I. There are several gastrointestinal (GI) diseases that present in an acute fashion and require critical care, that is, acute pancreatitis, intestinal perforation, ischemic bowel, acute bleeding, or following a major surgical procedure involving the GI tract. In addition, those critically ill may also develop acute GI complications as a result of the systemic inflammatory response. This chapter will focus on the most common GI diseases that may lead to ICU admission and those GI diseases that arise from being critically ill.
II. EVALUATION OF SUSPECTED GI PATHOLOGY: The diagnosis of a GI disorder in the critically ill can be challenging as these patients may not be able to articulate their symptoms and often do not present with classic clinical findings. A nonspecific finding such as a change in mental status, fever, leukocytosis, hypotension, or decreased urine output may be the only sign of intra-abdominal pathology. Physical examination may be unreliable, and the patient may be too unstable to leave the ICU to undergo diagnostic studies. Thus, clinicians must maintain a high index of suspicion for an undiagnosed GI problem as a cause of hemodynamic instability.
A. Signs and Symptoms that suggest GI pathology are numerous and can include abdominal pain, chest pain, bleeding (hematemesis, melena, rectal bleeding), emesis, change in bowel habits, and tube feed intolerance.
B. Initial Evaluation includes assessing for abdominal tenderness and distension, fever, tachycardia, hypotension, and/or change in vasopressor requirement. Correct positioning and function of existing nasogastric tubes and abdominal drains must be confirmed. Laboratory tests such as a complete blood count (CBC), serum chemistries, liver function tests (LFTs), and amylase may be helpful.
C. Increased vigilance is required when caring for solid organ transplant recipients or patients with chronic inflammatory or autoimmune disorders treated with immunosuppressive medications. These chronically immunosuppressed patients may have no signs or symptoms of ongoing GI pathology.
D. Further Diagnostic Studies and Procedures are often necessary to establish a diagnosis and are guided by the initial assessment. Portable plain radiographs can reveal the presence of pneumoperitoneum, intestinal obstruction, or confirm the correct placement of an enteral tube (Fig. 26.1). However, more sophisticated studies are often required to establish a diagnosis. The risks associated with a diagnostic study must be weighed against the expected benefits. Commonly performed tests such as an abdominal CT scan pose a higher risk to the critically ill patient who is at increased risk for contrast-induced nephropathy (see Chapter 25), ventilator-associated pneumonia, as well as airway and hemodynamic complications while traveling to the radiology department. Commonly available diagnostic modalities and their advantages and disadvantages in an ICU setting are summarized in Table 26.1.
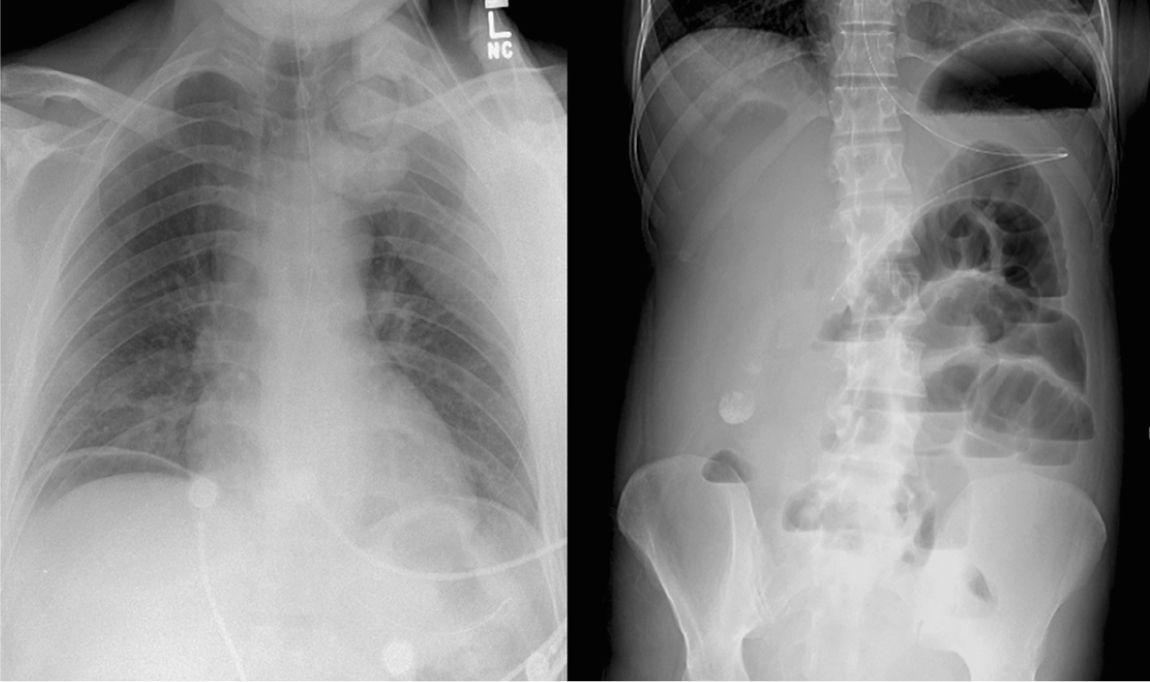
FIGURE 26.1 A plain chest radiograph demonstrating pneumoperitoneum (left) and an abdominal film demonstrating small bowel obstruction and nasogastric tube placement (right) (Courtesy of Hasan Alam, MD).
III. GI BLEEDING is commonly seen in an ICU setting (Table 26.2). GI bleeding may originate from the upper (proximal to the ligament of Treitz) or lower GI tract. Risk factors include liver disease, alcoholism, uremia, diverticulosis, peptic ulcer disease, and the intake of a variety of medications, including nonsteroidal anti-inflammatory drugs (NSAIDs), antiplatelet agents, and anticoagulants.
A. Signs and Symptoms include shock, hematemesis, coffee ground emesis, melena, hematochezia, or hematocrit drop with occult blood present in the stool.
B. Initial Evaluation may need to proceed simultaneously, with stabilization depending on the rate of blood loss and hemodynamic stability of the patient.
1. A history focusing on risk factors for GI bleeding, prior abdominal operations, and significant medical comorbidities should be obtained.
2. Physical examination should focus on assessing end-organ perfusion. Certain physical findings such as stigmata of liver disease may suggest an underlying etiology.
3. Laboratory studies including CBC, coagulation studies, and chemistries should be obtained. Initial hemoglobin values will not reflect the degree of acute blood loss.
C. Stabilization is accomplished by ensuring an adequate airway, hemodynamic stability, and adequate monitoring. A patient with an altered level of consciousness and copious hematemesis will likely require endotracheal intubation. Adequate peripheral intravenous access should be secured. Central venous access, an arterial catheter, and a urinary catheter placement may be appropriate for patients in shock. Initial resuscitation with isotonic crystalloid solution is appropriate while preparations for blood transfusions are made. Resuscitation with blood products in a 1:2::FFP:RBC ratio for those who require a massive blood transfusion (>10 units PRBC) is an appropriate goal. There is some controversy regarding the target hemoglobin but overall it appears that having a lower target (7 g/dL), at least in upper GI bleeds, was associated with less bleeding and lower mortality. Severe coagulopathy should be corrected (see Chapter 27).
| Diagnostic Modalities for Acute Gastrointestinal Diseases in the ICU | |
Modality | Advantages and Usefulness | Disadvantages |
Plain radiographs | • Portable and fast • Assess for free air • Confirm tube placement (nasogastric, postpyloric) | • Findings are usually nonspecific • Overlying tubes, monitors, cables, and other ICU equipment often obscure images |
Ultrasound | • Portable • Assess gall bladder/biliary tree • Can guide procedures (paracentesis, biliary drains) | • Image quality is operator dependent • Air-filled intestines can obscure images |
CT | • Characterizes many forms of intra-abdominal pathology • CT angiography can assess mesenteric vessels | • Patient transport out of the ICU • Optimum study requires IV contrast, which can cause nephropathy and allergic reactions |
MRI | • Noninvasive method to assess for choledocholithiasis | • Patient transport out of the ICU • Time-consuming • Requires patient cooperation |
Angiography | • Diagnostic and therapeutic in mesenteric ischemia • GI bleeding and intra-abdominal/pelvic bleeding following trauma | • Patient transport out of the ICU • Requires IV contrast • Risk of vascular injury (arterial injury at the access site, pseudoaneurysms, hematoma, bleeding, arterial dissection, and embolization of vessel plaque) |
Radionuclide imaging | • HIDA scan may help characterize suspected biliary pathology such as cholecystitis • Technetium scan can confirm ongoing GI bleeding | • Never an initial diagnostic test • Patient transport out of the ICU • Limited utility in an ICU setting |
Endoscopy | • Diagnostic and therapeutic • EGD and colonoscopy can be performed portably • ERCP can be life-saving in cholangitis | • Requires sedation • Colonoscopy requires colonic purge for optimum study • Small risk of intestinal perforation • ERCP can cause pancreatitis |
Bedside laparoscopy | • Direct visualization facilitates diagnosis of acalculous cholecystitis and mesenteric ischemia, according to several reports (small case series) • Can potentially avoid nontherapeutic laparotomy | • Invasive • Inability to evaluate some areas (retroperitoneum) • Patient must be mechanically ventilated and sedated • Utility as a diagnostic modality in the ICU has not been rigorously studied |
| Causes of Gastrointestinal (GI) Bleeding | |
Upper GI Source | Lower GI Source |
Variceal bleeding | Diverticulum |
• Esophageal, gastric, duodenal | • Small bowel, colonic |
Mallory–Weiss tears | Inflammatory bowel disease |
Peptic ulcers | Mesenteric ischemia, including ischemic colitis |
• Gastric, duodenal | Infectious colitis |
Gastritis and erosions | Malignancy |
Malignancy | Angiodysplasia |
Dieulafoy’s lesions | • Colonic, small bowel |
Arterial-enteric fistula • Rupture of aneurysm or ulcer • Postaortic surgery | Hemorrhoids |
Diverticulum (duodenal) | |
Angiodysplasia (duodenal) | |
Hemobilia | |
Pancreatic bleeding |
D. Localization of Bleeding
1. Nasogastric tube lavage of the stomach is easy to perform and is the first maneuver performed to rule out an upper GI source. A clear lavage of the stomach with some bile staining reassures the clinician that the source is not the upper GI tract.
2. Endoscopy
a. Esophagogastroduodenoscopy (EGD) is the diagnostic test of choice for suspected upper GI bleeding and is diagnostic in 90% to 95% of cases. Often, the precise source of bleeding is identified, allowing for immediate endoscopic therapy; however, this can be challenging if a copious amount of blood and clot is present.
b. Colonoscopy for lower GI bleeding is diagnostic in 53% to 97% of cases, depending on the patient population of each study. Colonoscopy is usually undertaken after a colonic purge to increase diagnostic accuracy and visualization. Again, it is possible to perform a therapeutic maneuver as well but these are less successful than the upper GI bleeds. Complications, including perforation, are rare, but the index of suspicion for their occurrence must be high in critically ill, elderly, and immunosuppressed patients.
3. Angiography may be required if endoscopy cannot localize bleeding.
a. Requires ongoing bleeding for successful angiographic detection (0.5–1 mL/min, approximately 3 U of blood per day).
b. Angiography may direct endoscopic or surgical treatment or may be therapeutic via embolization or selective intra-arterial vasopressin infusion. The risk of significant bowel ischemia as a result of embolization, even in the small or large bowel, is less than 5% but the patient must be monitored for ischemia with serial abdominal exams.
c. Other complications associated with angiography include contrast-induced nephropathy, distal embolization of vessel wall plaque, and access site complications.
d. Provocative angiography may be performed if no bleeding is able to be detected by conventional angiography. This technique involves the use of pressors to drive the systolic pressure high (160–180 mmHg), vasodilators to dilate the mesenteric vessels, and heparin. If heparin fails, a low dose of tPA may be considered. By provoking the bleeding, the angiographer may then perform the embolization. If angiographer cannot control the bleeding, the patient must go to the operating room for a resection immediately or if in a hybrid room have the procedure done in the same room. Therefore a multidisciplinary approach with radiology and surgery is key. A provocative angiogram demonstrates the bleeding in approximately 50% of the cases.
4. Radionuclide imaging involves the use of one or two available tracers: technetium-99m–labeled sulfur colloid (99mTc-SC), and technetium-99m–labeled red blood cells (99mTc-RBC). 99mTc-SC can be used immediately, but is taken up by the liver and spleen, which can obscure interpretation of the images when bleeding is located adjacent to these structures. 99mTc-RBC is not taken up by the liver and spleen, but requires preparation prior to use.
a. 99mTc-labeled scans are sensitive and can confirm ongoing bleeding. Their specificity in determining a precise anatomic site of bleeding is debatable.
b. Their utility in an ICU setting is limited, but may be helpful in hemodynamically stable patients with slow lower GI bleeding not localized by colonoscopy.
5. A capsule study can occasionally help identify a bleeding source in the small bowel of hemodynamically stable patients.
E. Specific Therapy for Common Causes of GI Bleeding
1. Upper GI bleeding
a. Variceal bleeding may originate in the esophagus, stomach, or duodenum and is usually the result of cirrhosis and portal hypertension. Splenic vein thrombosis can also result in the development of gastric varices.
1. Prognosis is related mainly to the severity of the patient’s underlying liver disease. Mortality following an episode of variceal bleeding ranges from 10% to 70%. The risk of rebleeding is 70% within 6 months.
2. Early endoscopy is crucial for diagnosis and management. Between 30% and 50% of patients with known varices bleed from other upper GI sources. Endoscopic variceal band ligation and sclerotherapy can control bleeding in 80% to 90% of cases.
3. Balloon tamponade is indicated when endoscopic interventions cannot control esophageal or gastric variceal bleeding. Because of the risk of pressure necrosis, balloon deflation must occur within 24 to 48 hours and should be followed by another attempt at endoscopy with band ligation or sclerotherapy. It is important to protect the airway with endotracheal intubation prior to placement of the balloon tube.
4. Medical management should occur concurrently with endoscopy or balloon tamponade. Somatostatin and its synthetic analog octreotide, as well as vasopressin, have been used.
5. After the bleeding has stopped and the patient is stabilized, treatment with nonselective β-blockers (propranolol or nadolol) should be initiated. These agents decrease the risk of rebleeding and have a proven survival benefit.
6. Transjugular intrahepatic portosystemic shunting (TIPS) controls refractory variceal bleeding in up to 90% of cases by reducing the portal pressure gradient to <15 mmHg. Using a trans-jugular approach, a stent is placed in the liver to connect a branch of the portal vein with a hepatic vein. Complications include occlusion, accelerated liver failure, and hepatic encephalopathy.
7. Surgical therapy for refractory bleeding includes splenorenal shunt and portacaval shunt and should generally be undertaken after the patient is determined to not be a liver transplant candidate.
8. Splenectomy is indicated in patients who are bleeding from gastric varices due to splenic vein thrombosis.
b. Mallory-Weiss tears are mucosal lacerations within 2 cm of the gastroesophageal (GE) junction that are likely due to increases in pressure occurring during vomiting. Most of these lesions stop bleeding spontaneously and have less than a 10% chance of rebleeding. However, actively bleeding Mallory-Weiss tears are best addressed endoscopically.
c. Peptic ulcer disease can manifest as duodenal or gastric ulcers.
1. EGD is the initial diagnostic and therapeutic choice. The endoscopic appearance of an ulcer has prognostic significance. Ulcers with a spurting artery or a visible vessel have a high rebleeding risk (50%–100%). Ulcers with adherent clot or a red or black spot are at moderate risk, while ulcers with a clean base are at low risk (<5%) for rebleeding. Repeat EGD may be necessary to control bleeding in some circumstances. In refractory cases, angiographic embolization has been reported to stop bleeding in 80% to 88% of cases. Uncontrolled bleeding in a hemodynamically unstable patient may necessitate surgical intervention.
2. Acid suppression with proton pump inhibitors (PPIs) has evolved as a crucial component in the management of ulcer disease. In the acute setting, PPIs are administered intravenously as a continuous infusion or twice-daily boluses. Patients are then transitioned to an oral regimen. PPIs produce more consistent acid suppression than histamine receptor (H2) blockers. Patients with large ulcerations >2 cm have a high risk of failing medical therapy, and surgical resection/acid reduction should be considered.
3. Helicobacter pylori infection (see Section IV.B.2), if present, should be eradicated to decreases the rate of future rebleeding.
4. Mucosal ulcerations may be due to neoplasm rather than peptic ulcer disease. The endoscopic appearance in conjunction with a biopsy will help establish the correct diagnosis.
d. Stress ulceration, also called stress-related erosive syndrome, erosive gastritis, or hemorrhagic gastritis, leads to upper GI bleeding in 1% to 7% of ICU patients. Mucosal hypoperfusion and increased gastric acidity occurring in critically ill patients are postulated to play a role in pathogenesis.
1. Endoscopic findings include mucosal erosions.
2. Management is with PPIs as discussed above. H. pylori infection should be excluded. Rarely, angiography with selective embolization (often of the left gastric artery) is necessary. Operative management is reserved for severe refractory hemorrhage.
e. Aortoenteric fistulae are communications between the GI tract, most commonly the distal duodenum, and either the native aorta (primary) or an aortic vascular graft (secondary). Secondary fistulas are more common, occurring in 0.6% to 1.5% of patients after open aortic reconstructive surgery. An initial “herald” bleed followed by massive hemorrhage has been described. Patients may also present with graft infection. Accurate and timely diagnosis is facilitated by a high index of suspicion.
1. Endoscopy demonstrating an eroding aortic graft is uncommon. CT scan is the diagnostic study of choice and may demonstrate hematoma or air at the aortic graft. Once the diagnosis is established, emergency operative exploration is indicated.
f. Dieulafoy’s lesion is an abnormally large artery protruding through the mucosa, most often into the gastric lumen (fundus or body). There is no mucosal ulceration. Bleeding can be massive and can recur in 5% to 15% despite endoscopic therapy. When rebleeding occurs despite endoscopic intervention, surgical ligation or excision of the vessel is indicated.
g. Duodenal diverticulum is a rare cause of upper GI bleeding. The diagnosis can be made endoscopically. Bleeding duodenal diverticula are treated with surgical excision.
h. Angiodysplasia can be located throughout the entire GI tract and can cause brisk bleeding. Endoscopy can detect and treat these lesions. Surgical excision is indicated when lesions continue to bleed despite medical management.
i. Pancreatic bleeding can occur from erosion of a pancreatic pseudocyst into an adjacent vessel, resulting in upper GI bleeding. This is most commonly associated with episodes of pancreatitis.
j. Hemobilia can result from trauma, procedures such as liver biopsy, biliary stent placement, malignancy, gallstones, or erosion of blood vessels into the biliary tree. Treatment of choice is angioembolization.
2. Lower GI bleeding accounts for approximately 25% of GI bleeding and has a mortality rate of 2% to 4%.
a. Intestinal ischemia can involve either the small bowel or the colon and can present with lower GI bleeding. Intestinal ischemia is discussed later in greater detail (Section IV.F.1).
b. Small bowel bleeding is suspected after two negative EGDs and a negative colonoscopy.
1. Angiodysplasia is the most common cause of GI hemorrhage between the ligament of Treitz and the ileocecal valve. Other causes include tumors, inflammatory bowel disease, Meckel’s diverticulum, NSAID-induced ulcers, and Dieulafoy’s lesions.
2. Diagnosis is difficult but may be made in approximately 50% to 70% of cases by push enteroscopy (which examines the jejunum) or capsule study. If the bleeding is brisk, radionuclide scans or angiography may be useful. Again, a provocative angiogram (see above) is an option followed by embolization or operative resection. The angiographer may leave the catheter in place and the surgeon may inject the catheter during the laparotomy with methylene blue to visualize the segment of bowel with the bleeding site.
3. Treatment of small intestinal sources of bleeding is directed at the underlying disease.
c. Diverticular bleeding accounts for 20% of colonic bleeding and is the most common cause of massive lower GI bleeding. Hemorrhage occurs when the vasa recta ruptures into an adjacent diverticular lumen. Bleeding is usually self-limited in 85% of cases but can recur in 14% to 53% of patients. Colonoscopy can locate the site of bleeding, but therapeutic maneuvers are difficult. Angiography can also localize the bleeding and is potentially therapeutic. Urgent operative resection is indicated when bleeding and hemodynamic instability persists. Elective colectomy is considered after multiple episodes of diverticular bleeding.
d. Colonic angiodysplasias are more frequent in patients above the age of 60. Most lesions are found in the ascending colon, and most patients have multiple lesions. Colonoscopy and angiography are very successful for diagnosis and treatment, and colon resection is reserved for failures of these modalities.
e. Colorectal neoplasm (adenocarcinoma as well as polyps) can present with acute lower GI bleeding, although chronic or occult blood loss and microcytic anemia is a more common presentation. Treatment is multidisciplinary, including surgical resection. Postpolypectomy bleeding can be treated with colonoscopy and coagulation.
f. Hemorrhoids and other benign anorectal diseases make up nearly 10% of acute hematochezia. In patients with portal hypertension, hemorrhoidal bleeding can be life threatening, and treatment must be aggressive and may involve a portosystemic shunting procedure.
g. Colitis may be ischemic, infectious, or a result of inflammatory bowel disease (Crohn’s disease or ulcerative colitis) and can present with bloody diarrhea, hematochezia, or melena. Colonoscopy usually reveals diffuse mucosal inflammation. Management of inflammatory bowel disease is best directed by a gastroenterologist and consists of hydration, bowel rest, and steroids. Ischemic and infectious colitis are discussed in Sections IV.F.1 and IV.F.4.
IV. SPECIFIC GI PROBLEMS BY ORGAN
A. Esophagus
1. Esophageal perforation is often iatrogenic and may follow upper endoscopy procedures, NG tube placement, balloon tamponade of bleeding varices, endotracheal tube placement, or transesophageal echocardiography. Abnormal anatomy such as a Zenker’s diverticulum may predispose the patient to this complication. Perforation can occur in the cervical, thoracic, or abdominal esophagus, leading to cervical abscess, mediastinitis, empyema, or peritonitis.
a. Diagnosis. Patients may have pain, fever, subcutaneous crepitus, leukocytosis, pneumomediastinum, or a pleural effusion. A contrast swallow study, CT scan, or esophagoscopy can confirm and localize esophageal perforations.
b. Treatment is urgent and includes broad-spectrum antibiotics, drainage, and gastric acid suppression. Some patients may be candidates for primary repair at the time of drainage. Most patients will remain npo for a prolonged period and will require nutritional support via a feeding tube.
2. Boerhaave’s syndrome refers to spontaneous esophageal perforation, which may have no obvious precipitant or may be related to retching/vomiting, blunt trauma, weightlifting, or childbirth. Predisposing factors include reflux esophagitis, esophageal infections, peptic ulcer disease, and alcoholism.
3. Ingestion of foreign bodies and caustic substances can cause significant esophageal injury.
a. Ingested foreign bodies, most commonly food boluses, can present with dysphagia, odynophagia, chest pain, or airway obstruction. Blunt objects less than 2 cm in size traverse the GI tract uneventfully, while objects larger than 6 cm will obstruct within the duodenum if not in the esophagus. Most objects that do not pass spontaneously may be removed endoscopically—this should be done early to minimize the risk of perforation from pressure-induced necrosis of the esophageal wall.
b. Acids (pH <2) and alkalis (pH >12) cause severe burns when ingested. Vomiting after the ingestion exposes the esophagus to the caustic substance a second time.
1. Initial management includes a careful assessment of the airway. Intraoral burns, edema of the uvula, and inability to swallow saliva may suggest impending airway compromise. Assessment of the airway should be ongoing as the injury evolves. Patients may also require significant resuscitation due to inflammation of the mediastinal tissues. Radiographs of the chest and abdomen are helpful to evaluate for perforation. There is no role for gastric lavage, induced emesis, or activated charcoal.
2. Endoscopy early in the patient’s course is controversial but can be helpful to assess the degree of injury. Endoscopy is helpful in evaluating and managing esophageal strictures that develop later on as a complication of caustic ingestions.
B. Stomach
1. Stress ulceration is discussed in Section III.E.1.
2. Peptic ulcer disease (PUD) refers to gastric and duodenal ulcers. Risk factors include H. pylori infection, NSAIDs, and aspirin use. Patient can present with pain, upper GI bleeding, obstruction, or peritonitis from perforation.
a. The diagnosis of active H. pylori infection can be made during endoscopy utilizing the biopsy specimen and include histology, culture, urease testing, and polymerase chain reaction.
b. Treatment of H. pylori is indicated in most patients on the basis of its association with peptic ulcers, gastric carcinoma, and gastric lymphoma. First-line regimens consist of a PPI plus two antibiotics such as amoxicillin + clarithromycin (first-line therapy), amoxicillin + metronidazole (for macrolide allergy), or metronidazole + clarithromycin (for penicillin allergy).
c. Complications of PUD include upper GI bleeding (Section III.E.1), perforation, and obstruction. Perforation requires an operation with either a laparoscopic or an open approach.
C. Pancreas
1. Acute pancreatitis
a. Most common etiologies are alcohol and gallstones (70%–80% of cases). Other causes include biliary reflux, contrast reflux, hypercalcemia, hyperlipidemia, trauma, and so on.
b. The pathogenesis of acute pancreatitis is related to the release of activated pancreatic enzymes that autodigest the pancreatic parenchyma and cause inflammation, microvascular injury, and necrosis. Activated enzymes may also circulate to distant organs, causing activation of the complement and coagulation cascades, vasodilatation, and endothelial injury. Systemic consequences may include shock, acute lung injury and acute respiratory distress syndrome (ALI/ARDS), and acute renal failure.
c. Symptoms of acute pancreatitis include severe epigastric pain radiating to the back, nausea and vomiting, and fever.
d. The diagnosis is established with a consistent history and physical examination and increased serum amylase or lipase levels. An abdominal CT scan demonstrating pancreatic inflammation, edema, or necrosis is not necessary for the initial diagnosis (Fig. 26.2).
e. The prognosis for the majority of patients is good and they experience mild, self-limiting disease. Severe acute pancreatitis, defined as acute pancreatitis with organ dysfunction, develops in 10% to 20% of patients, necessitating admission to the ICU. Various tools have been developed to assess the severity of acute pancreatitis—the most commonly used is Ranson’s criteria (Table 26.3).
f. Clinical course
1. The early phase is characterized by local inflammation, significant retroperitoneal fluid sequestration, and a systemic inflammatory response that can be robust and may lead to multiple organ system failure.
a. Initial treatment is largely supportive. Aggressive fluid resuscitation and electrolyte repletion should be undertaken. Nasogastric tube decompression can be helpful to alleviate nausea but does not shorten the clinical course. Pain relief, supplemental oxygen, invasive monitoring, mechanical ventilation, and inotropic support may be necessary.
b. Prophylactic antibiotics are not indicated in severe pancreatitis without overt signs of sepsis with a positive culture or aspiration. Even in patients with a large amount of necrosis (30% or more), routine use of antibiotics have been associated with no improvement in outcomes.
c. Nutrition should be provided. Several trials support the use of early enteral feeding via a nasogastric or nasojejunal tube within the first 48 hours. Total parenteral nutrition (TPN) should only be considered for patients who cannot tolerate adequate enteral nutrition.
d. Patients with mild gallstone pancreatitis should undergo cholecystectomy during the index hospitalization to prevent recurrence. The type of intervention and timing for patients with severe gallstone pancreatitis must be individualized. Patients who are too sick to undergo cholecystectomy may be treated with ERCP and sphincterotomy.
2. The later phase of severe acute pancreatitis is characterized by local complications and can carry on for weeks or months.
a. Pancreatic necrosis (Fig. 26.2) should be suspected in patients who fail to improve or suffer clinical deterioration. Necrosis is not present in the first 2 to 3 days of symptoms. The extent of pancreatic necrosis correlates well with the amount of devascularized pancreas as demonstrated by a contrast-enhanced CT scan. By the end of the second week, infection of the necrosis may occur. New organ failure, new fever, or an increasing leukocytosis should prompt CT-guided aspiration of necrotic tissue. Gram stain and culture aid in establishing the diagnosis of infected pancreatic necrosis. Patients with infected pancreatic necrosis should be treated with antibiotics and drainage. There has been evidence to suggest that percutaneous treatment as a bridge to definitive drainage is an option. The use of either transgastric endoscopic debridement or video-assisted retroperitoneal debridement are newer modalities that may be considered if debridement is needed. The morbidity is significantly less than a laparotomy. Early debridement may result in incomplete debridement and the need for a second operation. The ideal timing for debridement appears to be between 21 and 27 days but the later the better.
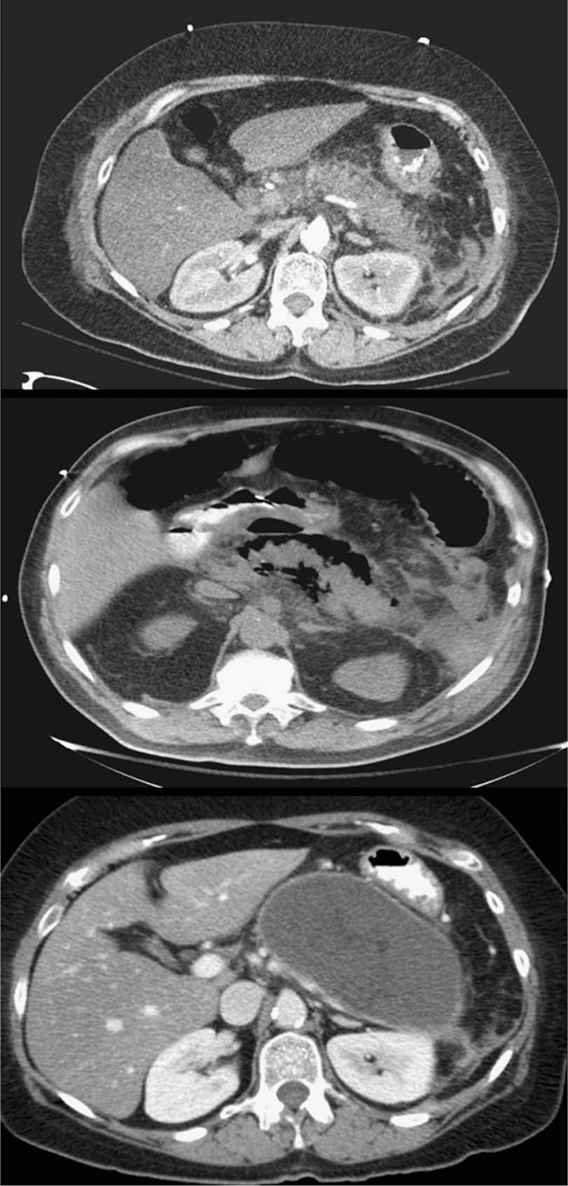
FIGURE 26.2 Acute pancreatitis. Axial CT images demonstrating: acute pancreatitis with prominent pancreatic inflammation (top), pancreatic necrosis with air around the pancreas (middle), and a giant pancreatic pseudocyst that developed several weeks after the acute episode (bottom) (Courtesy of Hasan Alam, MD).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








