and AV block may occur in isolation.17–20 Therefore, a routine metabolic evaluation is indicated in all patients with new conduction deficits. Case reports and animal studies suggest a link between metabolic acidosis and heart block, and metabolic acidosis frequently accompanies hyperkalemia in the setting of chronic kidney disease.21 The administration of sodium bicarbonate acutely raises extracellular pH and indirectly lowers extracellular potassium, and may improve responsiveness to vasopressors in emergent situations.22 Hyponatremia, hypokalemia, hypomagnesemia, and hypocalcemia have not been implicated in heart block. A number of drugs can cause severe sinus bradycardia, AV block, or both. β-Adrenergic blockers and calcium channel blockers are both negatively chronotropic and inotropic, and their administration can result in significant bradycardia and hypotension, particularly in overdose. Digoxin toxicity may present with high-grade or complete AV block, further compounded by atrial or ventricular tachycardia due to increased automaticity. Amiodarone, dronedarone, and sotalol—class III antiarrhythmic drugs with mixed antiarrhythmic effects—frequently cause bradycardia due to SA or AV conduction defects. Some studies suggest that drug-induced AV block—particularly at therapeutic levels—is a predictor of future conduction disorders.23 Clearly, the initial treatment is discontinuation of the offending drug(s). Directed therapy may occasionally be useful as well, but the evidence is largely anecdotal. β-Adrenergic blocker toxicity may respond to glucagon, and calcium channel blocker toxicity may respond to calcium or glucagon in refractory cases.24–28 Vasopressors are occasionally necessary because of the vasodilatory and negative inotropic effects of these drugs. Digoxin toxicity can be treated with digoxin antibody fragments (Digibind).29 If hemodynamic instability persists in the setting of severe bradycardia, temporary pacing is indicated. β-Adrenergic blockers and calcium channel blockers increase pacing thresholds in permanent devices; if failure to capture is noted in a patient with suspected overdose of these agents, a temporary increase in pacing output may solve the problem.30 Lyme disease is the most common tick-borne illness in North America.31 Erythema migrans is sufficient for diagnosis, but most patients cannot recall a tick exposure or rash.32 The diagnosis of Lyme carditis requires serologic testing for confirmation, and Borrelia burgdorferi IgM and IgG antibodies are positive in the vast majority of patients with the disease.33 Regardless of patient history, the diagnosis of Lyme carditis should always be entertained in a patient presenting with heart block in an endemic area. Typically, Lyme carditis affects the AV node and is associated with a stable, junctional escape rhythm, but more diffuse involvement of the His-Purkinje system with a slower, more unstable escape is also possible.34 Although conduction deficits regress rapidly and completely with appropriate antibiotic therapy, temporary pacing is occasionally necessary. Infective endocarditis can be complicated by a broad spectrum of conduction disturbances, including first-degree AV delay, bundle branch block, and complete heart block. Any new conduction deficit suggests perivalvular abscess, due to the proximity of the compact AV node and bundle branches to the membranous septum.35,36 Conduction disturbances most commonly complicate aortic valve endocarditis, but the tricuspid valve and mitral valve are also susceptible.37–39 Perivalvular abscess and heart block are indications for surgical repair.40 Serial 12-lead electrocardiography should be performed in all patients with endocarditis, and temporary pacing should be strongly considered for any progressive conduction disturbance. Lymphocytic and giant cell myocarditis are typically associated with acute systolic dysfunction, but they can also be complicated by severe electrical abnormalities, including complete heart block. Lymphocytic myocarditis carries a more favorable prognosis than giant cell myocarditis, but permanent pacemaker dependency is possible with either condition.41 Official guidelines for temporary and permanent pacing after ST-segment elevation myocardial infarction were last updated in 2004, and permanent pacemaker indications following myocardial infarction were updated in 2008 as previously discussed.1,42 Recommendations for temporary pacing are largely based on expert opinion, in addition to case reports, case series, and published summaries from before the reperfusion era.
Cardiac Pacing
Indications for Permanent Pacemaker Implantation
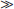 > Risk; the procedure/treatment should be performed/administered.
> Risk; the procedure/treatment should be performed/administered.
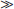 Risk; it is reasonable to perform the procedure/administer treatment, although additional studies with focused objectives are still needed.
Risk; it is reasonable to perform the procedure/administer treatment, although additional studies with focused objectives are still needed.
Pacing Indications for Sinus Node Dysfunction
Pacing Indications for Acquired Atrioventricular Block
Other Permanent Pacing Indications
Indications for Temporary Pacing
Atrioventricular Nodal Dysfunction
High-Grade and Paroxysmal Atrioventricular Block
Electrolyte and Metabolic Derangement
Drug Side Effects
Infectious Disease
After Myocardial Infarction
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cardiac Pacing