I. OVERVIEW. The intensivist needs to be well versed in advanced cardiopulmonary resuscitation (CPR) not only to administer care in the ICU but also to assist throughout the hospital. The algorithms and protocols presented here are based on the American Heart Association 2010 Guidelines for cardiopulmonary resuscitation. Formal training with routine recertification is essential to maintenance of skills. In addition to competence in resuscitation, the responsibilities of the intensivist include personnel and resource management that involves clear and deliberate communication and delegation of responsibilities in a crisis.
II. CARDIAC ARREST
A. Initial response to a cardiac arrest can be quite rapid in the hospital setting. In the ICU, because of continuous ECG monitoring, the frequent use of arterial blood pressure determinations, the optimal nurse-to-patient ratios, abnormalities in the circulation, and dysrhythmias are identified immediately.
B. Etiologies. Cardiac arrest in adults may be due to a number of causes, ranging from intrinsic cardiopulmonary problems to metabolic and anatomic abnormalities.
1. Myocardial infarction
2. Pericardial tamponade
3. Pulmonary embolus
4. Tension pneumothorax
5. Hypoxemia
6. Acid–base derangements
7. Hypovolemia
8. Hypothermia
9. Electrolyte abnormalities, including potassium, calcium, and magnesium
10. Adverse drug events
C. Pathophysiology. A cascade of events begins with the systemic hypoperfusion caused by cardiac arrest. Initially, hypoxemia leads to anaerobic metabolism and acidosis. This leads to systemic vasodilation, pulmonary vasoconstriction, and insensitivity to catecholamines. With resuscitation after a period of hypoxia, the different organs are susceptible to reperfusion injury.
III. ADULT RESUSCITATION
A. Basic Life Support (BLS) Primary Survey and Advanced Cardiac Life Support (ACLS) Secondary Survey. ACLS relies on proper BLS assessment and care, including high-quality CPR and defibrillation, as appropriate. ACLS achieves definitive treatment by adding drug therapy and advanced airway management. Immediately starting CPR and defibrillating without delay increases the chance of return of spontaneous circulation (ROSC) and survival to discharge for ventricular fibrillation (VF) sudden cardiac arrest (SCA), while interventions such as advanced airway management and pharmacologic therapy have not been shown to improve survival to discharge.
1. Circulation. Evaluation of appropriate circulation should take place immediately and involve palpation of the carotid pulse for at least 5, but not more than 10, seconds. If no definite pulse is palpated or the patient has a critically low blood pressure for the clinical situation, then chest compressions are begun at a rate of 100 compressions/min, alternating with ventilation in a 30:2 ratio. In the event of advanced airway placement, compressions continue at 100/min without stopping for ventilation at 8 to 10 breaths/min. Compressions should be performed with the rescuer’s hands on the sternum at the level of the nipple, depressing the chest at least 2 inches and allowing the chest to completely recoil after each compression. Adequacy of compressions may be assessed by palpating a pulse or observing the pressure using invasive arterial pressure monitoring. Furthermore, the patient should be placed onto a hard surface to facilitate the quality of compressions. The evaluation for ROSC should be done after every five cycles, or 2 minutes. After successful return to a perfusing rhythm, chest compressions must continue for an additional 2 minutes.
2. Airway. The ICU patient may already have a secured airway. Otherwise, the airway patency needs to be initially assessed with a head tilt–chin lift maneuver, jaw thrust, or artificial airway. The patient should be evaluated for spontaneous ventilation, using the algorithm—look for rise and fall of the chest, listen for exhalation, and feel for airflow. During the first minutes of VF SCA, rescue breaths are probably not as important as chest compressions.
3. Breathing. In the absence of adequate ventilation, the rescuer will initiate two breaths via bag-valve mask ventilation with 100% oxygen. At this point, breaths should be evaluated and maintained at a rate of 8 to 10 breaths/min, or alternating with compressions in a 30:2 ratio. If appropriate rise and fall of the chest is not achieved, then the airway should be repositioned and examined for a foreign body. Additionally, a definitive airway may be placed as long as it does not interfere with other resuscitative efforts, and it should be done by the most experienced person. Proper placement of the endotracheal tube (ETT) is confirmed with end-tidal CO2 measurement using a colorimetric CO2 indicator or continuous waveform capnography and auscultation of the chest. In addition to confirming tracheal tube placement in the airway, continuous capnography can be used as a guide for further CPR. An ETCO2 <10 mmHg indicates the CPR technique requires improvement (better chest excursions, higher rate of compressions). The persistence of ETCO2 <10 mmHg suggests that ROSC is unlikely while an abrupt increase to 35 to 40 mmHg demonstrates that ROSC has likely been achieved. Excessive ventilation is unnecessary and is harmful because it increases intrathoracic pressure, decreases venous return to the heart, and diminishes cardiac output and survival.
If intravenous access cannot be obtained immediately, epinephrine, atropine, naloxone, vasopressin, and lidocaine can be administered via the ETT. The dose is typically 2 to 2.5 times the intravenous dose, diluted in 5 to 10 mL of water or saline.
4. Defibrillation, with CPR, forms the basis of successful adult resuscitation for VF SCA, with the attention focused on minimal interruption of other interventions. As time progresses, the likelihood of ROSC decreases. The successful utilization of automated external defibrillators (AEDs) has resulted in the training of additional responders, such as police, fire personnel, security guards, airline attendants, and so on. AEDs are available in many public areas and include adhesive electrode pads for both delivery of shock and sensing of the rhythm. In light of evidence supporting minimizing interruption of chest compressions, the current recommendation is for a single shock between cycles of CPR and the palpation for a pulse after an additional 2 minutes of chest compressions. This is to minimize any delays in coronary perfusion, despite ROSC.
a. Biphasic waveform defibrillators utilize a positive current in one direction then reversed in the opposite direction. Unless there is documentation of previous successful defibrillations, 200 J is recommended for the first shock. As mentioned above, CPR should continue through the charging, and it is the responsibility of the operator to make sure that all personnel are “cleared,” or not in contact with the patient, during defibrillation.
b. Monophasic waveform defibrillators supply a shock in a single direction and have been replaced by biphasic defibrillators in most institutions.
5. Diagnosis and cardioversion of dysrhythmias. Although the most common nonperfusing waveforms are VF and ventricular tachycardia (VT), instability may be also due to bradycardia and supraventricular tachycardia (SVT). A useful diagnostic and therapeutic tool is adenosine that has the ability to reveal underlying atrial rhythms (Fig. 33.1) and possibly convert an SVT to sinus rhythm. This diagnosis is complex, differentiating a wide-complex SVT from VT, and thus should not be used in the event of VT. A more sensitive method for diagnosing the atrial activity is the atrial electrogram. If the patient is not dependent on atrial pacing and has intraoperatively placed atrial pacing wires, these can be connected to a precordial lead and monitored temporarily. Additionally, a transesophageal pacer can be utilized to obtain the atrial electrogram (Fig. 33.2). If this is not immediately available, then a transvenous pacing wire can be passed through a No. 4 endotracheal tube inserted in the esophagus for the same purpose. In contrast to defibrillation during which time electrical charges are administered on command when there are no discernible QRS complexes, with cardioversion, the shocks are synchronized to the R-wave. This prevents the electrical charge creating a lethal dsyrrhymia by falling on the T-wave. For synchronized cardioversion of a paroxysmal SVT (PSVT), the operator should choose a lower initial dose, such as 50 J (Fig. 33.3). With more resistant rhythms, such as atrial fibrillation (AF) and atrial flutter, an initial dose of 100 J may be indicated. For hemodynamically stable VT, an initial cardioversion at 100 J is warranted. In all dysrhythmias, the responder must be cognizant of the precipitating causes of the abnormality.
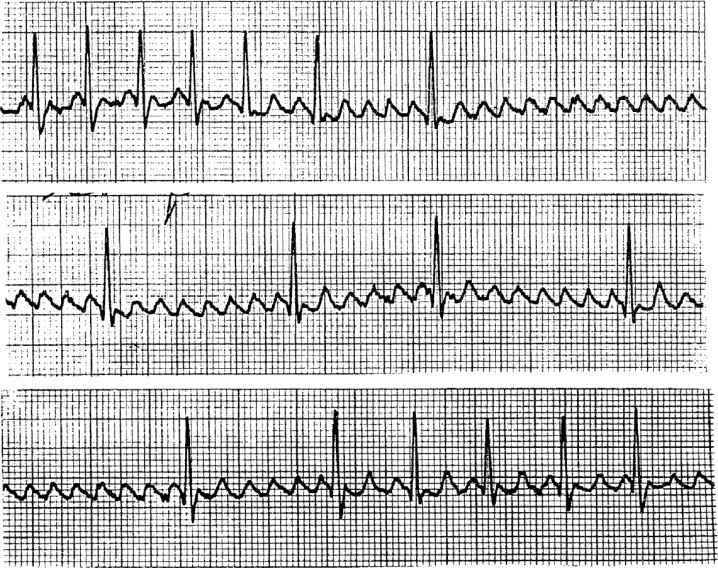
FIGURE 33.1 Diagnosis of a rhythm with adenosine. The initial ventricular response of 180 beats/min disappears after atrioventricular conduction is inhibited by adenosine, revealing an underlying rhythm of atrial flutter (300 beats/min, top), followed by a 6 to 8:1 block (middle), then a 2 to 3:1 block of atrial flutter with a ventricular rate of 120 beats/min.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








