CHAPTER 5

BASIC OPERATIVE TECHNIQUES IN TRAUMA AND ACUTE CARE SURGERY
Trauma and acute care surgery is an exciting field that offers a wide spectrum of operative experience, which ranges from elective to semielective to truly emergent, in body sites and cavities from head to toe and also from minimally invasive techniques to the “maximally” invasive procedures (e.g., “trap-door” approach to the left subclavian vessels). The basic principles of these divergent procedures are essentially the same: adequate exposure, control of bleeding and contamination, attention to patient physiology, and most importantly “do no harm.” The scope of acute care surgery practice is so vast that a discussion of it can only be a sketch. This chapter, therefore, outlines the accepted techniques of exposure and treatment of various injuries and diseases in different anatomic sites. A brief introduction into minimally invasive approach follows, since that will surely be the state-of-the-art in the next decade. The reader is encouraged to supplement this chapter with atlas of operative and anatomic details.
OPERATIVE APPROACH TO NECK TRAUMA
In a landmark 1969 article, Monson et al. arbitrarily divided the neck into three clinical zones.1 Zone I is defined as the area between the suprasternal notch and the cricoid cartilage, Zone II is the region between the cricoid and the angle of the mandible, and Zone III lies above the angle of the mandible. Zone II injuries are accessible via standard cervical approaches and do not present operative difficulty. Zone I injuries by definition involve the thoracic inlet with potential injuries to major vessels, and Zone III injuries are problematic because of the difficulty in obtaining distal control. Direct operative approach is therefore preferred for Zone II, while angiography may be helpful in the other zones.2,3
The patient should be placed in a prone position with both arms tucked, if possible. As long as there is no concurrent spine injury, a shoulder roll should be placed to extend the neck and the table placed in a semi-Fowler’s position to aid in the operative exposure. The entire thorax and abdomen should be prepped in case of Zone I injury for accessing the chest and abdomen for potential multicavitary injuries. The groin and both thighs are prepped and draped for possible saphenous vein harvest.
Innominate and Subclavian Vessel Injury
Operative exposure of Zone I vascular injuries varies with the stability of the patient. If the patient is hemodynamically unstable with a large hemothorax or excessive bleeding from the chest tube, an anterolateral thoracotomy (high in the 3rd or 4th intercostal space) will allow apical packing to tamponade the bleeding from innominate or proximal subclavian vessels. This can be done in the trauma bay of the emergency department for the patient in extremis. In the stable patient, a median sternotomy in the O.R. is a good approach for the innominate and the right subclavian vessels. For the left subclavian artery, the incision may be extended along the sternocleidomastoid or the clavicle. This approach is superior to the morbid “trap-door” incision. The second and third portions of the subclavian are best approached by an incision along the clavicle with extension along the sternocleidomastoid, if necessary (Fig. 5.1). Subperiosteal resection of the mid or medial one-third of the clavicle will allow excellent exposure. The majority of these vascular injuries may be managed by simple lateral repair or end-to-end anastomosis. Occasionally, a saphenous vein graft may be necessary. In stable patients with a subclavian pseudoaneurysm or intimal injuries, endovascular stent grafts are an increasingly attractive option.
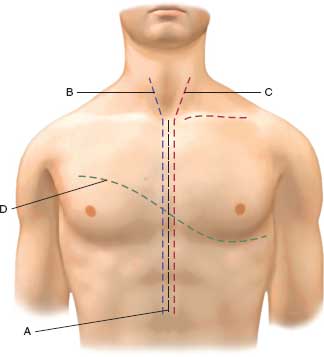
FIGURE 5.1. Operative approaches to neck and chest: A: standard median sternotomy for ascending aorta, innominate, cardiac injuries. B: Median sternotomy with extension to right neck for innominate, right subclavian, and common carotid artery injuries. Extension along the right clavicle will provide access to distal right subclavian vessels. C: Median sternotomy with left neck extension for left common carotid, aortic arch, and left subclavian vessel injuries. Extension along the left clavicle will provide access to distal left subclavian vessel injuries. D: Left anterolateral thoracotomy with extension to right chest as a clamshell incision providing access to all chest structures
Carotid Artery Injury
Carotid exposure is obtained via a skin incision made along the anterior border of the sternocleidomastoid muscle (Figs. 5.1–5.3). The carotid sheath and its contents are readily identifi able. The venous and lymphatic structures are retracted in a lateral direction. Proximal and distal exposure of the carotid arteries is obtained after identifying the ansa cervicalis and 12th cranial nerve (Fig. 5.2). Digital pressure or a sidebiting vascular clamp can be used to control hemorrhage while obtaining control. Proximal injury to the carotid at its aortic or subclavian origin requires more extensive exposure than the simple neck incision. Median sternotomy is the most commonly employed approach. A supraclavicular incision is useful for exposure in some cases, and dislocation or resection of the clavicle may improve the exposure (Fig. 5.1).
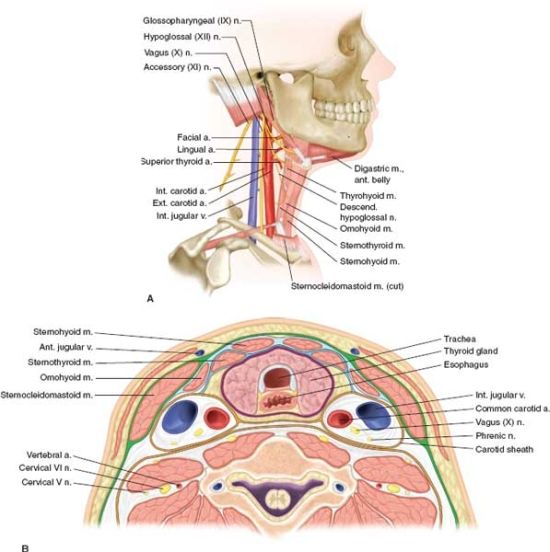
FIGURE 5.2. A,B: Anatomy of neck demonstrating the important structures in neck exploration. The cross section shows the carotid sheath and its contents and the anatomy of trachea and esophagus.
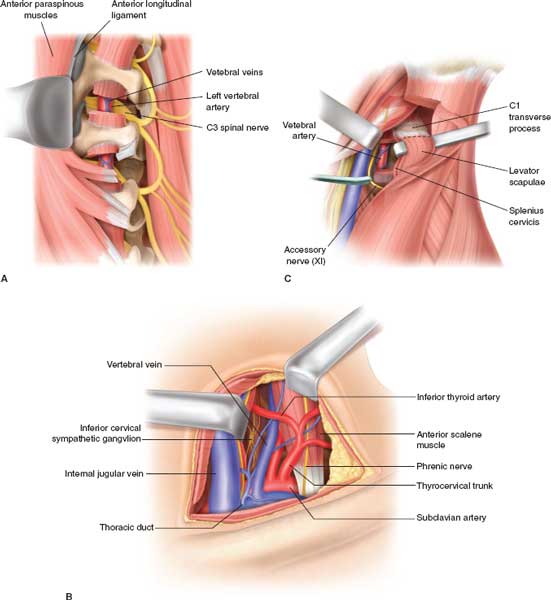
FIGURES 5.3. A: Anatomy of the vertebral artery, showing the course surrounded by cervical transverse processes. Exposure is facilitated by retraction of the anterior paraspinous muscles and anterior longitudinal ligament. B: Exposure of proximal vertebral artery by a supraclavicular incision, exposing the thyrocervical trunk from the subclavian artery. C: Exposure of the distal vertebral artery by sternocleidomastoid incision, dividing its origin from the mastoid process and dividing the levator scapulae and the splenius cervicis muscles.
High Zone III injuries resulting in distal internal carotid laceration or disruption can be problematic in exposure and control. Anterior subluxation of the mandible can improve exposure, but only by about 2 cm. Osteotomy of the mandibular ramus may provide better exposure and mobility. Placement of a Fogarty balloon catheter to provide distal vascular control can be lifesaving during these maneuvers. Depending on the surgeon’s experience in operating around the base of the skull, intraoperative assistance from either a maxillofacial surgeon or a neurosurgeon is advisable in difficult cases. Once proximal and distal control are secured, a Fogarty balloon catheter is carefully passed to remove any thrombus, and both proximal and distal ends are flushed with heparinized saline. A 5-O or 6-O monofilament polypropylene suture is used for the repair and handled with appropriate vascular technique. Except for tangential lacerations where primary repair is possible by lateral arteriorrhaphy, end-to-end anastomosis is often difficult, because the transected ends of the artery retract. Care must be taken to inspect for and repair any intimal flap at this time. This can often be done by incorporating the intimal defect into the laceration repair by utilizing a series of interrupted sutures. Also, the lumen of the artery must not be narrowed by this primary repair; if so, vein patch or interposition graft will be required. If there is a considerable destruction of the carotid artery, interposition grafting is warranted, preferably utilizing autologous tissue. Saphenous vein is generally the ideal conduit. If adequate autologous conduit is not available, synthetic material may be used. The choice between woven Dacron and ePTFE is based on a surgeon’s preference, as neither represents an ideal substitute for a vein graft.3 If anatomy permits, the external carotid artery can be divided at a distal location, and transposed to the internal carotid artery. The use of a temporary shunt is not mandated by the available data. Increasingly, percutaneous transluminal placement of endovascular devices has become an alternative option to surgical repair.
Vertebral Artery Injury4
Vertebral artery injury,4 fortunately, is not common. As warned by Matas “A glance at the surgical anatomy of this vessel as it lies deeply hidden in the skeleton of the neck, only escaping at short intervals from its osseous canal, to become immediately invested by the very important and vital cervical nerves as they issue from the spinal foramina, will at once remind us of the magnitude of the purely technical difficulties in the way of its atypical ligation, and of the errors of diagnosis that must be incurred.”
If the identification and exposure of the vertebral artery injury is uncomplicated during neck exploration, proximal and distal surgical ligation of the injured vessel is performed. If encountered at neck exploration, the following steps are indicated: (1) Gauze packing at the site of bleeding and (2) exposure of the subclavian artery by dividing the origin of sternomastoid from the clavicle and proximal control of the origin of the subclavian artery. The neck incision is carried posterior to the ear with division of the attachment of sternomastoid and splenius capitis muscle. Distal ligation may be performed by dividing the splenius capitis and sternomastoid attachments to the mastoid, palpation of the transverse process of the atlas, and exposing the vertebral artery between the axis and atlas. Bone wax or other hemostatic agents can be used to pack and compress this area, or “blind” application of surgical clips deep into the wound may staunch the bleeding (Fig. 5.3A–C). The difficult dissection of the vertebral artery makes angiographic embolization an attractive option. Current data support the conclusion that most vertebral artery injuries can safely be managed without an operation or by angiographic embolization. Surgical intervention should be reserved for patients with severe bleeding or where embolization has failed.
Venous Injury
Injury to the innominate, subclavian, axillary, or internal jugular veins may be the source of severe hemorrhage. These veins can be ligated if the destruction is severe. In the case of severe bilateral jugular venous injury, ligation will carry significant clinical consequences. In this setting, reconstruction with an autologous conduit is advisable.3
Cervical Esophagus
Exposure is obtained by a left neck incision along the anterior border of the sternocleidomastoid muscle with medial retraction of the carotid vessels. Adequate mobilization behind the trachea and palpation of the nasogastric tube facilitate identification of the esophagus. The recurrent laryngeal nerve needs to be protected in the dissection and frequently may be palpated or visualized (Fig. 5.2). The esophageal perforation is identified either by direct visualization or with the help of intraluminal saline or dye. The perforation is repaired in either one or two layers. Neither the number of suture layers nor the type of suture material (absorbable or nonabsorbable) seem to influence the incidence of fistulization after the repair. If the operative exploration is delayed, repair may be difficult because of extensive inflammation in the area. In either instance (early or delayed operation), wide drainage is the key to success. Closed suction drains (Jackson- Pratt) usually are preferred.5
Laryngotracheal injury in the neck is uncommon but can be produced by blunt trauma, seat belt injury, or penetrating injuries. These injuries should be suspected with subcutaneous emphysema, soft tissue air in x-rays, or changes in phonation and blood in the aerodigestive tract. Primary repair with absorbable sutures without tracheostomy is the ideal approach. Tracheostomy through the injury, as once recommended, is not the preferred option. More complex injuries to the larynx are best handled with the help of ENT surgeons because of long-term complications and the need for corrective procedures.6
OPERATIVE APPROACHES TO CHEST TRAUMA
Emergency Department Thoracotomy
The usual incison is a left anterolateral thoracotomy (Fig. 5.1). For right-sided penetrating wounds and suspected injuries in the right chest, thoracotomy may be commenced as a right anterolateral thoracotomy and, if cardiac arrest has occurred and open cardiac massage is needed, a separate left anterolateral thoracotomy may be added. The usual incision is in the 4th or 5th intercostal space, extending as a sweeping curve into the axilla. If exposure is inadequate or for bilateral thoracic injuries, the incision may be extended across the sternum into the right chest as a “clam shell” thoracotomy. This provides outstanding exposure but one has to look for the transected internal mammary arteries and ligate them. When a patient is in extremis from a penetrating injury at the thoracic inlet and a massive hemothorax, a higher (3rd intercostal space) incision provides access to the thoracic vessels at the inlet that can be compressed manually while the patient is transported to the Operating Room.
Once inside the chest, a rapid assessment of the pericardium is made.7 Tense, bluish pericardium suggests tamponade and is relieved by a knife anterior to the phrenic nerve. The pericardiotomy is then extended with a scissors parallel to the nerve. In the presence of a cardiac laceration, cardiorrhaphy may be achieved by staples, sutures, or gentle traction on a Foley catheter balloon inserted through the laceration (Figs. 5.4 and 5.5). Active hemorrhage from pulmonary lacerations is controlled by clamping the hilum of the lung manually with fingers or by placing a vascular clamp across. Some surgeons recommend “a lung twist” after the inferior pulmonary ligament is divided. These maneuvers also aid in the prevention of air embolism and aspiration of blood into the uninjured lung. Active hemorrhage from the apex of the pleural cavity suggests a subclavian vessel injury. Temporary tamponade of the vessels may be achieved by packing with large lap pads or pressure with a fist. The patient, if resuscitated, is then moved to the operating room. Cardiorrhaphy is completed by interrupted sutures with pledgets, taking care not to occlude the coronary vessels, if the wounds are adjacent to them. Most bleeding pulmonary injuries are best treated by resectional debridement.8 In penetrating abdominal wounds with continuing hemorrhage, the descending thoracic aorta may be occluded initially by finger compression over the spine. Blunt finger dissection of the aorta must be performed for a proper application of a thoracic aortic clamp. A Penrose drain around the aorta applied as a tourniquet is a useful alternative technique. While doing these maneuvers, beware of injury to the esophagus or the intercostal vessels. Open cardiac massage, if needed, is best performed by a two-handed technique. Beware of the surgeon’s fingers causing a rent in the soft, hypoxic ventricle.
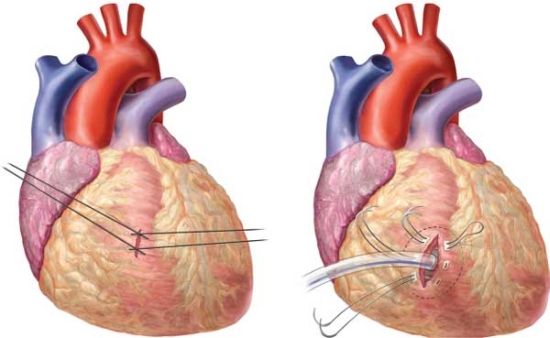
FIGURES 5.4 AND 5.5. Cardiac repair with sutures crisscrossed to close the laceration and control of hemorrhage with placement of a Foley catheter with the balloon infl ated.
Pitfalls of ERT: The potential problems of ERT include improperly placed incisions with severe limitation of exposure, incision through the female breast, iatrogenic injury to the lung, intercostal or internal mammary vessels, pericardium, lung, phrenic nerve, and coronary vessels. Hasty and inexperienced efforts at descending thoracic aortic occlusion may result in injury to the aorta, intercostal vessels, or the esophagus. The uncontrolled, desperate nature of Emergency Department Thoracotomy is a setup for injury to the operating team by sharp objects.
Injury to the Thoracic Esophagus
Operative repair is the treatment of choice for free thoracic perforations, for injuries diagnosed both early (<24 hours) or late (>24 hours). The presence of systemic sepsis, pneumothorax, pneumomediastinum or pneumoperitoneum is indication for early thoracotomy and repair.5
The operative approach consists of thoracotomy on the side of the leak (left thoracotomy for lower esophageal injury and right thoracotomy for upper esophageal injury), exposure of the esophagus, and thorough debridement of all necrotic tissue. The perforation is identified and closed. In penetrating trauma, multiple perforations are not uncommon and should be sought diligently. The choice of suture material for closure of the perforation is variable between surgeons, as is the necessity for a two-layered closure with inner absorbable and outer nonabsorbable sutures. While various tissues are recommended as buttress for the suture, Richardson5 used buttress by either muscle or pleura. Sternocleidomastoid muscle is used to buttress or primarily close the defects in the neck, and a flap of diaphragm is often used for thoracic perforation. Patients with perforated cancer or severe underlying disease may need esophagectomy. Whichever method is used, it is critical to drain the area extensively, usually with large caliber chest tubes placed in the vicinity of the esophageal repair.
Treatment of delayed recognition of the perforation: The problems of delayed treatment involve extensive mediastinitis, necrosis of the esophageal wall, and the difficulty of effectively closing the perforation, even with various buttressing methods. Even when repair is technically feasible, subsequent breakdown of the repair is the rule rather than the exception. It is in such patients that “exclusion” procedures were practiced at one time, largely replaced in recent times by esophageal stenting. In selected patients with controlled sepsis, esophageal irrigation and wide drainage of the mediastinum and pleural cavity are viable options.5
Control of Intrathoracic Bleeding
Pulmonary lacerations are best approached by localized debridement–resection of the injured lung. Staplers are useful to accomplish this rapidly and effectively. Only rarely is formal lobectomy needed. Pneumonectomy is reserved for extensive injury to the hilum and is even more uncommon. Intercostal vessel bleeding requiring operative control can easily be performed through a thoracotomy.
Damage-control Procedures in the Chest
These are not uncommon with success of early transport and emergency resuscitation in patients with severe injury.
As described by Wall and associates, “Damage control in the chest consists of different technical maneuvers to use quicker and technically less demanding operations to accomplish the same goal.”9 These include resuscitative thoracotomy, nonanatomically stapled lung resections, pulmonary tractotomy, en masse lobectomy/pneumonectomy, intravascular shunts and vascular ligation instead of repair. The chest incision is provisionally closed by prosthetic patches or towel clips.
Tracheobronchial Injury
Richardson6 presented a personal series of laryngotracheal injuries recently and pointed out the infrequency of these injuries and the resultant limitation of experience. This report is mandatory reading to be prepared to deal with these injuries. The basic lessons are clear: tracheal injury in the neck from blunt trauma needs resection and reanastomosis of the trachea. The need for complete excision of damaged cartilage was emphasized. The repair may be performed through the neck with a sternal extension. For injury near the carina, a right thoracotomy is preferred. Mainstem bronchial injury represents a major problem, especially from penetrating trauma. Involvement of the pulmonary hilum with major vascular injuries forces a pneumonectomy, a highly lethal procedure, especially when performed on the patient in shock. For blunt bronchial injury, end-to-end anastomosis is feasible. Preservation of pulmonary tissue is clearly preferable. Richardson6 emphasized the poor long-term prognosis of these patients. Fortunately, these injuries are relatively uncommon and the inexperienced surgeon is admonished to call senior thoracic surgeons for help.
OPERATIVE APPROACH TO ABDOMINAL TRAUMA
Solid Organ Injury
Exploratory celiotomy is clearly indicated in patients with suspected hollow viscus injury or hemodynamic instability with solid organ injury. Prep the patient from chin to midthighs and table-to-table laterally (be prepared for possible resuscitative thoracotomy, median sternotomy, or availability of the groins for vascular access or saphenous vein harvesting). The abdomen is explored by a long midline incision. After evacuation of the peritoneal cavity of all blood, the abdomen is packed in all the quadrants and serially explored. Control the most active bleeding first.
Liver. Perihepatic packs and manual compression of the liver by an assistant will reduce bleeding from the liver. Exploration of the abdominal cavity is rapidly completed. Intestinal perforation, if present, is swiftly closed to prevent continued contamination of the peritoneal cavity. The various options in the operative treatment of the injured liver can then be considered.
Approximately 50%-60% of the liver lacerations, especially after penetrating trauma, are minor capsular tears that have stopped bleeding at the time of laparotomy. These capsular tears and minor parenchymal lacerations may be left alone. Grade II injuries are deeper lacerations, especially central in location and may be actively bleeding at operation. Hemostasis can temporarily be achieved by pressure. If bleeding resumes after releasing the tamponade, liver sutures of an absorbable material may be applied in an interlocking mattress fashion. A better alternative is to explore the laceration by a hepatotomy and individually ligate the bleeding vessels. If a diffuse bleeding continues, absorbable mattress sutures buttressed by pledgets of gelfoam can be used to compress the parenchyma and achieve hemostasis. The majority of liver injuries, fortunately, can be controlled by these simple methods.
Burst injuries due to blunt trauma and large-caliber missile wounds cause greater destruction of the parenchyma of the liver, often involving both lobes and require more “complex repairs.” Ancillary techniques as autotransfusion by a cell saver, blood warmers to combat hypothermia and massive transfusion protocols with emphasis on high Fresh Frozen Plasma (FFP) to packed cell ratios, and appropriate resuscitation are crucial for a successful outcome. Adequate mobilization of the liver by dividing its ligamentous attachments enables the organ to be delivered into the operative wound and facilitate manual compression of the liver.
Occlusion of the portal triad or Pringle’s maneuver reduces vascular inflow to the liver and consequently may reduce bleeding from the liver laceration. It consists of sliding the operator’s hand into the lesser sac and occluding the free edge of the lesser omentum and its contained portal and hepatic vessels between the fingers. If the procedure is effective in slowing the hemorrhage from the liver laceration, a vascular clamp may be applied across the free edge of the omentum. With reduction in the rate of bleeding by portal occlusion, the liver laceration can be evaluated to control bleeding vessels by direct visualization and ligation. Deeper lacerations may be explored by the finger-fracture technique popularized by Pachter et al.10 The parenchyma of the liver is teased between the surgeon’s fingers so that the hepatic vessels are exposed as threads traversing the teased liver substance (Fig. 5.6). These can then be individually ligated. The process is continued till the entire laceration is explored and hemostasis is achieved. The staplers are an invaluable adjunct in this setting.
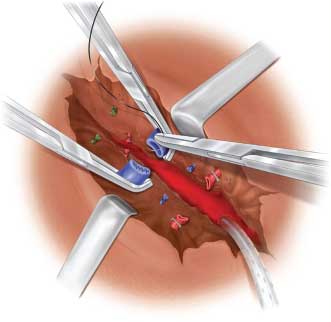
FIGURE 5.6. Hepatotomy with vascular ligation.
In the patient with large stellate fractures or deep lacerations of the liver, the finger-fracture technique of hemostasis and debridement of nonviable liver tissue inevitably results in large defects in the hepatic lobes. Minor bleeding, usually of venous origin, may persist from the raw surfaces of the divided liver. A graft of viable omentum with an intact vascular supply can be used to fill in this dead space and control the venous bleeding (Fig. 5.7). This living tissue acts not only as a pack to control bleeding, but may serve also as a rich source of macrophages to help minimize the risk of subsequent sepsis.
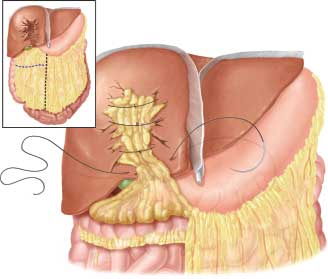
FIGURE 5.7. Omental packing after debridement–resection of liver.
HEPATIC RESECTION.
Anatomic lobectomy, or formal hepatic resection even though underemphasized in recent years, has its role. Polanco et al.11 recently analyzed 216 patients with complex liver injury and noted 21 anatomic segmentectomies, 23 nonanatomic resections, 3 left lobectomies, 8 right lobectomies, and 1 hepatectomy with orthotopic liver transplant. The overall mortality was 17.8%, but only 9% from liver injury. Despite these impressive results, the consensus opinion in this country favors resectional debridement. Selective vascular ligation of hepatic artery, once popular, is now less commonly applied to hepatic trauma. If active bleeding persists after resectional debridement or packing, angiographic embolization is the preferred method. On the other hand, do not leave the operating room if control of surgical bleeding has not been achieved.
Despite these various techniques, in a small number of patients, approximately 2%-5%, profuse bleeding persists and may relate to the onset of coagulopathy. Massive red cell transfusion, hypothermia, and acidosis contribute to the development of coagulation defects. The liver injury, which may have stopped bleeding, may start to bleed diffusely without controllable points of hemorrhage. “Liver packing” has emerged as an important advance to control this “nonmechanical” bleeding.12 In such patients, perihepatic packing, abbreviated laparotomy, damage control resuscitation with hepatic angiography as an adjunct have emerged as the treatment of choice. The technique of packing is by Kerlix rolls tightly in the crevices of the liver injury and over the raw areas of the resected liver. Additional packs are placed around the liver and between the organ and the diaphragm and the lateral abdominal wall. The laparotomy is abbreviated, and the patient is taken to the ICU for resuscitation. The packs are removed in 24-48 hours, when the coagulation factors have been replaced and the coagulopathy corrected. Pack removal is accomplished by copious irrigation with normal saline. The raw areas of the liver are carefully inspected and nonviable tissue is debrided. The liver laceration is drained extensively with closed suction drains.
As opposed to external tamponade, internal tamponade is a valuable method in patients with missile wounds of the liver that traverse the entire thickness of the lobes, an injury too extensive to control without a major resection. In such patients, a method of internal tamponade by a “Penrose pack,” made by passage of a red rubber catheter through the missile tract and pulling a wad of Penrose drains tied to the end of the catheter through the laceration. The Penrose pack acts as a tamponading plug and controls the bleeding. The drains, brought to the exterior through the flank, also serve to drain the laceration. A similar result may be achieved by using a sterilized Sengstaken-Blakemore tube. The management of retrohepatic venous injuries is discussed under the section on abdominal vascular trauma.
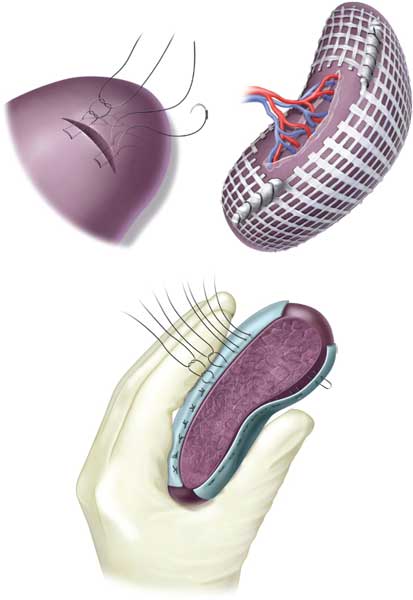
Spleen. Except for high-grade splenic injuries with hemodynamic instability, nonoperative management of splenic trauma is the rule. In patients with penetrating trauma with multiple injuries and in unstable patients, splenectomy is the preferred treatment. Complete mobilization of the spleen from its ligaments, control and division of the splenic vessels at hilum, attention to the short gastric vessels and the tail of the pancreas are some of the technical details that need emphasis. Splenorrhaphy is an option when circumstances are favorable for repair: hemodynamic stability and absence of major associated injuries. Techniques include sutures with buttress of omentum, strips of gelfoam or absorbable mesh; partial splenectomy or splenic wrap with absorbable mesh13 (Figs. 5.8–5.10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree