Key Concepts
 In a patient with an acute asthma attack, a normal or high Paco2 indicates that the patient can no longer maintain the work of breathing and is often a sign of impending respiratory failure. A pulsus paradoxus and electrocardiographic signs of right ventricular strain (ST-segment changes, right axis deviation, and right bundle branch block) are also indicative of severe airway obstruction.
In a patient with an acute asthma attack, a normal or high Paco2 indicates that the patient can no longer maintain the work of breathing and is often a sign of impending respiratory failure. A pulsus paradoxus and electrocardiographic signs of right ventricular strain (ST-segment changes, right axis deviation, and right bundle branch block) are also indicative of severe airway obstruction.
 Asthmatic patients with active bronchospasm presenting for emergency surgery should be treated aggressively. Supplemental oxygen, aerosolized β2-agonists, and intravenous glucocorticoids can dramatically improve lung function in a few hours.
Asthmatic patients with active bronchospasm presenting for emergency surgery should be treated aggressively. Supplemental oxygen, aerosolized β2-agonists, and intravenous glucocorticoids can dramatically improve lung function in a few hours.
 Intraoperative bronchospasm is usually manifested as wheezing, increasing peak airway pressures (plateau pressure may remain unchanged), decreasing exhaled tidal volumes, or a slowly rising waveform on the capnograph.
Intraoperative bronchospasm is usually manifested as wheezing, increasing peak airway pressures (plateau pressure may remain unchanged), decreasing exhaled tidal volumes, or a slowly rising waveform on the capnograph.
 Other causes, such as obstruction of the tracheal tube from kinking, secretions, or an overinflated balloon; bronchial intubation; active expiratory efforts (straining); pulmonary edema or embolism; and pneumothorax, can simulate bronchospasm.
Other causes, such as obstruction of the tracheal tube from kinking, secretions, or an overinflated balloon; bronchial intubation; active expiratory efforts (straining); pulmonary edema or embolism; and pneumothorax, can simulate bronchospasm.
 Chronic obstructive pulmonary disease (COPD) is currently defined as a disease state characterized by airflow limitation that is not fully reversible. The chronic airflow limitation of this disease is due to a mixture of small and large airway disease (chronic bronchitis/bronchiolitis) and parenchymal destruction (emphysema), with the representation of these two components varying from patient to patient.
Chronic obstructive pulmonary disease (COPD) is currently defined as a disease state characterized by airflow limitation that is not fully reversible. The chronic airflow limitation of this disease is due to a mixture of small and large airway disease (chronic bronchitis/bronchiolitis) and parenchymal destruction (emphysema), with the representation of these two components varying from patient to patient.
 Cessation of smoking is the long-term intervention that has been shown to reduce the rate of decline in lung function.
Cessation of smoking is the long-term intervention that has been shown to reduce the rate of decline in lung function.
 Preoperative interventions in patients with COPD aimed at correcting hypoxemia, relieving bronchospasm, mobilizing and reducing secretions, and treating infections may decrease the incidence of postoperative pulmonary complications. Patients at greatest risk of complications are those with preoperative pulmonary function measurements less than 50% of predicted.
Preoperative interventions in patients with COPD aimed at correcting hypoxemia, relieving bronchospasm, mobilizing and reducing secretions, and treating infections may decrease the incidence of postoperative pulmonary complications. Patients at greatest risk of complications are those with preoperative pulmonary function measurements less than 50% of predicted.
 Restrictive pulmonary diseases are characterized by decreased lung compliance. Lung volumes are typically reduced, with preservation of normal expiratory flow rates. Thus, both forced expiratory volume in 1 sec (FEV1) and forced vital capacity (FVC) are reduced, but the FEV1/FVC ratio is normal.
Restrictive pulmonary diseases are characterized by decreased lung compliance. Lung volumes are typically reduced, with preservation of normal expiratory flow rates. Thus, both forced expiratory volume in 1 sec (FEV1) and forced vital capacity (FVC) are reduced, but the FEV1/FVC ratio is normal.
Anesthesia for Patients with Respiratory Disease: Introduction
The impact of preexisting pulmonary disease on respiratory function during anesthesia and in the postoperative period is predictable: Greater degrees of preoperative pulmonary impairment are associated with more marked intraoperative alterations in respiratory function and higher rates of postoperative pulmonary complications. Failure to recognize patients who are at increased risk is a frequent contributory factor leading to complications, as patients may not receive appropriate preoperative and intraoperative care. This chapter examines pulmonary risk in general and then reviews the anesthetic approach in patients with the most common types of respiratory disease.
Certain risk factors (Table 24-1) may predispose patients to postoperative pulmonary complications. The incidence of atelectasis, pneumonia, pulmonary embolism, and respiratory failure following surgery is quite high, but varies widely (from 6% to 60%), depending on the patient population studied and the surgical procedures performed. The two strongest predictors of complications seem to be operative site and a history of dyspnea, which correlate with the degree of preexisting pulmonary disease.
| Patient-related Factors1 | Procedure-related Factors1 |
|---|---|
| Supported by good evidence | |
| Aortic aneurysm repair
|
| Supported by fair evidence | |
|
|
| Good evidence against being a risk factor | |
|
|
| Insufficient data | |
|
|
The association between smoking and respiratory disease is well established; abnormalities in maximal midexpiratory flow (MMEF) rates are often demonstrable well before symptoms of COPD appear. Although abnormalities can be demonstrated on pulmonary function tests (PFTs), because most patients who smoke do not have PFTs performed preoperatively, it is best to assume that such patients have some degree of pulmonary compromise. Even in normal individuals, advancing age is associated with an increasing prevalence of pulmonary disease and an increase in closing capacity. Obesity decreases functional residual capacity (FRC), increases the work of breathing, and predisposes patients to deep venous thrombosis.
Thoracic and upper abdominal surgical procedures can have marked effects on pulmonary function. Operations near the diaphragm often result in diaphragmatic dysfunction and a restrictive ventilatory defect (see below). Upper abdominal procedures consistently decrease FRC (60% to 70%); the effect is maximal on the first postoperative day and usually lasts 7-10 days. Rapid shallow breathing with an ineffective cough caused by pain (splinting), a decrease in the number of sighs, and impaired mucociliary clearance lead to microatelectasis and loss of lung volume. Intrapulmonary shunting promotes hypoxemia. Residual anesthetic effects, the recumbent position, sedation from opioids, abdominal distention, and restrictive dressings may also be contributory. Complete relief of pain with regional anesthesia can decrease, but does not completely reverse these abnormalities. Persistent microatelectasis and retention of secretions favor the development of postoperative pneumonia.
Although many adverse effects of general anesthesia on pulmonary function have been described, the superiority of regional over general anesthesia in patients with pulmonary impairment is not firmly established.
Because of the prevalence of smoking and obesity, many patients may be at increased risk of developing postoperative pulmonary dysfunction. The risk of complications increases if the patient is having a thoracotomy or laparotomy, even if the patient has no risk factors. Patients with known disease should have their pulmonary function optimized preoperatively, with careful consideration given to the choice of general versus regional anesthesia.
The American College of Physicians has established guidelines to assist in the preoperative assessment of patients with pulmonary disease (see Table 24-2).
Recommendation 1:
|
Recommendation 2:
|
Recommendation 3:
|
Recommendation 4:
|
Recommendation 5:
|
Recommendation 6:
|
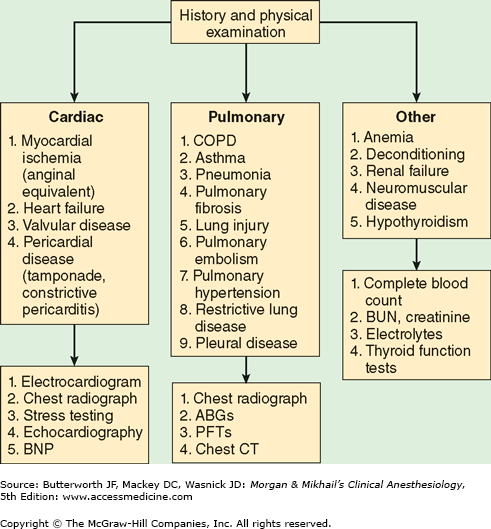
When patients with a history of dyspnea present without the benefit of a previous workup, the differential diagnosis can be quite broad and may include both primary pulmonary and cardiac pathologies. Diagnostic approaches to evaluating such patients are summarized in Figure 24-1.
Figure 24-1
Evaluation of dyspnea. ABGs, arterial blood gases; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CT, computed tomography; PFTs, pulmonary function tests. (Reproduced, with permission, from Sweitzer BJ, Smetana GW: Identification and evaluation of the patient with lung disease. Anesthesiol Clin 2009;27:673.)
Obstructive Pulmonary Disease
Obstructive and restrictive breathing are the two most common abnormal patterns, as determined by PFTs. Obstructive lung diseases are the most common form of pulmonary dysfunction. They include asthma, emphysema, chronic bronchitis, cystic fibrosis, bronchiectasis, and bronchiolitis. The primary characteristic of these disorders is resistance to airflow. An MMEF of <70% (forced expiratory flow [FEF25-75%]) is often the only abnormality early in the course of these disorders. Values for FEF25-75% in adult males and females are normally >2.0 and >1.6 L/sec, respectively. As the disease progresses, both forced expiratory volume in 1 sec (FEV1) and the FEV1/FVC (forced vital capacity) ratio are less than 70% of the predicted values.
Elevated airway resistance and air trapping increase the work of breathing; respiratory gas exchange is impaired because of ventilation/perfusion 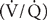 imbalance. The predominance of expiratory airflow resistance results in air trapping; residual volume and total lung capacity (TLC) increase. Wheezing is a common finding and represents turbulent airflow. It is often absent with mild obstruction that may be manifested initially only by prolonged exhalation. Progressive obstruction typically results first in expiratory wheezing only, and then in both inspiratory and expiratory wheezing. With marked obstruction, wheezing may be absent when airflow has nearly ceased.
imbalance. The predominance of expiratory airflow resistance results in air trapping; residual volume and total lung capacity (TLC) increase. Wheezing is a common finding and represents turbulent airflow. It is often absent with mild obstruction that may be manifested initially only by prolonged exhalation. Progressive obstruction typically results first in expiratory wheezing only, and then in both inspiratory and expiratory wheezing. With marked obstruction, wheezing may be absent when airflow has nearly ceased.
Asthma is a common disorder, affecting 5% to 7% of the population. Its primary characteristic is airway (bronchiolar) inflammation and hyperreactivity in response to a variety of stimuli. Clinically, asthma is manifested by episodic attacks of dyspnea, cough, and wheezing. Airway obstruction, which is generally reversible, is the result of bronchial smooth muscle constriction, edema, and increased secretions. Classically, the obstruction is precipitated by a variety of airborne substances, including pollens, animal dander, dusts, pollutants, and various chemicals. Some patients also develop bronchospasm following ingestion of aspirin, nonsteroidal antiinflammatory agents, sulfites, or tartrazine and other dyes. Exercise, emotional excitement, and viral infections also precipitate bronchospasm in many patients. Asthma is classified as acute or chronic. Chronic asthma is further classified as intermittent (mild) and mild, moderate, and severe persistent disease.
The terms extrinsic (allergic) asthma (attacks related to environmental exposures) and intrinsic (idiosyncratic) asthma (attacks usually occurring without provocation) were used in the past, but these classifications were imperfect; many patients show features of both forms. Moreover, overlap with chronic bronchitis (see below) is common.
The pathophysiology of asthma involves the local release of various chemical mediators in the airway, and, possibly, overactivity of the parasympathetic nervous system. Inhaled substances can initiate bronchospasm through both specific and nonspecific immune mechanisms by degranulating bronchial mast cells. In classic allergic asthma, antigen binding to immunoglobulin E (IgE) on the surface of mast cells causes degranulation. Bronchoconstriction is the result of the subsequent release of histamine; bradykinin; leukotrienes C, D, and E; platelet-activating factor; prostaglandins (PG) PGE2, PGF2α, and PGD2; and neutrophil and eosinophil chemotactic factors. The parasympathetic nervous system plays a major role in maintaining normal bronchial tone; a normal diurnal variation in tone is recognized in most individuals, with peak airway resistance occurring early in the morning (at about 6:00 am). Vagal afferents in the bronchi are sensitive to histamine and multiple noxious stimuli, including cold air, inhaled irritants, and instrumentation (eg, tracheal intubation). Reflex vagal activation results in bronchoconstriction, which is mediated by an increase in intracellular cyclic guanosine monophosphate (cGMP).
During an asthma attack, bronchoconstriction, mucosal edema, and secretions increase resistance to gas flow at all levels of the lower airways. As an attack resolves, airway resistance normalizes first in the larger airways (main-stem, lobar, segmental, and subsegmental bronchi), and then in more peripheral airways. Consequently, expiratory flow rates are initially decreased throughout an entire forced exhalation, but during resolution of the attack, the expiratory flow rate is reduced only at low lung volumes. TLC, residual volume (RV), and FRC are all increased. In acutely ill patients, RV and FRC are often increased by more than 400% and 100%, respectively. Prolonged or severe attacks markedly increase the work of breathing and can fatigue respiratory muscles. The number of alveolar units with low  ratios increases, resulting in hypoxemia. Tachypnea is likely due to stimulation of bronchial receptors and typically produces hypocapnia.
ratios increases, resulting in hypoxemia. Tachypnea is likely due to stimulation of bronchial receptors and typically produces hypocapnia.  A normal or high Paco2 indicates that the patient can no longer maintain the work of breathing and is often a sign of impending respiratory failure. A pulsus paradoxus and electrocardiographic signs of right ventricular strain (ST-segment changes, right axis deviation, and right bundle branch block) are also indicative of severe airway obstruction.
A normal or high Paco2 indicates that the patient can no longer maintain the work of breathing and is often a sign of impending respiratory failure. A pulsus paradoxus and electrocardiographic signs of right ventricular strain (ST-segment changes, right axis deviation, and right bundle branch block) are also indicative of severe airway obstruction.
Drugs used to treat asthma include β-adrenergic agonists, methylxanthines, glucocorticoids, anticholinergics, leukotriene blockers, and mast cell-stabilizing agents; with the exception of the last, these drugs may be used for either acute or chronic treatment of asthma. Although devoid of any bronchodilating properties, cromolyn sodium and nedocromil are effective in preventing bronchospasm by blocking the degranulation of mast cells.
Sympathomimetic agents (Table 24-3) are the most commonly used for acute exacerbations. They produce bronchodilation via β2-agonist activity. Activation of β2-adrenergic receptors on bronchiolar smooth muscle stimulates the activity of adenylate cyclase, which results in the formation of intracellular cyclic adenosine monophosphate (cAMP). These agents are usually administered via a metered-dose inhaler or by aerosol. Use of more selective β2-agonists, such as terbutaline or albuterol, may decrease the incidence of undesirable β1 cardiac effects, but are often not particularly selective in high doses.
| Adrenergic Activity | ||
|---|---|---|
| Agent | β1 | β2 |
| Albuterol (Ventolin) | + | +++ |
| Bitolterol (Tornalate) | + | ++++ |
| Epinephrine | ++++ | ++ |
| Fenoterol (Berotec) | + | +++ |
| Formaterol (Foradil) | + | ++++ |
| Isoetharine (Bronkosol) | ++ | +++ |
| Isoproterenol (Isuprel) | ++++ | — |
| Metaproterenol (Alupent) | + | + |
| Pirbuterol (Maxair) | + | ++++ |
| Salmeterol (Serevent) | + | ++++ |
| Terbutaline (Brethaire) | + | +++ |
Traditionally, methylxanthines are thought to produce bronchodilation by inhibiting phosphodiesterase, the enzyme responsible for the breakdown of cAMP. Their pulmonary effects seem much more complex and include catecholamine release, blockade of histamine release, and diaphragmatic stimulation. Oral long-acting theophylline preparations are used for patients with nocturnal symptoms. Unfortunately, theophylline has a narrow therapeutic range; therapeutic blood levels are considered to be 10-20 mcg/mL. Lower levels, however, may be effective. Aminophylline is the only available intravenous theophylline preparation.
Glucocorticoids are used for both acute treatment and maintenance therapy of patients with asthma because of their antiinflammatory and membrane-stabilizing effects. Beclomethasone, triamcinolone, fluticasone, and budesonide are synthetic steroids commonly used in metered-dose inhalers for maintenance therapy. Although they are associated with a low incidence of undesirable systemic effects, their use does not necessarily prevent adrenal suppression. Intravenous hydrocortisone or methylprednisolone is used acutely for severe attacks, followed by tapering doses of oral prednisone. Glucocorticoids usually require several hours to become effective.
Anticholinergic agents produce bronchodilation through their antimuscarinic action and may block reflex bronchoconstriction. Ipratropium, a congener of atropine that can be given by a metered-dose inhaler or aerosol, is a moderately effective bronchodilator without appreciable systemic anticholinergic effects.
The emphasis in evaluating patients with asthma should be on determining the recent course of the disease and whether the patient has ever been hospitalized for an acute asthma attack, as well as on ascertaining that the patient is in optimal condition. Patients with poorly controlled asthma or wheezing at the time of anesthesia induction have a higher risk of perioperative complications. Conversely, well-controlled asthma has not been shown to be a risk factor for intraoperative or postoperative complications. A thorough history and physical examination are of critical importance. The patient should have no or minimal dyspnea, wheezing, or cough. Complete resolution of recent exacerbations should be confirmed by chest auscultation. Patients with frequent or chronic bronchospasm should be placed on an optimal bronchodilating regimen. A chest radiograph identifies air trapping; hyperinflation results in a flattened diaphragm, a small-appearing heart, and hyperlucent lung fields. PFTs—particularly expiratory airflow measurements such as FEV1, FEV1/FVC, FEF25-75%, and peak expiratory flow rate—help in assessing the severity of airway obstruction and reversibility after bronchodilator treatment. Comparisons with previous measurements are invaluable.
 Asthmatic patients with active bronchospasm presenting for emergency surgery should be treated aggressively. Supplemental oxygen, aerosolized β2-agonists, and intravenous glucocorticoids can dramatically improve lung function in a few hours. Arterial blood gases may be useful in managing severe cases. Hypoxemia and hypercapnia are typical of moderate and severe disease; even slight hypercapnia is indicative of severe air trapping and may be a sign of impending respiratory failure.
Asthmatic patients with active bronchospasm presenting for emergency surgery should be treated aggressively. Supplemental oxygen, aerosolized β2-agonists, and intravenous glucocorticoids can dramatically improve lung function in a few hours. Arterial blood gases may be useful in managing severe cases. Hypoxemia and hypercapnia are typical of moderate and severe disease; even slight hypercapnia is indicative of severe air trapping and may be a sign of impending respiratory failure.
Some degree of preoperative sedation may be desirable in asthmatic patients presenting for elective surgery—particularly in patients whose disease has an emotional component. In general, benzodiazepines are the most satisfactory agents for premedication. Anticholinergic agents are not customarily given unless very copious secretions are present or if ketamine is to be used for induction of anesthesia. In typical intramuscular doses, anticholinergics are not effective in preventing reflex bronchospasm following intubation. The use of an H2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree