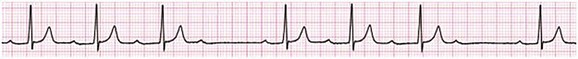
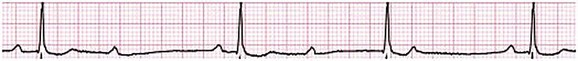
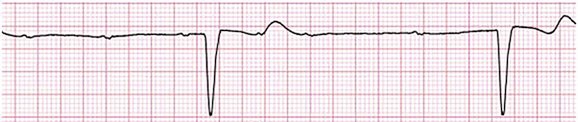
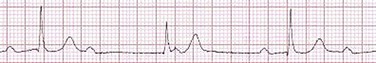
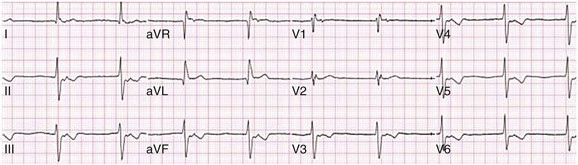
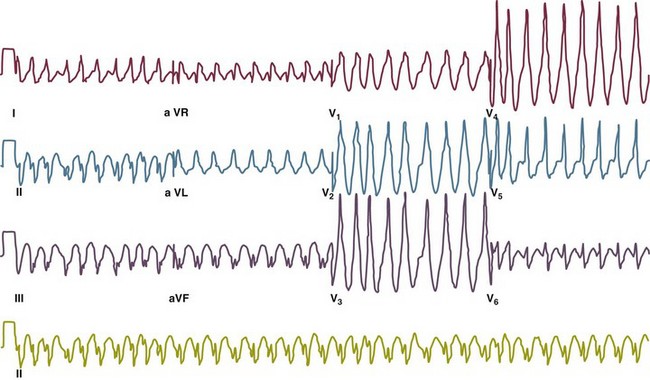
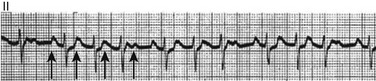
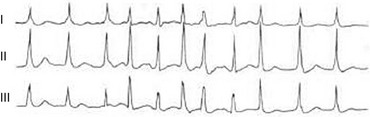
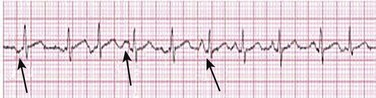
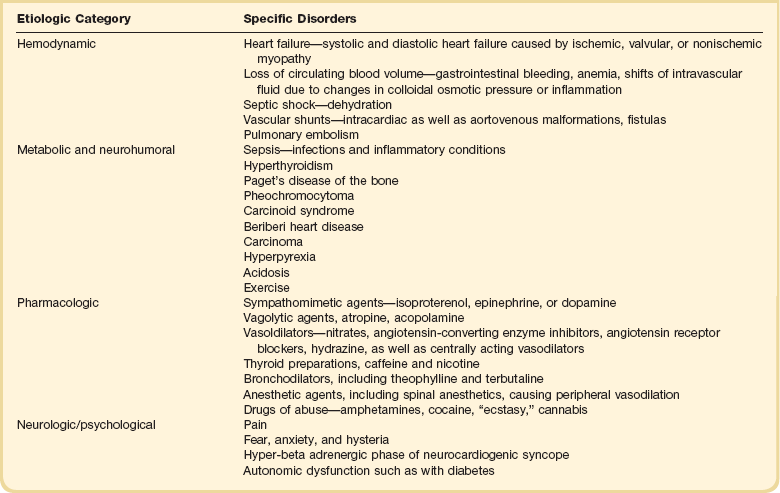
31 Sinus Bradycardia and Sinus Node Dysfunction Vagally Mediated Sinus Arrest, Bradycardias, and Heart Block Paroxysmal Supraventricular Tachycardia Approach to Paroxysmal Supraventricular Tachycardia Therapy Wolff-Parkinson-White Syndrome and Its Variants Nonparoxysmal Atrioventricular Junctional Tachycardia, Paroxysmal Atrial Tachycardia with Block, and Automatic Atrioventricular Junctional Tachycardia A BRIEF REVIEW OF ANTIARRHYTHMIC DRUGS Metabolic Disturbances and Ischemia Differential Diagnosis of Wide QRS Tachycardia Approach to Ventricular Arrhythmias in the Critically Ill Specific Ventricular Arrhythmias CARDIAC ARREST AND ELECTRICAL STORM CATHETER ABLATION OF CARDIAC ARRHYTHMIAS PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS ELECTROCARDIOGRAPHIC PATTERNS INTENSIVISTS SHOULD RECOGNIZE Critical care physicians deal with a plethora of medical and surgical problems in their patients. Arrhythmias may be the primary abnormality or may be secondary to myocardial ischemia, electrolyte imbalance, or toxic/metabolic disturbances. Optimal management of arrhythmias requires expertise in electrocardiography and clinical pharmacology as well as knowledge of arrhythmia precipitants, including proarrhythmia caused by antiarrhythmic drugs.1 The responsibility of the critical care physician is to facilitate transition from acute to chronic care by referring the patient with an arrhythmia to a cardiologist or an electrophysiologist. Therefore, the major emphasis of this chapter is on acute care of the patients with arrhythmias. Bradyarrhythmias usually present as either sinus node dysfunction (SND) or atrioventricular blockade (AV block). Bradyarrhythmias and indications and techniques for temporary cardiac pacing are reviewed extensively in Chapter 5. A brief overview is included here to highlight important issues for the intensivist. Sinus bradycardia is generally defined as periods of sinus rhythm with rates less than 60 beats per minute. In the absence of symptoms, it usually is benign and requires no treatment. Sinus bradycardia is common in young adults (particularly the physically fit). Nocturnal rates of 35 to 40 beats per minute and pauses during sleep of 2 seconds or longer are not uncommon. Sinus arrhythmia (Fig. 31.1) is a normal variant in which there are respirophasic changes in the RR interval on electrocardiogram (ECG) (prolongation of RR intervals during expiration). Sinus bradycardia may also be a manifestation of certain pathologic conditions such as increased intracranial pressure, oculocardiac reflex after ophthalmologic surgery, cervical and mediastinal tumors, hypothyroidism, hypothermia, gram-negative sepsis, Chagas’ disease, depression, and anorexia nervosa. Sinus bradycardia is often seen after cardiac transplantation. Beta blockers, parasympathomimetic agents, calcium antagonists, amiodarone, and lithium commonly produce sinus bradycardia. Digoxin, in therapeutic doses, usually does not markedly affect the sinus node and is relatively safe to use in patients with SND.2 Sinus bradycardia complicates 10% to 15% of acute myocardial infarctions and is most common with inferior infarcts. It also may be seen after successful thrombolysis. In the absence of hemodynamic compromise, it is associated with a more favorable prognosis than sinus tachycardia.3 Sinus bradycardia (with or without AV block) may occur during periods of autonomic instability. Examples include carotid hypersensitivity and neurocardiogenic syncope. These syndromes have cardioinhibitory (bradycardic), vasodepressor (vasodilatory), and mixed forms. Permanent pacing (which must include the ability to pace the right ventricle for heart block) is a well-established therapy for cardioinhibitory carotid sinus hypersensitivity. Neurocardiogenic syncope generally is a benign condition that usually can be managed without permanent pacemaker therapy. However, treatment for patients with frequent and severe cardioinhibitory spells, especially those in whom asystolic periods exceeding 5 seconds can be demonstrated clinically or during head-up tilt table testing, may include palliative pacemaker therapy.4 Second-degree AV block is classified as Mobitz type I (Wenckebach), Mobitz type II, 2 : 1 AV block, or high-degree AV block. In Mobitz I (Wenckebach) block, PP intervals are constant, with gradual PR prolongation before failure of impulse conduction (nonconducted P wave) (Fig. 31.2). Although classic Wenckebach block involves simultaneous shortening of successive RR intervals before AV block, atypical alternations in RR intervals actually are more common. In younger people, Mobitz I AV block with normal QRS complexes generally is benign and does not progress to more advanced AV conduction disturbances. In older patients, the prognosis may be similar to that with Mobitz II block. Mobitz I second-degree AV block may accompany inferior myocardial infarction. The condition is benign, with a favorable prognosis, in the absence of hemodynamic compromise. The conduction disturbance usually is transient, and permanent pacing is not required. Mobitz II AV block is characterized by sudden failure of atrial impulse conduction without prior PR prolongation (Fig. 31.3). This form of AV block frequently heralds development of complete AV block and Adams-Stokes syncope. Mobitz II second-degree AV block in the setting of anterior infarction is associated with pump failure and high mortality rates. Survivors should receive permanent pacemakers. It is important to remember a few general rules to avoid common ECG misinterpretations of second-degree AV block. The 2 : 1 form of AV block may be nodal or infranodal. 2 : 1 block associated with narrow QRS complexes generally results from AV nodal block (Fig. 31.4). Wide QRS complexes are compatible with block in either the AV node or His-Purkinje system. If more than one P wave is not conducted to the ventricle, the term high-grade AV block is used (Fig. 31.5). Complete AV block is diagnosed by the presence of independent atrial and ventricular activity on the ECG where the atrial rate is faster than the ventricular rate. When the atrial rhythm is sinus, AV dissociation is present, with the sinus rate exceeding the ventricular rate. The PP interval is constant. The RR interval is constant. The PR interval is variable in a random, nonrecurring pattern (Fig. 31.6). Complete AV block may also be present during all varieties of atrial tachycardia. Acquired complete AV block most commonly occurs distal to the His bundle, usually is secondary to a trifasicular conduction disturbance, is potentially life-threatening, and generally is irreversible. A wide QRS escape rhythm with ventricular rates less than 40 beats per minute is the rule. An exception is seen in the setting of inferior infarction, in which recovery of complete (narrow QRS) AV nodal block occurs in greater than 90% of patients (time to recovery 30 minutes to 16 days).5 Drug toxicity, coronary artery disease, and degenerative disease of the conduction system are the most common causes of AV block in adults. Surgery, electrolyte disturbances (such as hyperkalemia), endocarditis, myocarditis (Lyme carditis), tumors, myxedema, rheumatoid nodules, Chagas’ disease, calcific aortic stenosis, polymyositis, amyloidosis, sarcoidosis, scleroderma, and vagotonic reflexes all may result in AV block. In truth, the number of factors and conditions that may result in AV block is nearly endless. “Hypervagal” responses (carotid hypersensitivity, neurocardiogenic syncope) may produce transient AV block (see later).3 When the sinus node does not depolarize the atrium for any reason (high vagal tone, SND), the cells near the AV junction (AV node, His bundle) may take over as the active pacemaker. Retrograde activation of the atrium, commonly manifest as negative P waves in the inferior leads (II, III, aVF), may be seen (Fig. 31.7). If the junctional rhythm goes faster than 60 beats per minute, it is termed an accelerated junctional rhythm. This is a common manifestation of digitalis toxicity. The most common cause of nonconducted P waves during telemetry or Holter recordings is bradycardia-associated AV block. This manifests as sudden (usually nocturnal) block of one or more P waves with or without antecedent PR prolongation. This phenomenon is characterized by PP prolongation before AV block and is the result of transient increases in vagal tone. Vagally mediated sinus arrest, bradycardia, and heart block often occur in the intensive care unit (ICU) setting as a result of suctioning, gagging, femoral vessel compression (for hemostasis), and a variety of other triggers (Box 31.1). Vagal stimulation may lower blood pressure with or without significant bradycardia. Bradyarrhythmias and hypotension usually resolve when vagal stimulation ceases. Persistent bradycardia or hypotension mediated by vagal tone may require placing the patient in the Trendelenburg position, temporary saline infusion, or intravenous administration of atropine to fully resolve the episode. Tachyarrhythmias often occur in critically ill patients. Conditions such as hypoxemia, electrolyte imbalance, catecholamine excess (endogenous and exogenous), and other metabolic disturbances predispose patients (with or without preexisting arrhythmic substrates) to tachyarrhythmias. Intensivists must be prepared for acute management of supraventricular tachycardia. Knowledge of arrhythmia mechanisms, appropriate choices for acute pharmacotherapy, and indications for urgent or emergent direct current cardioversion are requisite.6 We will discuss the mechanisms of supraventricular tachyarrhythmias, give an ECG-guided approach to their diagnosis, and cover specific treatments for these dysrrhythmias (pharmacologic and catheter ablation). AVNRT is by far the most common and in the past accounted for 50% to 60% of PSVTs evaluated at referral centers.7 The precise reentrant circuit is not defined; however, it is clear that the anterior and posterior AV nodal approaches and the perinodal atrial tissue are involved. In 76% to 90% of cases, antegrade conduction proceeds along the posterior (slow) AV nodal approach (pathway) and retrograde conduction along the anterior (fast) AV nodal pathway.7,8 This is slow-fast AVNRT. Because retrograde conduction is so rapid, atrial and ventricular activation are virtually simultaneous. P waves are usually not visible on the surface ECG or may appear in the terminal portion of the QRS complex (pseudo R′ in lead V1 or pseudo-S waves in the inferior leads). Atrial contraction on a closed AV valve may produce neck pounding.9 Less common (so-called “unusual”) variants (fast-slow, slow-slow, and slow–sort of slow) of AV nodal reentry also exist.8 AVNRT usually manifests after the age of 20 years8 and is more common in women than in men. The typical heart rate in AVNRT ranges from 150 to 250 beats per minute. Palpitations, lightheadedness, and near-syncope may accompany an episode. True syncope is unusual. Neck pounding (see previously) is virtually pathognomonic,8 but its absence does not exclude AVNRT. Before catheter-based cures became routine, AVRT was the next most common (accounting for 30%) PSVT mechanism.7 AVRT (also commonly referred to as orthodromic tachycardia) manifests (on average) at a somewhat earlier age than that typical for AVNRT. The antegrade limb of the circuit proceeds down the normal AV nodal His-Purkinje system. The retrograde limb uses an accessory pathway that usually is located along the mitral or tricuspid valve annulus. Because the accessory pathway conducts in only retrograde fashion, it is concealed (not seen on surface ECG). Because AVRT proceeds normally antegradely, the QRS complex is generally narrow. The AVRT reentry circuit travels antegradely through the AV node and His-Purkinje system to the ventricles before retrograde activation of the atria occurs via the bypass tract. The extra time taken to travel by way of the ventricles creates a longer RP interval during tachycardia compared with that seen in AVNRT. Because AVNRT and AVRT activate periannular atrial tissue first, P waves (if visible on surface ECG) will be negative in the inferior leads. Upright P waves in these leads indicate atrial (or sinus) tachycardia. AVRT tends to go faster than AVNRT and is more prone to manifest with QRS alternans or left bundle branch block (LBBB) aberrancy.10,11 A decrease in tachycardia rate on development of bundle branch block ipsilateral to the pathway is characteristic of AVRT. AV block is unusual during AVNRT and excludes the diagnosis of AVRT (which requires both atrial and ventricular participation). The presence of AV block strongly suggests the diagnosis of atrial tachycardia. In the past, intra-atrial reentry, automatic atrial tachycardia, and sinus nodal reentry accounted for the remaining 8% to 10% of PSVTs.7 Sinus node reentry rarely occurs as an isolated phenomenon.12 Approximately 50% of patients with intra-atrial reentry have evidence of structural heart disease.13 This tachycardia is particularly prone to develop after surgery for congenital cardiac anomalies. Reentry occurs around structural barriers, such as suture lines. In patients without clear-cut structural disease, subtle changes such as scarring and fibrosis provide the substrate for reentry. Automatic atrial tachycardias occur along the crista terminalis, near the ostium of the coronary sinus, along the tricuspid and mitral annulus, in both atrial appendages, and within or in close proximity to the pulmonary veins. Automatic atrial tachycardias are exquisitely sensitive to catecholamines. Although these tachycardias may manifest in the absence of structural heart disease or obvious precipitants, they also are commonly associated with chronic lung disease, pneumonia, myocardial (atrial) infarction, and acute alcoholic binges. Amphetamine or cocaine abuse also may precipitate automatic atrial tachyarrhythmias. Automatic atrial tachycardia is difficult to manage with pharmacotherapy. Precipitants should be treated or eliminated whenever possible. Beta blockers may slow atrial rate but rarely restore sinus rhythm. Adenosine may produce sinus rhythm; however, tachycardia may resume as soon as the drug is metabolized.14 Vagal maneuvers may produce AV block but do not terminate these arrhythmias. Clinical successes have been obtained with class IC agents and amiodarone. Flecainide should be avoided in patients with coronary artery disease or significant left ventricular dysfunction. Intravenous flecainide is not available in the United States (see later). Amiodarone is available for intravenous administration. Intravenous amiodarone may result in hypotension (vasodilation) but usually does not exacerbate heart failure or cause proarrhythmia in the setting of preexisting left ventricular dysfunction. The ECG pattern of Wolff-Parkinson-White syndrome (see “Electrocardiographic Patterns Intensivists Should Recognize”), short PR interval with preexcitation (delta wave), has a reported prevalence of 0.1% to 0.3% in the general population. It is twice as common in men as in women. Classic Wolff-Parkinson-White syndrome occurs when the accessory AV pathway is capable of bidirectional conduction (AV and ventriculoatrial). Symptomatic presentation usually is during the teenage years or early adulthood. Pregnancy may exacerbate symptoms. The most common tachycardia is AV reentry (down the AV node and His-Purkinje system, up the bypass tract), identical to AVRT involving a concealed bypass tract. Approximately 25% of patients with a Wolff-Parkinson-White ECG pattern are incapable of retrograde conduction via the accessory pathway (and therefore do not have orthodromic AVRT). Asymptomatic patients generally have a benign prognosis; however, the initial presentation may be ventricular fibrillation (see later).15 Accessory pathways generally have conduction properties similar to those of myocardium. Decremental conduction, (AV conduction delay or block) which is characteristic of the AV node, is uncommon. Pathways may therefore be capable of very rapid antegrade (AV) conduction. In these instances, atrial fibrillation may be associated with irregular wide QRS tachycardia and ventricular rates in excess of 300 beats per minute (Fig. 31.8). Syncope or SCD (degeneration to ventricular fibrillation) may ensue. Intravenous ibutilide (1 mg infused over 10 minutes; a second 1-mg dose may be given by infusion after a 10-minute wait, if necessary) also blocks antegrade accessory pathway conduction and is more likely to terminate acute episodes of atrial fibrillation or flutter (see later).16 Atriofascicular pathways (Mahaim fibers) connect the right atrium and the right bundle branch. During sinus rhythm, preexcitation is minimal or absent. Typical Mahaim reentry travels antegradely down the bypass tract and retrogradely through the normal conduction system (usually beginning with the right bundle branch). A regular wide QRS typical LBBB pattern is seen.17 These pathways conduct antegrade in a decremental fashion (retrograde accessory pathway conduction is absent) and occur much less frequently than typical AV accessory pathways. Patients with atriofascicular fibers frequently have multiple accessory pathways or AVNRT.15,18 Patients whose supine systolic blood pressure is greater than 90 mm Hg can be given intravenous adenosine. It is administered in the same manner as described previously. Tachycardia termination suggests a supraventricular mechanism that includes the AV node as a requisite part of the circuit. Transient AV block suggests an atrial tachycardia. No response to adenosine suggests a ventricular origin.19,20 Digitalis toxicity also may precipitate atrial tachycardia (so-called paroxysmal atrial tachycardia with block) (Fig. 31.9). This tachycardia usually is managed by withholding digoxin and administering potassium. Lidocaine, phenytoin, and digoxin-specific antigen-binding fragments also may be used. This tachyarrhythmia results in marked hemodynamic deterioration after corrective surgery for congenital heart disease. It generally appears within 12 hours postoperatively and terminates within a few days if the patient survives. Digitalis, beta blockers, and class IA antiarrhythmics are ineffective in children. Amiodarone (which may suppress tachycardia or control its rate) should be administered when rates less than 150 beats per minute cannot be achieved by other means.21 In adults, beta blockade may successfully control the rate. Adult automatic AV junctional tachycardia may be difficult to manage medically (Fig. 31.10). Recent reports suggest that catheter ablation can eliminate tachycardia while preserving AV conduction.22–24 MAT is an automatic tachyarrhythmia. It is characterized by three or more morphologically distinct (nonsinus) P waves, atrial rates of 100 to 130 beats per minute, and variable AV block (Fig. 31.11). MAT is commonly associated with respiratory disease and CHF. It has been reported in patients with cancer, lactic acidosis, pulmonary emboli, renal disease, and infection. Hypoxemia frequently is present. MAT may be exacerbated by digitalis or theophylline toxicity, hypokalemia, hypomagnesemia, and hyponatremia. These precipitants usually do not result in MAT if respiratory decompensation is absent. Although MAT is (in general) an uncommon arrhythmia, it is relatively common in the critical care setting. Treatment of MAT usually is directed at elimination of the underlying precipitants. Metoprolol (used cautiously when bronchospasm is present) or verapamil may provide (atrial and ventricular) rate control and occasionally restore sinus rhythm.25,26 Potassium and magnesium supplements may help suppress MAT. Amiodarone has also been useful in restoring sinus rhythm. MAT may, superficially, resemble atrial fibrillation. Careful examination of a 12-lead ECG may be required to distinguish between these two entities. Differentiation is important for proper patient management. As noted, MAT does not respond to direct current cardioversion and is not amenable to catheter ablation. Sinus tachycardia usually is a normal reflex response to changes in physiologic, pharmacologic, or pathophysiologic stimuli such as exercise, emotional upset, fever, hemodynamic or respiratory compromise, anemia, thyrotoxicosis, poor physical conditioning, sympathomimetic or vagolytic agents, and abnormal hemoglobins.27 The resulting increase in cardiac output usually is beneficial. Heart rate generally does not exceed 180 beats per minute, except in young patients, who may achieve rates higher than 200 beats per minute during vigorous exercise.3 Tachycardia resolves when conditions return to baseline. The differential diagnosis for sinus tachycardia is presented in Table 31.1. Although the precise reentrant circuit is unknown, typical atrial flutter traverses (with either counterclockwise or clockwise rotation) through an isthmus formed by the inferior vena cava, tricuspid valve, eustachian ridge, and coronary sinus ostia. Counterclockwise rotation is more common and results in negative “flutter” waves in ECG leads II, III, aVF, and V6. Atrial activity in lead V1 is positively directed. Clockwise atrial flutter produces oppositely directed flutter waves in these leads. Atrial rates generally range between 250 and 350 beats per minute; however, slower rates may be seen in the presence of specific pharmacotherapy (which slows conduction within the circuit) or marked right atrial enlargement (presumably caused by a larger circuit).28 Atrial flutter usually manifests with 2 : 1 AV block and ventricular rates of approximately 150 beats per minute. Atrial fibrillation most often is associated with structural cardiac (diffuse atrial) disease. Unlike in typical atrial flutter, left atrial enlargement is more important than right atrial enlargement in the pathogenesis of atrial fibrillation.29 The chaotic ECG appearance of this arrhythmia usually is the result of shifting reentrant circuits (multiple wavelet hypotheses). Atrial fibrillation may have focal triggers (usually in one or more pulmonary veins).30 Causes of atrial fibrillation are listed in Box 31.2. Although atrial fibrillation is the most common sustained arrhythmia, there is no consensus on optimal atrial fibrillation management. In the critically ill, atrial fibrillation may be a “sign” (perhaps of disease severity) rather than an arrhythmic disease entity (as seen in noncritically ill patients with recurrent paroxysmal, persistent, and permanent atrial fibrillation). Critically ill patients are often in a hyperadrenergic state, which may increase ectopic triggers and shorten atrial refractoriness. This is likely the mechanism of atrial fibrillation in younger patients without chronic structural heart disease. In older patients with structural disease, increased adrenergic tone (and triggers) may precipitate atrial fibrillation when fibrosis has already created a suitable reentrant substrate.31 1. Is the diagnosis of atrial fibrillation correct? 2. Are there causes/precipitants (see Box 31.2) that can be eliminated or corrected? 3. Is it necessary to restore and maintain sinus rhythm? 4. Is atrial fibrillation causing hemodynamic impairment (angina, heart failure, hypotension)? 5. What are the potential adverse effects of the various therapeutic options?31 It is important to carefully analyze a 12-lead ECG because, as previously noted, MAT may be misinterpreted as atrial fibrillation on a rhythm strip. The treatment of choice for MAT remains correction of precipitants such as hypoxemia, digitalis or theophylline toxicity, hypokalemia, or hypomagnesemia. Atrial fibrillation may, likewise, terminate (and be less likely to recur) when precipitants are removed or corrected.31 Atrial fibrillation that does not compromise the patient may not require aggressive therapy. Rate control strategies may suffice. Spontaneous atrial fibrillation termination may be difficult to distinguish from a clear-cut benefit of specific antiarrhythmic pharmacotherapy. Hemodynamic impairment should be treated with urgent or emergent direct current cardioversion.31 Serial direct current shocks are not appropriate for recurrent (within hours or days) paroxysms (self-terminating episodes) of atrial fibrillation. This scenario is relatively common in ICUs or after cardiac surgery. It is also important to avoid repetitive, futile shocks or delivery of direct current to an inadequately sedated patient because both of these may heighten the hyperadrenergic state and create or exacerbate a downward clinical spiral.31–33 Restoration of sinus rhythm may be very difficult and impractical when a severe metabolic derangement or multisystem organ failure is present.32 Control of the ventricular rate (Table 31.2) is most frequently achieved using digoxin, beta blockers, calcium channel blockers (verapamil or diltiazem), or combinations of these agents. Verapamil should be administered cautiously to patients with significant left ventricular dysfunction. Although digoxin is effective in controlling rates at rest, exercise rate control is not often achieved. Digoxin remains appropriate therapy for patients with concomitant left ventricular dysfunction and CHF. Intravenous diltiazem is effective and well tolerated. Diltiazem may be administered by continuous intravenous infusion. The combination of efficacy, ease of parenteral delivery, and tolerance makes this agent an attractive option in the critical care setting. Table 31.2 Intravenous Drugs for Atrial Fibrillation †Metoprolol and propranolol also may be used. ‡Amiodarone also is effective for rate control. Adapted from Falk RH: Control of the ventricular rate in atrial fibrillation. In Falk RH, Podrid PJ (eds): Atrial Fibrillation: Mechanisms and Management. New York, Raven Press, 1992. It has become relatively common to treat acute episodes with intravenous ibutilide. This unique class III agent prolongs action potential by blocking the rapid component of the delayed rectifier current.34,35 This increase results in QT interval prolongation. Patients receiving intravenous ibutilide should be carefully monitored34 (on telemetry for 4 to 8 hours) for development of torsades de pointes. Direct comparisons of intravenous procainamide and ibutilide have demonstrated clear superiority of ibutilide in conversion of atrial fibrillation and atrial flutter. Restoration of sinus rhythm with ibutilide occurred in 32% to 51% (atrial fibrillation) and 64% to 76% (atrial flutter) patients, compared with 0% to 5% (atrial fibrillation) and 0% to 14% (atrial flutter) after intravenous procainamide.34,36,37 As a result of this data, use of procainamide for this indication has become passé. Ibutilide is suitable for acute cardioversion; however, prolonged intravenous or oral dosing is not available to prevent arrhythmia recurrence. Ibutilide may be administered safely to patients on concomitant antiarrhythmic agents.38 Intravenous amiodarone is (initially) primarily a calcium channel and beta blocker. It may be effective for rate control when other agents fail. The temptation to use intravenous amiodarone to restore sinus rhythm should be tempered by knowledge of its acute electrophysiologic effects. Its class I and, particularly, class III effects take time to occur, making this a poor choice for rapid conversion. Bolus treatment with intravenous amiodarone has been very disappointing (4% conversion) for acute conversion of atrial fibrillation. By contrast, in approximately 20% to 50% of patients with persistent atrial fibrillation (lasting longer than 24 to 48 hours), reversion to sinus rhythm is achieved with sustained administration (loading periods of up to 4 weeks) of oral amiodarone.39 Intravenous amiodarone may result in less hypotension when used for rate control in the ICU than diltiazem.40,41 Intravenous class IC agents (such as flecainide and propafenone) are the most effective drugs for converting atrial fibrillation of recent onset. Unfortunately, they are not available in the United States. Ibutilide is more effective than intravenous class IC agents for restoration of sinus rhythm in atrial flutter.39 Electrical cardioversion remains the most effective way of restoring sinus rhythm in patients with atrial fibrillation. As noted, urgent electrical cardioversion should be contemplated for sustained tachycardias that precipitate angina, heart failure, or hypotension. There are many pitfalls associated with acute atrial fibrillation management. Intravenous beta and calcium channel blockers may result in bradycardia, hypotension, and heart failure. Beta blockers may also aggravate or precipitate bronchospasm. Ibutilide may be proarrhythmic (torsades de pointes). Amiodarone is unlikely to be proarrhythmic in the absence of electrolyte abnormalities (hypokalemia, hypomagnesemia) or other drugs that have already prolonged the rate-corrected QT interval (QTc).31,34,41 In the absence of clear-cut evidence from randomized controlled trials, appropriate management should include treatment (or elimination) of potential precipitants and beta blockade (given the hyperadrenergic state of many ICU patients) as the initial pharmacotherapy of choice. If atrial fibrillation recurrence needs to be prevented and sinus rhythm maintained, institution of specific antiarrhythmic therapy (intravenous procainamide, intravenous or oral amiodarone, oral dofetilide, or sotalol) may be effective. We favor adding intravenous amiodarone. This recommendation is based on 100% bioavailability of the intravenous preparation (critically ill patients may not absorb oral drugs), amiodarone’s noncompetitive β-antagonistic effects, its benefit in the perioperative period of cardiac surgery42 (a time when endogenous and exogenous catecholamine levels are often high), and its efficacy in preventing atrial fibrillation recurrence.31,41 A recent meta-analysis of perioperative prophylactic amiodarone demonstrated decreased incidence of atrial fibrillation and flutter, ventricular tachyarrhythmias, stroke, and reduced length of stay after cardiac surgery.42 Not all studies included used beta blockade, and the course of therapy was inconsistent among trials. The Prophylactic Oral Amiodarone for the Prevention of Arrhythmias That Begin Early after Revascularization, Valve Replacement, or Repair (PAPABEAR), a large randomized controlled trial, compared perioperative amiodarone with placebo and showed significant reduction in postoperative atrial tachyarrhythmias.43 Toxicity risks were reduced because amiodarone was used for a short duration. Neither study demonstrated reduction in mortality rates. The data for perioperative amiodarone in cardiac surgery are compelling; however, incremental benefit beyond beta blockade alone remains unclear. It may still be reasonable to reserve amiodarone for postoperative atrial fibrillation in patients already receiving beta blockers and to limit use of amiodarone to 6 to 12 weeks postoperatively to prevent side effects. The intensivist must balance complicated issues before undertaking direct current cardioversion. Strong effort should be focused on avoidance of low-yield attempts. Repeated doses of anesthesia and multiple shocks will ultimately result in further deterioration of critically ill patients. Optimal management of precipitants, careful choices, and monitoring of antiarrhythmic therapy, as well as a solid understanding of cardioversion and defibrillation techniques (see later), will maximize success. In some cases of atrial fibrillation, rapid ventricular rate cannot be controlled by pharmacotherapy. Radiofrequency ablation of the AV junction and permanent pacing may be required when medical therapy is ineffective.44 Prevention of embolic strokes remains the most important goal of therapy for atrial fibrillation. Anticoagulation plays a pivotal role in minimizing the risk of emboli (and strokes) during elective cardioversion of atrial fibrillation.45 Classic recommendations for management of atrial fibrillation of more than 48 hours’ duration include 3 weeks of therapeutic warfarin (to achieve a prothrombin time [PT]/international normalized ratio [INR] of 2.0-3.0) before direct current shock administration and (at least) 4 more weeks of warfarin after the procedure. Although emboli may be less frequent with atrial flutter,45 it is clear that they occur,46 and the recommendations are the same as for atrial fibrillation. Novel oral anticoagulants have recently been introduced for stroke prophylaxis in atrial fibrillation (see later). Short-term therapeutic anticoagulation with heparin before cardioversion (followed by warfarin in the usual manner) combined with transesophageal echocardiography (TEE) has gained acceptance as an alternative approach.47 Data from the ACUTE trial suggested similar embolic rates (0.5% versus 0.8%) comparing conventional and TEE-guided approaches.48 TEE is useful for detecting left atrial thrombi. It provides an excellent, minimally invasive view of the left atrial appendage. Patients with obvious thrombi should be anticoagulated for up to 8 weeks and have demonstrable resolution of clot before cardioversion is attempted.47 There are well-described risk factors that help the clinician balance the risk of anticoagulation and the risk of stroke from atrial fibrillation. The CHADS2 and CHA2DS2-VASc (Congestive heart failure, Hypertension, Age ≥ 75 years [doubled risk weight], Diabetes mellitus, previous Stroke/transient ischemic attack [doubled risk weight], Vascular disease, Age 65 to 74 years, female Sex) risk scores as well as a novel bleeding risk score, HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke history, Bleeding history or predisposition, Labile international normalized ratio, Elderly [≥65 years], Drugs/alcohol concomitantly) aid the clinician in balancing a patient’s embolic stroke risk with the risk for bleeding.49 Patients who are younger than age 65 years with normal hearts and “lone” atrial fibrillation (i.e., with none of the aforementioned risk factors) can be anticoagulated with aspirin 325 mg daily or perhaps not at all. Patients with 1 point should have individualized treatment, and patients with 2 points should be anticoagulated. Patients with rheumatic mitral stenosis or the presence of a prosthetic heart valve are among the highest risk for stroke and should be anticoagulated regardless of the CHADS2 score.50 Recently, two classes of drugs, the direct thrombin (factor IIa) inhibitors and the factor Xa inhibitors (collectively termed “novel anticoagulants”) have emerged as options for prophylaxis against stroke in patients with nonvalvular atrial fibrillation. As a majority of patients in atrial fibrillation are willing to consider switching to these medications, intensivists will undoubtedly encounter patients on these drugs and deal with issues that arise from their use.51 Dabigatran, a direct thombin inhibitor, is an oral anticoagulant dosed twice a day. The drug was studied in comparision to warfarin for reduction of strokes in patients with atrial fibrillation. The RE-LY trial found a decreased incidence of stroke with the 150 mg dose of dabigatran and similar risk of bleeding.52 Dabigatran’s half-life is 14 to 18 hours and it is recommended that it be stopped 2 to 3 days prior to an elective surgery (4 to 5 days seems more prudent). There is no specific antidote to this drug. Local measures may suffice for minor bleeding. Dialysis and dabigatran’s relatively short half-life usually allow discontinuation of the drug to reverse the bleeding diathesis. The only current reversal option for dabigatran is emergency dialysis. Performing dialysis rapidly in unstable patients with bleeding or in those with large intracranial hemorrhage will present a very great challenge, even at level 1 trauma centers.53
Cardiac Arrhythmias
Bradycardias
Sinus Bradycardia and Sinus Node Dysfunction
Atrioventricular Block
Junctional Rhythm
Vagally Mediated Sinus Arrest, Bradycardia, and Heart Block
Supraventricular Tachycardia
Overview
Paroxysmal Supraventricular Tachycardia
Approach to Paroxysmal Supraventricular Tachycardia Therapy
Wolff-Parkinson-White Syndrome and Its Variants
Acute Management of Tachycardia Associated with Wolff-Parkinson-White Syndrome
Nonparoxysmal Atrioventricular Junctional Tachycardia, Paroxysmal Atrial Tachycardia with Block, and Automatic Atrioventricular Junctional Tachycardia
Multifocal Atrial Tachycardia
Sinus Tachycardia
Atrial Flutter
Atrial Fibrillation
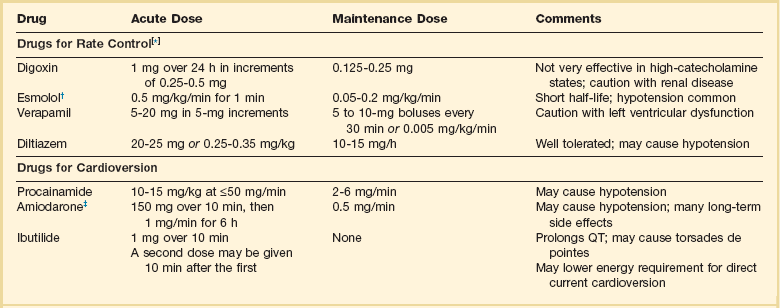
Acute Management of Atrial Fibrillation
Special Considerations for the Intensivist
Anticoagulants for Stroke Prevention in Atrial Fibrillation.
Anticoagulation in the ICU.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree