CHAPTER 38

ACUTE PANCREATITIS
Acute pancreatitis (AP) is an inflammatory process readily diagnosed by the combination of epigastric abdominal pain, abnormal elevation of pancreatic enzymes (amylase or lipase) in the serum, or computed tomography evidence of pancreatic inflammation.1 In 75% of affected patients, AP is a self-limited process presenting as mild pain with rapid, spontaneous resolution. However, AP is a severe, life-threatening condition in the remaining quartile of patients, characterized by a systemic inflammatory response syndrome (SIRS) associated with the potential of multiple organ system failure (MOSF) and other complications including death. Thus, optimal treatment varies considerably, driven by accurate assessment of the severity of the disease, determination of the underlying etiology, and identification of potential complications such as pseudocyst, necrosis, infection, hemorrhage, and abdominal compartment syndrome (ACS).
EPIDEMIOLOGY
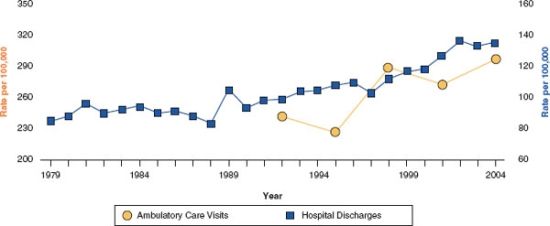
The annual incidence of AP varies among populations, ranging between 4.9 and 35 per 100,000 persons per year.2 Russo et al3. reported 218,188 patients admitted in the United States for AP, with an overall mortality rate of 1.8% in the year 2000. In fact, the incidence of AP is increasing in western countries, resulting in an increasing rate of outpatient visits and admissions for acute and chronic pancreatitis (Fig. 38.1) in the United States. This is attributed to increasing alcohol consumption, and an increased incidence of gallbladder disease and obesity in this population.4 AP can occur at any age, but the mean age for the first attack is the sixth decade.
FIGURE 38.1 Age-adjusted rates of ambulatory care visits and hospital discharges with Pancreatitis in the United States of America 1979–2004. Source: National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care and National Hospital Discharge Survey. With permission from Everhart JE et al., Gastroenterology, 2009;136:1134–1144.
ETIOLOGY AND PATHOGENESIS
Calculus biliary tract disease is the most common cause of AP and when combined with ethanol alcohol abuse as the second most common causative factor, these etiologies account for over 80% of the cases. The other etiologies of AP are rare and can be difficult to definitively diagnose. Table 38.1 summarizes these various etiologies.
Mechanical
The most common cause of AP is the passage of biliary tract precipitates in the form of crystalline sludge or frank gallstones from the gallbladder into the common bile duct, leading to temporary or persistent obstruction of the pancreatic duct at the level of the ampulla of Vater. Data are inconclusive as to whether the ensuing AP is due to a chemical injury from reflux of bile or enteric contents or an increase in hydrostatic pressure. Biliary AP is more common in females.
The second most common mechanical etiology of AP is endoscopic retrograde cholangiopancreatography (ERCP). Asymptomatic hyperamylasemia is observed following ERCP in 35%-70% of patients. However, clinical symptoms consistent with pancreatitis occur in fewer than 5% of the cases,5,6 and the current frequency of pancreatitis following ERCP is considerably lower through efforts to avoid pancreatic duct injection when unnecessary and avoidance of vigorous injection of contrast when the duct does need to be visualized.
Anatomical abnormalities of the pancreas can also lead to mechanical causes of AP. Periampullary neoplasms or diverticuli, and duodenal duplication cysts may rarely lead to AP. Pancreas divisum is a relatively common anatomic variant of the pancreas seen in 5%-7% of the healthy population and is due to failure of the dorsal and ventral ducts to fuse during embryogenesis. This abnormality is thought to result in a relative stenosis of the lesser ampulla, resulting in ductal hypertension and subsequent AP. Annular pancreas is often associated with aberrant ductal anatomy, but rarely presents with pancreatitis.
Finally, AP occurs in 5% of patients with blunt abdominal trauma; hyperamylasemia without frank AP is present in a substantial percentage of other blunt trauma patients. Penetrating trauma involving the pancreas also causes AP; the energy distribution of a firearm missile places this mechanism at considerably higher risk for pancreatitis than that of relatively low-energy stab injuries.7
Toxic
Alcohol-induced pancreatitis is more common among men and is the second most common cause of AP, but only a small percentage of alcoholic subjects develop pancreatitis. This disparity is thought to be due to a combination of environmental and genetic factors which are poorly understand. Alcohol-induced pancreatitis may present as AP or as chronic pancreatitis. Not all alcoholic pancreatitis patients progress to chronic pancreatitis; even with continued alcohol abuse, some patients have nonprogressive, repetitive AP.8
Pharmacologic
Certain pharmacologic agents cause AP in idiosyncratic fashion due to poorly understood pharmacogenetic factors (e.g., aminosalicylates, 6-mercaptopurine) or direct, dose-related toxic effects (e.g., diuretics, sulfonamides). Table 38.1 lists the most common agents associated with drug-induced AP. The estimated incidence of drug-induced pancreatitis is 0.1%-2%. Balani et al.9 described certain subpopulations who may be at higher risk such as children, women, the elderly, and patients with advanced HIV infection or inflammatory bowel disease. Most cases of drug-induced pancreatitis are mild and self-limiting, and the diagnosis of the etiology is usually implied rather than proven.
Metabolic
Hypertriglyceridemia and hypercalcemia are the two most common metabolic disorders associated with AP, but account for fewer than 3% of AP cases. Serum triglyceride concentrations must be markedly elevated, above 1,000 mg/dL to provoke AP. Thus, this etiology of AP is usually associated with type I, II, and V hyperlipidemia, but elevated triglycerides secondary to obesity, diabetes, estrogen or tamoxifen therapy, or nephrotic syndrome can also provoke AP. Triglyceride concentrations need to be measured at admission; after fluid resuscitation, the serum values may reduce considerably and thus prove misleading. Hypercalcemia of any etiology can occasionally cause AP, representing <1% of cases, and not clearly correlating with the degree of hypercalcemia. This disorder is usually a manifestation of hyperparathyroidism, but can also be seen in patients with excessive doses of vitamin D, familial hypocalciuric hypercalcemia, metastatic cancer, and total parenteral nutrition (TPN).
Infectious
Although several infectious agents can cause AP, this etiology accounts for <1% cases of AP.10 The viruses, Mumps, Coxsackievirus, Hepatitis B, Cytomegalovirus, Varicella-Zoster and Herpes simplex can induce AP. More recently, HIV infection has been related to AP. Although it is hypothesized that AP can be caused by HIV itself, most commonly it is associated with opportunistic infections or medications (e.g., didanosine). Bacterial infections including Mycoplasma, Legionella, Leptospira and Salmonella, and fungal infections (Aspergillus) and parasites (Toxoplasma, Cryptosporidium, and Ascaris) have been reported as agents causing AP.
Hereditary Pancreatitis
Some genetic mutations are associated with AP, most notably mutations of the serine protease 1 (prss1) and serine protease inhibitor Kazal 1 (spink1) genes. SPINK1 is described as disease modifier and can promote AP in combination with alcohol abuse. Also, at least one mutant variant of the cystic fibrosis transmembrane conductance regulator (cftr) gene has been identified in some patients with idiopathic chronic and acute recurrent pancreatitis. These possible inherited forms of AP should be suspected when the following criteria are present: Idiopathic recurrent pancreatitis, relatives with pancreatitis-associated mutations or family history of recurrent pancreatitis, idiopathic chronic pancreatitis, or pancreatitis in childhood.11 Genetic testing and counseling are recommended for this group of patients.
Autoimmune
Autoimmune pancreatitis is poorly understood, but is often associated with elevations in serum IgG4 concentrations, and resection specimens stain strongly for IgG4 expression. The process can be localized, or involve the entire gland. These patients typically present with a constellation of symptoms and findings consistent with pancreatic cancer including pancreatic and biliary strictures and low-attenuation masses by CT, rather than AP.
Vascular
AP secondary to ischemia is a very rare event but it has been described as a complication of lupus and other etiologies of vasculitis, atheroembolic events, intraoperative hypotension, and hemorrhagic shock. This etiology of AP needs to be particularly considered in patients that develop AP following cardiopulmonary bypass status.
PATHOGENESIS
The inflammatory processes that occur with AP are extraordinarily similar regardless of the etiology, and include both structural and biochemical changes. Early events are characterized by changes in secretion, intracellular activation of enzymes, and generation of inflammatory mediators. Intra-acinar activation of trypsinogen, uncontrolled by endogenous antagonists such as SPINK 1, alpha-1 antitrypsin, and alpha-2-macroglobulin, leads to activation of other enzymes such as proelastase, procarboxypeptidase, prophospholipase A2 and other cascades including kallikrein–kinin, complement components, and fibrinolysis. Pancreatic digestion characterized by necrosis, apoptosis, and autophagy ensues. Microcirculatory injury and leukocyte chemoattraction with release of cytokines further mediate these processes.
The release into the circulation of activated pancreatic enzymes and cytokines (phospholipase, elastase, TNF, IL-2, platelet-activated factor—PAF, complement, etc.) mediate the SIRS seen in patients with severe pancreatitis. In these patients, these mediators affect distant organs, leading to increased vascular permeability, vasodilation, liver injury, pulmonary injury, myocardial depression, and acute kidney injury. Thus, severe AP can lead to MOSF.12 Why a subset of individuals develop severe AP with SIRS is poorly understood and is an area of intense investigation.
CLINICAL FINDINGS
The most consistent symptom of AP is severe epigastric pain, often extending through or with band-like radiation to the back. Anorexia, nausea, and vomiting are also reported by the majority of patients. Systemic findings such as fever, tachycardia, dehydration, and in severe cases, shock and coma subsequently may be present. The typical, temporal history of biliary pancreatitis is onset of pain within 2 hours of a large meal, usually high in fat content. The onset of symptoms from alcohol-induced AP typically ensues 1–3 days after a drinking binge.
Importantly, findings on the initial physical exam are rarely indicative of the severity of the disease. Hemodynamic changes or signs of pulmonary, hepatic, or renal injury should not be expected at presentation, nor should their absence suggest the patient is likely to follow a self-limited clinical course. Epigastric tenderness is a constant finding. Guarding, abdominal distension, decreased bowel sounds, and even rebound tenderness may be present. The mucous membranes may appear dry and the urine typically appears concentrated.
Later in the disease course, if the AP process is caused by choledocholithiasis or liver injury leading to cholestasis has occurred, jaundice or scleral icterus may be noted. Similarly, physical findings of pulmonary injury will develop in severe AP including rales and decreased breath sounds, usually in left base associated with a pleural effusion. Without adequate volume replacement or in the setting of severe renal injury, oliguria or anuria may be observed. Although classically described, Grey-Turner sign (ecchymotic discoloration of flank) and Cullen sign (ecchymotic discoloration of periumbilical region) are seen in only 1%-2% of patients and indicate severe AP with retroperitoneal hemorrhage dissecting into these areas. These findings have been associated with a mortality rate of 37%.13
DIAGNOSIS OF AP: BIOCHEMICAL MARKERS AND IMAGING
The diagnosis of AP is first suspected clinically then confirmed by biochemical or radiologic evidence. According to guidelines published by the American College of Gastroenterology, the diagnosis of AP requires two of the following three features: (1) abdominal pain characteristic of AP, (2) serum amylase or lipase ≥3 times the upper limit of normal, and (3) characteristic findings of AP on CT scan.14
Biochemical Markers
Amylase and lipase are the most accepted confirmatory biomarkers in AP. Serum amylase concentrations rise within 6–12 hours of the onset of pain and with a half-life of approximately 10 hours. Serum lipase concentrations more slowly elevate, typically peaking 24 hours after the onset of pain, and then normalize over 2–3 weeks. In episodes of mild and uncomplicated pancreatitis, serum amylase should normalize within 3–5 days. Elevated serum amylase is considered a sensitive but nonspecific test for AP. Hyperamylasemia can be found in diseases that affect other organs, which produce amylase, such as salivary glands (e.g., parotiditis, trauma, calculi) and fallopian tubes (ectopic pregnancy and salpingitis). Hyperamylasemia can be observed in chronic renal failure patients or found in almost any peripancreatic inflammatory process including intestinal ischemia or a perforated viscus. In contrast, elevated serum lipase is both sensitive (85%-100%) and specific for the diagnosis of AP. Thus, it is ultimately a superior diagnostic tool except early in the clinical course; additional, later sampling may be necessary.15 Importantly, the level of pancreatic enzyme elevation does not correlate with severity of disease and has no value in the assessment of clinical progress or prognosis; therefore, daily measurement of amylase and lipase is not recommended.
Several other biochemical tests or biomarkers have been forwarded as having merit in the diagnosis of AP, but for a variety of reasons, none have been routinely employed. Urine amylase and clearance of amylase can be diagnostic tools for AP, but are only used to diagnose macroamylasemia (in which urine amylase is normal). The serum elevation of other pancreatic enzymes (phospholipase A, trypsin, carboxipeptidase A, colipase, and carboxylester lipase) currently lacks clinical utility. Pancreatitis-associated protein, Trypsinogen activation peptide (TAP), and Trypsinogen-2 are nonenzymatic pancreatic peptides that are elevated in AP. Serum concentrations of TAP have been described as a predictor of disease severity.16
IMAGING
Recent advances in imaging have led to significant progress in the diagnosis, assessment, prognostication, and treatment of AP and associated complications. Of historical interest, a series of abdominal roentograms was once the only option for imaging patients with abdominal pain and suspicion of AP. Classically, the findings of AP are localized or may be only a segmental ileus called the “sentinel loop sign” and the “colon cut-off sign.” In severe cases, a generalized ileus can be observed.
Contrast-enhanced Computed Tomography (CT) is currently the preferred imaging tool for the diagnosis of AP and assessment of severity. CT at the initial evaluation of a patient with abdominal pain can exclude pancreatitis and many other acute intra-abdominal processes. However, CT is not necessary to implement appropriate initial treatment for a patient with even severe AP, and CT is not cost-effective when patients present with clear clinical and biochemical features of mild AP. If CT is obtained in a patient with self-limited AP, the findings range from a normal appearing pancreas without peripancreatic abnormalities to diffuse enlargement of the gland with heterogeneous attenuation of the parenchyma and stranding of the peripancreatic fat.17 Focal involvement of the gland can be seen in up to 18% of patients and this pattern is commonly seen in patients with chronic alcohol abuse.18
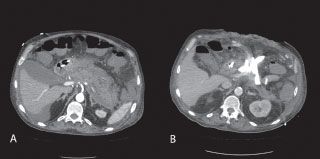
If a patient presents with clinical features predictive of a severe episode or the patient’s clinical condition deteriorates despite adequate initial medical management, a dynamic, contrast-enhanced CT should be obtained. In severe AP, all the findings described above can be observed, but the true value of dynamic contrast administration is the potential identification of regions of the gland that lack arterial enhancement representing an area(s) of necrosis. Beger et al. showed an overall accuracy of 87% for detection of necrosis (confirmed by surgical intervention) with 100% sensitivity for the detection of extended pancreatic necrosis, but only 50% sensitivity for the detection of minor necrotic areas identified at surgery; the specificity of CT for necrosis was 100% in this series.
The normal pancreatic parenchyma attenuates x-rays to 40–50 Hounsfield units (HU) on unenhanced CT images; this increases to 100–150 HU during the arterial phase of contrast administration. Typically, delineation of necrosis by dynamic, contrast-enhanced CT becomes identifiable 24–48 hours after the initiation of symptoms (Fig. 38.2). Thus, early CT (within 12–24 hours of initial presentation) may be equivocal or may underestimate the amount of necrosis and severity of disease, and more informative results are obtained with a dynamic, contrast-enhanced CT 48–72 hours after the onset of an acute attack of pancreatitis.19 This timing also allows intravascular resuscitation prior to the administration of intravenous contrast, thus reducing the risk of contrast-induced renal injury. If contrast cannot be given due to severe allergy or tenuous renal function, the criterion of pancreatic parenchyma displaying attenuation <30 HU correlates to regions of ischemia, and the development of necrosis has been described.19
FIGURE 38.2 Computed tomography of pancreatic necrosis. A: Arterial phase image of the pancreas from a dynamic, contrast-enhanced CT shows lack of enhancement of nearly the entire pancreas, suggesting necrosis of more than 80% of the Gland. B: CT of the same patient after open debridement and external drainage demonstrating control of peripancreatic fluid collections.
CT also plays an essential role in the identification and management of AP complications, such as focal fluid collections, abscess, and pseudocyst. Throughout the course of severe AP, repeated CT studies may be needed according to clinical evolution or anticipation of complications. However, routine serial CT scan is not recommended unless there has been a change in the patient’s clinical condition.
Ultrasound (US) evaluation is appropriately obtained in patients with AP when cholelithiasis is a possible etiology. A diffusely enlarged pancreas with hypoechoic areas are distinctive ultrasonographic findings of AP. However, in almost a third of the patients, the interposition of bowel gas obscures the pancreas. For this reason, US has a limited role in diagnosis and assessment of the severity of AP.20 US is helpful in the assessment of the intra- and extrahepatic biliary tree. A dilated common bile duct may suggest impaction rather than spontaneous passage of the biliary and pancreatic duct obstruction, and should prompt evaluation for the appropriateness of ERCP.
Magnetic Resonance Imaging (MRI)/Magnetic Resonance Cholangiopancreatography (MRCP) is similar in accuracy to contrast-enhanced CT in diagnosis and staging the severity of AP. MRI/MRCP is not the standard imaging technique for AP and its use will depend on availability, local expertise, and institutional algorithms. MRI is a good alternative when intravenous contrast cannot be administered and necrosis is suspected. Purported advantages of MRI include no radiation exposure, lack of nephrotoxicity of gadolinium, superior tissue characterization, and potential ability to detect pancreatic duct disruptions.21 Disadvantages include patient intolerance or ineligibility, significantly longer durations of image acquisition, and variability in technique and access. MRCP is the only noninvasive method to assess the pancreatic duct and is appropriate when delineation of biliary and pancreatic duct anatomy is important or to identify the potential etiology of AP such as biliary calculi and pancreas divisum, or there is clinical need to assess the pancreatic duct for evidence of disruption or stricture (Fig. 38.3). If pancreatic stricture is suspected, the sensitivity of the technique is dependent upon imaging before and after the administration of secretin.
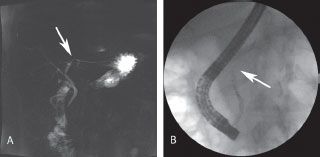
FIGURE 38.3 Disrupted pancreatic duct. Pancreatitis can result in disruption and/or stricture of the main pancreatic duct, complicating care. A: MRCP suggests a stricture or disruption of the pancreatic duct near the neck of the gland. B: Subsequent ERCP confirms disruption with an abrupt termination of filling and inability to visualize the proximal duct with contrast injection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree