
AIRWAY MANAGEMENT
HISTORY, DEFINITIONS, AND BASICS
“There is nothing living that does not breathe, and nothing breathing that does not live.”
—Lectures on the Whole of Anatomy (Harvey, 1653)
William Harvey’s recognition of the importance of ventilation marked a turning point in medical history. Prior generations of practitioners followed the teachings of Galen who opined that the lungs existed to cool the heart. Following Harvey, 18th Century European communities organized “Rescue Societies” to save drowning victims.
As airway structures were increasingly recognized for their specific roles as conduits of gases destined for the alveoli; oral and nasal devices were developed for the specific purpose of resuscitation. These were naturally adapted to stabilize airways for use with first-generation inhalational anesthetics such as chloroform. Periodically, airway emergencies would occur. Tracheostomy, which had fallen into disrepute during the middle ages, reappeared as an essential tool for anesthesiologists when performed by John Snow (who famously delivered chloroform as an obstetrical anesthetic to Queen Victoria) to save a patient’s life. As anesthesia itself disseminated through surgical practice, so did the need for surgical airway skills.
Fortunately, technology has made the need for emergent surgical airways rare. Airway management progressed from natural airways and “blind” techniques, through direct visualization, and is now facilitated by video tools including videolaryngoscopy and fiberoptic laryngobronchoscopy. As emergent surgical airways are performed less frequently, some of those skills have been lost, yet operative teams (surgeons and anesthesiologists) must find common strategies to ensure that the procedure can be performed safely when needed. The need is invariably urgent or emergent. Thus, consideration of alternative airway strategies (Plan A, Plan B…, surgical airway) is an essential part of every airway plan.
IS AN ARTIFICIAL AIRWAY NECESSARY?
Before selecting a strategy for securing an artificial airway in an acute care situation, practitioners must first ask themselves whether airway manipulation is necessary. Adequate surgical anesthesia can often be accomplished by either peripheral nerve blockade or neuraxial (spinal/epidural) techniques, especially where limb or pelvic surgery is required. While these skills require advanced training and in some cases specialized equipment not immediately available in all situations, infiltrative local anesthesia should also always be considered as an option for the procedurally trained acute care physician, as it has been used successfully in several trauma situations including cesarean section,1 craniotomy for epidural hematoma,2 and facial injury after motor vehicle accidents that include zygomatic arch fractures and multiple lacerations.3 Irrespective of the situation, training in airway management is crucial for every acute care physician, because unexpected events are frequent occurrences4 and preparedness is a high priority.
Herein, we define a “secured airway device” to be a patent conduit for gas exchange that is unlikely to fail through conventional manipulation of a patient’s body position encountered during a perioperative period (including transport to/from stretchers, ICU beds, and operating room tables). A “definitive airway device” is defined as a patent conduit for gas exchange that involves the trachea and a specialized plastic connection, which universally fits most ventilators and manual ventilation equipment. Current advanced cardiac life support (ACLS) guidelines use a slightly broader term (the “advanced airway device”) to describe tubular conduits that facilitate ventilation.5 By this definition, some advanced airway devices require advanced skill and training (endotracheal tubes), while others require minimal skill and training (such as the supraglottic airways [see laryngeal mask airways (LMAs) and Combi-tubes below]). In most acute care situations, a definitive airway such as an oral endotracheal tube is preferred over the supraglottic advanced airway devices. However, certain scenarios necessitate the use of an LMA or Combi-tube (or even mask) on a temporary basis.
After placement, the position and patency of all airway devices must be confirmed as soon as possible by either direct means (visualization of conduit in trachea) or indirect means (e.g., end-tidal CO2, blood gas results, or chest x-ray). The “gold standard” for accepting any airway device as functional is end-tidal CO2 monitoring as it provides nearly instant physiologic confirmation of cardiac output and successful gas exchange. Because a secured airway device known to exist in the trachea is a definitive airway device and a definitive airway device becomes secured with either strong adhesive tape or through the use of a mechanical strap holder, these two terms will often be used interchangeably in clinical settings. Moreover, in clinical practice of acute care surgery, the word “device” is often omitted, as in “the patient’s airway was secured.”
To avoid confusion with specific anatomical structures, also referred to as airways, this shortening will be avoided. For this chapter, we will assume all secured airway devices are confirmed and all definitive airway devices are confirmed and subsequently secured unless explicitly noted.
COMMON DEVICES AND TIMING OF PLACEMENT
The three most common definitive airway devices in acute surgical care patients are the oral endotracheal tube (oral ETT), the nasal ETT, and the tracheostomy tube.6 Besides specialized semipermanent tracheostomy tubes, these devices all contain an inflatable bulb designed as a barrier to prevent the entry of stomach contents or copious oral secretions into the conducting airways (see aspiration below). The choice of device will depend on several factors such as procedure site, ease of placement, and hemodynamic stability of the patient. In most patients, the oral ETT is the most efficient way to establish a definitive airway (see below for difficult airway considerations and intubation techniques). Because placement of an oral ETT is facilitated with neck extension (and in some instances flexion), precautions must be taken in patients with known or suspected cervical instability. Basal skull fractures are an absolute contraindication to nasal ETTs. Tracheostomy or needle cricothyrotomy as a bridge to tracheostomy (see end of Chapter) constitutes the default backup plan for any acutely ill patient in whom a definitive airway device cannot be established by other means. Although the oral ETTs are the most common airways in these patients, a tracheostomy is an acceptable (and sometimes desirable) plan “A” in patients with a suspected or known difficult airway who are likely to require mechanical ventilation for extended periods of time.
Protection of the airway is often used as a justification of the establishment of a definitive airway. Common wisdom holds that as a patient progresses through depressed levels of consciousness, the airway reflexes attenuate and the inability to cough and swallow secretions may lead to an increased risk of aspiration of stomach contents. It is prudent to consider the use of a definitive airway device in a patient whose Glasgow Coma Scale (GCS) is deteriorating. Many clinicians regard descent through a GCS of 9 as an indication to establish a definitive airway device, although the patient’s history and baseline mental status must also be considered. While generally accepted as an indication for endotracheal intubation, a GCS of <9 is not necessarily a contraindication to extubation.
Aspiration has traditionally been associated with poor outcomes in trauma patients.7,8 High morbidity and mortality is associated with the development of chemical pneumonitis or aspiration pneumonia. Prevention of aspiration is therefore a goal of airway protection. It is prudent to delay elective surgery until a patient has fasted for 8 hours or more, to allow time for gastric emptying. Some controversy surrounds the specific time interval. In 2011, the American Society of Anesthesiologists (ASA) revised their published guidelines to include special considerations for otherwise healthy patients not at increased risk for delayed gastric emptying. For these patients, the recommended minimum fasting period for clear liquids is 2 hours, and 6 hours for nonhuman milk and for light meals not containing fried or fatty food.9 Regardless, major trauma can slow gastric emptying (as can diabetes and hiatal hernias) and, if possible, prophylactic treatment with antacids, H2-blockers, and agents that speed gastric motility are recommended as long as their administration does not impede more urgent treatments.
Other risks and complications associated with intubation are both less common and less severe than the failure either to mask ventilate or to establish a secure airway. Tooth, gum, and lip damage can occur with any oral manipulation. Nasal ETTs are traumatic to the nasal mucosa. Bleeding can be minimized with a topical vasoconstrictor, such as phenylephrine. The posterior pharynx can be inadvertently penetrated.10 Such penetration is a major concern in patient who had previously received radiation treatment to the head, neck, or face.
MASK VENTILATION
Mask ventilation is the most fundamental and essential skill of airway management, yet it is undervalued from educational and clinical perspectives. Whether a patient requires sedation for a minor procedure or is facing pending respiratory failure, evaluation for potentially difficult mask ventilation should occur in tandem with evaluation for potential difficult intubation (see Tables 7.1 and 7.2). It is easy for mask ventilation to become an afterthought in the acute care setting since most patients in trauma surgery are generally intubated without masking to minimize aspiration risk (see section on Rapid Sequence Induction). When intubation fails in a patient who is easy to mask ventilate, there is time to call for help, retrieve additional equipment, or prepare for a surgical airway. In contrast, failed intubation of a patient who is difficult or impossible to mask ventilate can rapidly become a fatal situation. Thus, even in the setting of emergency surgery where time is of the essence, identification of the patient who is a potentially difficult to mask ventilate should prompt a discussion between the surgeons and anesthesiologists about contingency plans for failed intubation.
TABLE 7.1
SUMMARY OF POOLED SENSITIVITY AND SPECIFICITY OF COMMONLY USED METHODS OF AIRWAY EVALUATION
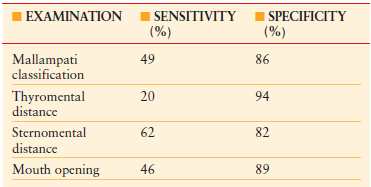
Data derived from Wilson ME, Spiegelhalter D, Robertson J, et al. Predicting difficult intubation. Br J Anaesth. 1988;61:211.
TABLE 7.2
ASSESSMENT AND PREDICTABILITY OF DIFFICULT MASK VENTILATION
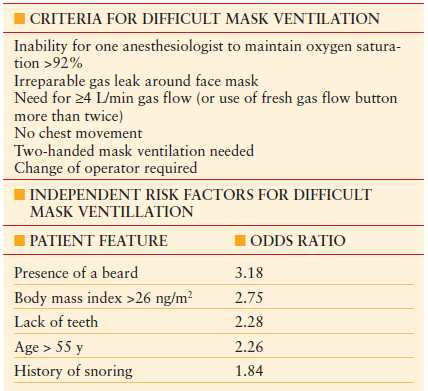
From Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229.
It is critical to rapidly assess the adequacy of mask ventilation and make adjustments (or abandon attempts at masking) in a timely fashion. As discussed in a recent review,11 adequacy can be difficult to define. What is considered safe in the operating room may be of questionable security in the trauma helicopter or hospital elevator. The modern anesthesia machine in most operating rooms not only provides inhaled anesthesia but is well-equipped with several monitors capable of assessing adequacy of ventilation. The flexible bag connected to the anesthesia machine will deflate and inflate with the spontaneous ventilation of a patient as long as the mask is well-placed (“sealed”) to the face. It is important to recognize that the bag for manual ventilation in the operating room performs differently from the more familiar self-inflating (Ambu TM) bag. During manual ventilation in the apneic patient, the bag in the operating room will fail to reinflate with leakage of air, and often this is a clue that the mask may need to be readjusted (see C-E technique below). The digital readout of the exhaled tidal volume can also be used to confirm delivery of manually or machine-delivered ventilated breaths. Most importantly, a persistent end-tidal CO2 (etCO2) waveform on the capnograph signals gas exchange. With most sampling systems, etCO2 will only appear after several breaths. Importantly, the appearance of etCO2 verifies both ventilation and perfusion. This makes etCO2 monitoring an especially important tool.
Outside of the operating room, it can be challenging to assess the adequacy of mask ventilation. Bag movement is unreliable as most emergency carts are stocked with self-inflating bags. Physical examination should reveal chest rise and breath sounds with spontaneous or manual ventilation. Although less reliable than etCO2, the observation of condensation in the mask is a good sign that gas exchange is taking place. Similarly, maintenance or improvement in oxygen saturation (as measured by arterial blood gas or pulse oximetry) is another indirect method that provides confidence in one’s ventilation technique. However, etCO2 is quickly being recognized as the gold standard monitor of ventilation. The ASA’s 2010 monitoring guidelines recommend etCO2 monitoring for deep sedation and the 2010 ACLS guidelines encourage its use in CPR.5 These guidelines may translate to increased availability of these monitors outside the operating room. Local administrators and their clinical leaders should include etCO2 monitoring capability as they evaluate purchasing of new equipment.
Problems with mask ventilation can generally be classified into one of two categories: (1) difficulty creating an adequate seal or (2) obstruction to airflow. Examples of the former include patients with thick beards, edentulous patients, and patients with severe facial trauma. Obstruction to airflow may occur anywhere along the airway, from bronchoconstriction or masses within the lungs to larygnospasm to collapse of the upper airway in a patient with obstructive sleep apnea. Even in the absence of sleep apnea, morbid obesity can lead to enough extrathoracic pressure to impede mask ventilation. Chest compressions performed as part of cardiopulmonary resuscitation can divert mask ventilation breaths from the lungs to the stomach, and for this reason, current (as of 2010) ACLS guidelines mandate that cycles of 30 compressions followed by two quick breaths be continued until an advanced airway is in place.5 Subtle improvements in the technique of mask ventilation as well as airway adjuncts can alleviate many cases of difficult mask ventilation.
In traditional one-handed masking, the practitioner’s left hand is responsible not only for creating an adequate seal, but also for minimizing upper airway obstruction by providing jaw thrust. The left thumb and index finger form a “C” and apply the mask to the face, taking care not to compress the bridge of the nose or interfere with the eyes. The remaining three fingers are spread along the mandible to form an “E,” with the fifth digit behind the angle of the jaw to maximize vertical thrust. Novices and trainees need to be constantly reminded to “lift the face to the mask” rather than pressing the mask on the face. It is also important to remember that the jaw thrust is very uncomfortable and should not be performed on conscious patients whose airway muscular tone is adequate to prevent their tongue from obstructing their airway.
At the first sign of difficulty with one-handed masking, two adjustment techniques are available to the lone practitioner. One is to use both hands to readjust the mask seal and provide maximal jaw thrust, as sometimes it is easier to maintain a strong jaw thrust with one hand than it is to create one with a single hand. Having optimized the seal and jaw thrust, the right hand goes back to squeezing the bag. The second technique is placement of airway adjuncts (orally or nasally), often followed by the two-handed readjustment described above.
Airway Adjuncts
Placement of an oral airway is often facilitated by using a wooden tongue depressor. Without a depressor, the oral airway should initially be inserted with the curve pointing away from the tongue, and then rotated to follow the curve of the tongue as the tip reaches the posterior pharynx. Either way, care must be taken to bring the tongue forward to open the airway, but not so far that it rests between the lower teeth and the oral airway (which could lead to laceration). It is important to select a size appropriate to the patient, though a size 10 (100 mm) oral airway is adequate for most adults. A properly placed oral airway is typically very effective at facilitating mask ventilation, but this adjunct should only be placed in patients with depressed levels of consciousness, as it sits at the base of the tongue and can be uncomfortable and stimulate a gag reflex.
Nasopharyngeal airways (“nasal trumpets”) are better tolerated in awake patients, though they should be avoided in patients with known or suspected skull base fractures. The nasal airway should be long enough to extend past soft tissue obstruction but not so long as it contacts the base of the tongue as the trumpet could be occluded by a large tongue or stimulate a gag reflex. Usually, size 30-34 is appropriate for adults, but it is prudent to check the fit by holding it against the side of the face to see if it extends from the nare to the angle of the mandible using its natural curve.
When repositioning and airway adjuncts do not allow adequate one-handed masking, you should progress to two-handed masking or alternative approaches to airway management. Even experienced anesthesia providers using oral airways in routine cases deliver larger tidal volumes with two-handed masking, and a two-handed technique should be used as soon as practicable.12 Most acute care situations involve either a self-inflating mask bag or an anesthesia machine, which necessitates the aid of a second provider to squeeze the bag. There are several approaches to two-handed masking, but one common method is to apply a seal with both thumbs and use the remaining fingers to maximize jaw thrust. In some situations, a third person is needed to depress the mask at an area of leak.
It is important to remember that the total volume of most adult-sized bags is 2 L and adverse effects of delivering the entire volume in some situations can occur. In patients with a full stomach, every attempt should be made to avoid delivering high volumes and pressures to prevent emesis from stomach distention. It is estimated that delivering pressures of over 20 cm H2O can open the lower esophageal sphincter in patients with normal esophageal anatomy. Normal tidal volumes vary between 4 and 8 mL per kg of ideal body weight. Positive-end expiratory pressure (PEEP), most commonly accomplished through the use of a specialized valve, is a preferred method for maintaining recruitment of end alveoli during manual ventilation as opposed to delivering large tidal volumes. The respiratory rate must allow for an appropriate time of exhalation, especially in patients with chronic obstructive pulmonary disease who may be more prone to “breath-stacking” or auto-PEEP.
In an emergency situation, it is time-consuming and dangerous to progress through each of these modifications in a stepwise fashion. For these cases, it is prudent to immediately place a nasal or oral airway adjunct. Outside of the operating room where patient positioning may be suboptimal and monitors of the adequacy of ventilation are less accessible, it is appropriate to begin with two-handed masking if enough help is available. In situations of cardiac arrest, the acute care surgeon is often supervising resuscitation efforts and should be sure that ventilation is being optimized with oral airways and two-handed masking. As part of the quality improvement program at our level 1 trauma hospital, we found that these techniques were being used in only 25% of cardiac arrests in a 6-month sample set (manuscript in progress). Finally, it is important to remember that adequate mask ventilation should not be considered a long-term solution for most acute care situations as blood in the airway, facial edema and fluid shifts, or provider fatigue can quickly turn adequate mask ventilation into an airway emergency.
ENDOTRACHEAL INTUBATION
Direct Laryngoscopy: Evaluation and Performance
Should a patient require an advanced airway, whether for elective surgery or respiratory failure, an endotracheal tube is often the first choice. As with all aspects of airway management, planning and preparation are paramount even in critical situations. After taking the patient history and clinical situation into account, the next step in the process is evaluation of the airway.
Despite ongoing attempts to apply evidence-based medicine to the prediction of difficult intubations, existing tests suffer from low specificity.13 We favor an airway evaluation that can be completed quickly and without complicated anatomical measurements or calculations. Bedside measurements in finger breadths are common. Every practitioner should be aware of exactly how many centimeters are spanned with two and with three finger breadths of his/her hand. Potentially difficult intubations are suggested by limited mouth opening, limited neck extension, limited ability to prognath the jaw, immobility of anterior neck tissues (e.g., radiation changes), and a short thyromental distance (<5 cm). The Mallampati classification (Fig. 7.1) refers to the view obtained by asking a patient sitting upright to open his mouth maximally and protrude his tongue. A view of the tonsillar pillars constitutes class I, view of part but not the entire uvula is class II, soft palate only is class III, and hard palate only is class IV. Identification of a class III or IV view raises concern for potentially difficult intubation. The test is properly performed without the patient phonating, but if a poor view does not improve with phonation, it is particularly worrisome. Practitioners must be aware that serial attempts at laryngoscopy lead to trauma, bleeding, and edema, all of which conspire to make the next attempt more difficult: every effort should be made to optimize conditions for the first attempt.
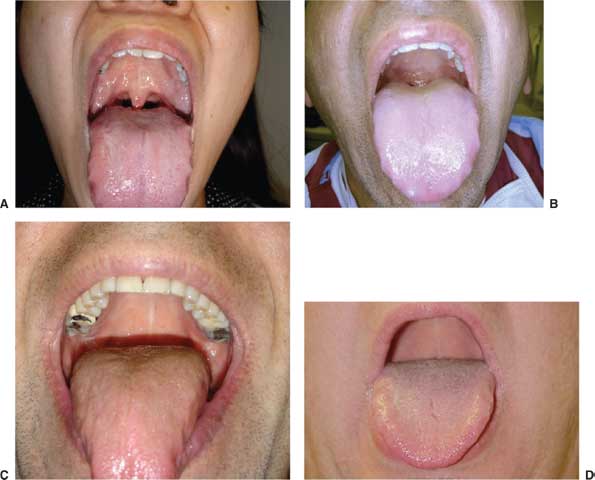
FIGURE 7.1. Photographic examples of Mallampati class. A–D: Mallampati I-IV. (From Barash PG, Cullen BF, Stoelting RK, et al. Clinical Anesthesia, 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








