
ACUTE CARE SURGERY: SKELETAL AND SOFT-TISSUE INJURY
Expert care of musculoskeletal injury in the polytrauma patient will lead to optimized long-term clinical outcomes. After the initial assessment and stabilization of the patient, the orthopedic trauma surgeon has the responsibility of thoroughly evaluating the pelvis and extremities for both bone and soft-tissue injury during the secondary survey. A collaborative effort, however, between the general trauma surgeon and the orthopedic surgeon is required to coordinate care and promote safe and efficacious treatment of the multiply injured patient.1
GENERAL PRINCIPLES
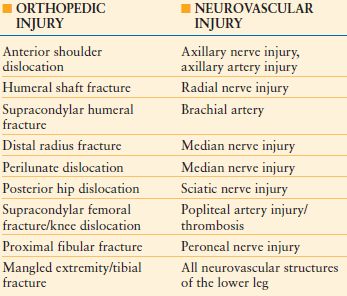
The evaluation of the trauma patient with musculoskeletal injury includes a complete history and physical with an extensive neurologic and vascular examination. Specific fractures and dislocations will pose a greater risk to surrounding neurovascular structures, and the status of these structures should be noted (Table 30.1). The soft tissue surrounding the fracture site needs to be scrutinized for any defects to determine if the fracture is open or closed. Musculoskeletal emergencies such as compartment syndrome or vascular injury need to be promptly identified.
TABLE 30.1
NEUROVASCULAR INJURIES ASSOCIATED WITH ORTHOPEDIC TRAUMA
Further, a tertiary survey is of the utmost importance considering that 15–20% of musculoskeletal injuries are initially missed in the polytrauma patient.2 This should be performed after the initial 24–48 hours. At this point of time in the patient’s treatment course, distracting injuries would have been stabilized and the patient can sometimes assist with localizing further sites of musculoskeletal trauma.
Beyond physical examination, imaging is a critical aspect of the orthopedic workup. At least two orthogonal radiographs that include the proximal and distal joints of the injured extremity should be obtained. Further dedicated radiographs and imaging modalities (computed tomography and magnet resonance imaging) should be ordered by the consulting orthopedist.
Initial Care: Realignment and Reduction
After achieving the diagnosis of fracture or dislocation, prompt delivery of orthopedic care is essential to stop the cycle of injury. Prompt reduction/relocation and splinting protects the soft-tissue envelope, including nerves and vasculature from further traumatic insult. Reductions and relocations should be performed after completion of the secondary survey. Fractures can be immobilized with a well-padded, plaster splint spanning the joint proximal and distal to the fracture site.
Open Fractures
Open fractures in a trauma patient are a site for bacterial proliferation and need to be treated with debridement of debris and necrotic tissue followed by irrigation to decrease bacterial loads. The energy imparted to produce an open fracture causes major stripping of muscle and periosteum with considerable devascularization to the bone. Inadequate debridement increases the risk of developing osteomyelitis, nonunion, and potential loss of limb. Antibiotics are an adjunct treatment to a thorough debridement of an open wound and cannot be used independently.
The Gustilo and Anderson classification continues to be the most commonly used system to describe and guide treatment of open fractures.3 The grade of the fracture is determined by the extent of soft-tissue disruption, degree of contamination of the wound, and severity of the fracture. In applying the classification system, the extent of soft-tissue disruption can only be determined after a thorough debridement has been performed. A grade I fracture is the result of low-energy trauma with a wound that is <1 cm that was caused by protrusion of the bone through the skin or a low-velocity bullet. A wound >1 cm with minimal devitalized soft tissue and contamination that was caused by moderate energy is classified as a grade II fracture. Grade III fractures are caused by high-energy trauma and have extensive soft-tissue injury, gross contamination, a laceration >10 cm, or a segmental fracture. High-power rifles and close-range shotgun blasts impact substantial soft-tissue injury and should be treated as grade III fractures. The presence of adequate soft-tissue coverage with intact periosteum, substantial soft-tissue loss with exposed bone that will require tissue transfer for coverage, and the presence of a vascular injury requiring repair for limb preservation subdivide grade III fractures into grade IIIa, IIIb, and IIIc, respectively.
The initial management of open fractures begins in the trauma resuscitation area. Gross contamination should be removed from the wound. Following the application of a dressing and fracture reduction, fractures should be splinted to decrease pain, reduce hemorrhage, and prevent further soft-tissue injury. Patients with open wounds need antibiotic prophylaxis with tetanus toxoid and a first-generation cephalosporin. Grade III fractures and contaminated wounds also receive an aminoglycoside to prevent gram-negative infection. Barnyard injuries warrant prophylaxis against anaerobes, such as clostridium, with penicillin. In the operating room, it is recommended to debride tissue that lacks contractility, consistency, and the ability to bleed.4 Loose, devitalized bone should be removed regardless of whether or not it is crucial to stabilization. It has been shown that delays of up to 24 hours do not influence infection rates; however, it is recommended to not delay surgical care of open fractures in a stable patient and perform the initial debridement within 6 hours.5 The combination of low-pressure irrigation with mechanical scrubbing is effective in reducing bacterial loads.6 There is no consensus regarding the use of additives in the irrigation fluid. Bacitracin has been shown to not degrade in irrigation solutions; it interferes in the cell wall synthesis of microbes without the risk of cell necrosis and delayed wound healing seen with povidone–iodine and chlorhexidine.7 Definitive fixation can be performed with an infection rate of <2% following debridement and irrigation in grade I fractures and grade II fractures with minimal soft-tissue loss.3 Grade III fractures and wounds with severe soft-tissue damage are treated in a staged fashion with temporary immobilization with an external fixator.
Following a thorough debridement, soft-tissue defects can be temporarily covered with an antibiotic bead pouch or a vacuum-assisted wound closure. High concentrations of antibiotics can be delivered to a wound through the use of antibiotic cement. Traditionally, tobramycin or vancomycin have been combined with one package of bone cement. The antibiotic cement can then be formed into beads on a stainless-steel wire and placed within a soft-tissue defect at an open fracture. If there is inadequate tissue to close the wound, a bead pouch can be formed by sealing the wound with an adhesive drape (i.e., Ioban and Tegaderm).8 The use of vacuum-assisted wound closure (VAC) has gained popularity in the treatment of traumatic wounds. Vacuum-assisted wound dressing devices have been shown to dramatically increase the formation of granulation tissue, decrease the wound bacterial count, and decrease edema following fasciotomies.9,10 The rate of deep infections following open tibial fractures has been shown to decrease fivefold when using VAC dressings rather than standard wound care.11 External fixators can be used to span fracture sites and maintain immobilization while allowing for monitoring of soft tissues. By restoring limb length and alignment, improved circulation promotes healing, reduces inflammation, and increases revascularization of devitalized tissue.
Wounds should continue to be taken to the operating room for repeat irrigation and debridement every 48–72 hours. Debridements need to be thorough with early removal of nonviable tissue to allow for timely preparation of the wound for soft-tissue coverage. Decreased infection and flap complication rates occur when flap coverage is performed within 7 days of injury.12 The early involvement of a plastic surgeon will aid in the planning of reconstructive options.
Compartment Syndrome
Compartment syndrome is a surgical emergency. The diagnosis of compartment syndrome can be challenging, especially in a trauma patient who is sedated or intubated. The devastating outcomes associated with a missed compartment syndrome stress the importance of early diagnosis and fasciotomies (Fig. 30.1A). Compartment syndrome is the result of an elevated pressure within a closed myofascial compartment that results in microcirculatory failure and tissue ischemia. Fifty percent of compartment syndromes are associated with a fracture and are caused by hemorrhage and edema in the injured soft tissue around the fracture. Other etiologies include vascular injuries with reperfusion injury and crush injuries. A constrictive dressing or splint can cause circumferential constriction of an extremity with resulting compartment syndrome that generally resolves with loosening of the dressings. The leg and the forearm are the most common sites for compartment syndrome secondary to tighter, more robust fascial boundaries. Compartment syndrome can also occur in the hand or foot, and rarely does it occur in the deltoid, arm, buttock, or thigh.13 The presence of an open fracture does not exclude the possibility of a concurrent compartment syndrome. Compartment syndrome has been noted to be present in 9% of open tibial fractures.14
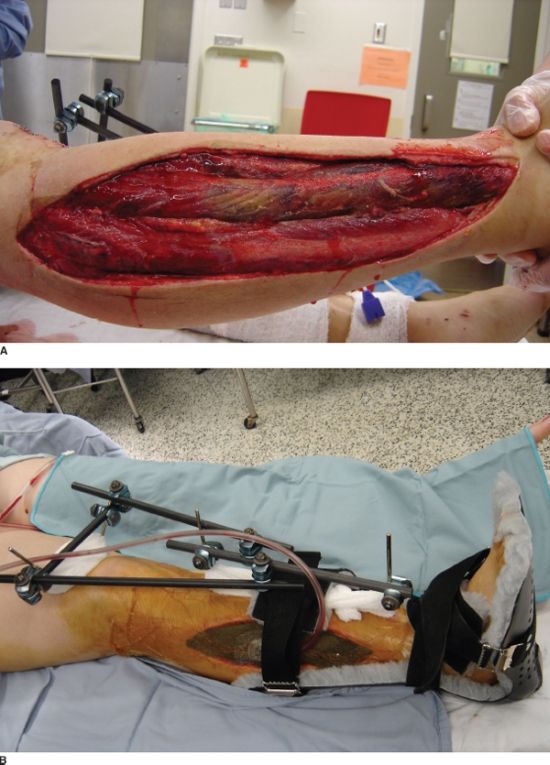
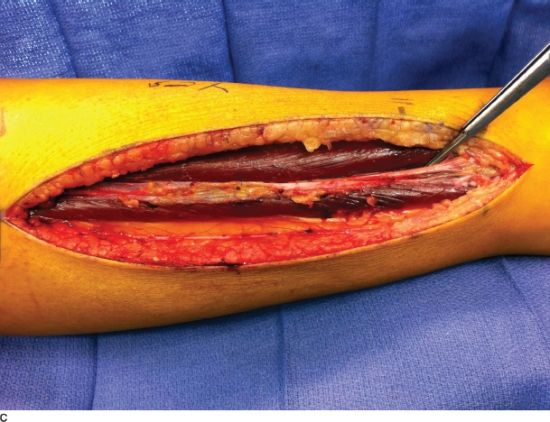
FIGURE 30.1. A: Necrotic muscle (anterior compartment) identified at the time of fasciotomy after delayed presentation of a compartment syndrome that resulted in permanent foot drop. B: Lateral-based longitudinal incision for release of both the anterior and lateral compartments of the leg in a patient with a segmental tibial shaft fracture. The fracture was provisionally stabilized with a spanning external fixation and a VAC was utilized to help decreased edema. C: The forceps marks the superficial peroneal nerve as it pierces through the fascia of the lateral compartment.
The diagnosis is based on clinical examination findings. Compartment pressure measurements are reserved for uncooperative, intoxicated, sedated, or obtunded patients. The classic signs of compartment syndrome are the five Ps: pressure, paresthesia, pain, paralysis, and pulselessness. The presence of tense, noncompressible compartments is generally the presenting sign that will initiate evaluation for compartment syndrome. Pain that is out of proportion to what would be expected for a particular injury and pain with passive stretch of muscles are key signs that confirm the diagnosis. Increasing demands for pain medication are also indicative of a developing compartment syndrome. Pulselessness, paralysis, and paresthesias are late signs that indicate a missed compartment syndrome. The presence of pulses and a normal neurologic examination should not be used to exclude a compartment syndrome.
Invasive compartment measurements can be used to assist in making the diagnosis when clinical findings are questionable. The delta P can be calculated by the difference between the diastolic blood pressure and compartment pressure. Values of <30 mm Hg are associated with inadequate oxygen delivery to tissues and require fasciotomies for treatment of compartment syndrome.15 The use of continuous monitoring of compartment pressures has been advocated for the obtunded patient.16 In general, compartment pressures of >20 mm Hg should raise suspicion for the development of a compartment syndrome. If suspicion of a compartment syndrome is high, it is better to perform a preemptive fasciotomy rather than miss a compartment syndrome and face potential limb loss.
The treatment of compartment syndrome is emergent decompressive fasciotomies. In compartment syndromes involving the leg, the anterior, lateral, and deep posterior compartments are the most frequently involved. The two-incision technique provides a reliable method for decompressing all four compartments. A lateral incision is made between the tibial crest and the fibula to release the anterior and lateral compartments. Longitudinal fasciotomies are made over both compartments until the muscles are soft (Figs. 30.1B and 30.2). Care should be taken to protect the superficial peroneal nerve as it courses posterior to the lateral intermuscular septum (Fig. 30.1C). A medial incision is then made 2 cm posterior to the posterior boarder of the tibia. The proximal aspect of the soleus is released from the tibia to allow access to the deep posterior compartment. Longitudinal fasciotomies are then made through the deep and superficial posterior compartments.
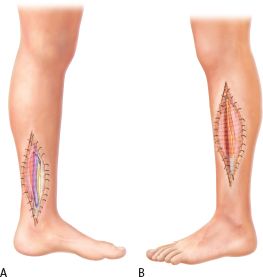
FIGURE 30.2. A: A lateral fasciotomy of the leg is performed by making a longitudinal incision from the proximal end of the fibula to the lateral malleolus and decompresses the anterior and lateral compartments. B: A medial incision situated between the anterior and posterior crests of the tibia provides a decompressive fasciotomy for the deep and superficial posterior compartments of the leg.
Compartment syndrome in the forearm also requires decompressive fasciotomies with two incisions. A curvilinear incision is made over the volar aspect of the forearm from the antecubital fossa to the palm (Figs. 30.3 and 30.4). The superficial fascia, deep intramuscular fascia, and carpal tunnel should be released. If the dorsal compartments remain tense following volar fasciotomies, a longitudinal incision is made over the dorsal aspect of the forearm. Fasciotomies are then performed over the extensor compartment and mobile wad.
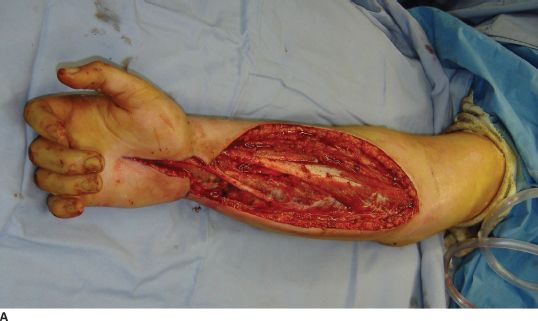
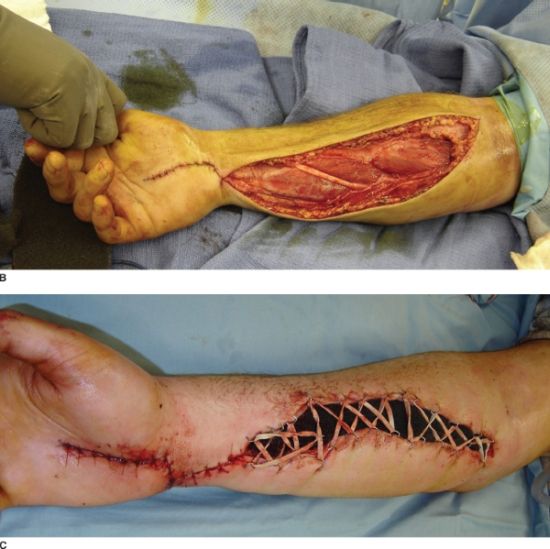
FIGURE 30.3. A: A patient with compartment syndrome of the forearm that was treated with complete release of the superficial fascia, deep intramuscular fascia, and carpal tunnel. B: Only the carpal tunnel portion could be reapproximated. C: A Jacob’s ladder, utilizing vessel loop and staples, was used to approximate the tissues and minimize the area that would ultimately require a skin graft.
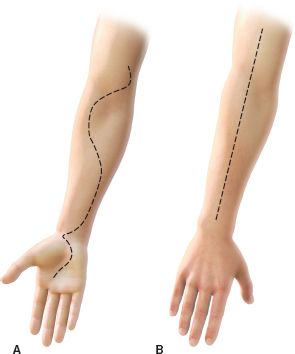
FIGURE 30.4. A: A curvilinear incision spanning over the volar aspect of the forearm from the antecubital fossa to the palm is used to decompress the superficial and deep compartments of the volar forearm. B: The dorsal compartments are released by performing a longitudinal incision over the dorsal aspect of the forearm.
Vacuum-assisted dressing application following fasciotomies has been shown to significantly decrease the time to definitive wound closure (delayed primary closure or skin grafting) when compared to not using a VAC, 6.7 days and 16.1 days, respectively.17 Irrigation and debridement of fasciotomy sites should be performed every 48–72 hours until delayed closure or skin grafting is performed.
Vascular Injury
A vascular consultation should be obtained if distal pulses are not equal to the contralateral extremity after reduction. In a patient in shock, pulses may be difficult to palpate and further evaluation with an ankle–brachial index (ABI) or an arteriogram can identify a vascular injury. If revascularization is deemed necessary, it should be emergently performed within 6 hours. Temporary or definitive fracture-dislocation stabilization needs to be performed prior to vascular repair to prevent reinjury or inappropriate tensioning of the vessel. An external fixator provides immediate, portable stabilization and can be applied quickly. The external fixator restores length and stability to facilitate vascular repair. Prophylactic fasciotomies should be performed to prevent reperfusion compartment syndromes.
Deep Vein Thrombosis and Pulmonary Embolism Prevention
The risk factors associated with the development of deep venous thrombosis (DVT) and pulmonary embolism (PE) are surgery, immobilization, fractures, and coagulopathy.18 All of these risk factors are present in a multiply injured patient. The mortality rate for an untreated PE is 30%.18 There continues to be controversy regarding the best prophylaxis for DVT. No significant decrease in the occurrence of DVT has been noted with the use of low-dose heparin, pneumatic compression devices, or arteriovenous foot pumps in the trauma patient.19 Low-molecular-weight heparin has been indicated for DVT prevention in patients with pelvic fractures, complex lower extremity fractures that require surgical fixation, prolonged bed rest, and spinal cord injuries with complete or incomplete paralysis.19 The use of vena cava filters has been recommended in patients who cannot receive anticoagulation secondary to ongoing bleeding or severe head injuries.20
MANAGEMENT OF INJURIES
Extremity Fractures
Fractures that involve the extremities can result in significant morbidity, decreased mobility, and post-traumatic arthritis. Extremity fractures can be seen in young patients who sustain high-energy trauma as well as elderly patients who sustain insufficiency fractures from low-energy mechanisms. The treatment modality and timing of surgical intervention for these injuries varies depending on the physiologic status of the multiply injured patient.
Damage Control Orthopedics
Early total care is the concept of stabilizing long-bone fractures acutely, especially for polytrauma patients. By stabilizing long-bone fractures, there is a subsequent decrease in fat emboli syndrome, mortality rate, and length of hospital stay. Over time, it was noted that some patients did not tolerate early total care and worsened with immediate definitive fracture treatments. This has led to the emergence of damage control orthopedics (DCO), with patients at risk of developing complications with acute fracture management being initially stabilized with provisional external fixation and less evasive procedures. However, it is still unclear what parameters can be used to identify at-risk patients who will benefit from DCO rather than early total care.
Patients with multiple trauma who survive the initial insult and the acute resuscitation period are subsequently faced with a 50% mortality rate from infection, acute respiratory distress syndrome (ARDS), and multiple organ failure.21,22 Early total care is the preferred treatment in most polytrauma patients; however, an unfavorable immunologic response will be seen in a rare subset of these patients following acute definitive fracture fixation within the first 24 hours after injury.23 The initial trauma has been referred to as the “first-hit” and the impact of the subsequent surgical burden as the “second-hit” phenomenon. To reduce the negative impact of the second hit, the principles of DCO were introduced.24,25 The “second-hit” phenomenon may impact in particular those patients with more severe injury and initial physiologic derangement.24 It appears the timing of secondary-hit phenomena may be most evident between days 3 and 5 following injury.26
Diaphyseal fractures are frequently associated with high-energy traumas and many have associated injuries. Surgical goals are to restore limb length, alignment, and rotation while allowing for early mobilization of the patient. The use of provisional external fixation with delayed definitive treatment has been recommended to limit adverse surgical insult in unstable polytrauma patients. In the absence of pin-site infections, provisional fracture fixation with an external fixator for <2 weeks does not adversely affect definitive treatment with IMN.27,28
Initial imaging modalities of a grossly deformed lower extremity include orthogonal radiographs of the diaphysis and the proximal and distal joints. Fractures involving the articular margins are frequently seen with diaphyseal fractures. A computed tomography scan should be obtained prior to proceeding with surgical intervention if there is any concern for an articular fracture that would alter management. Femoral neck fractures are associated with 10% of femoral shaft fractures and approximately 30% are missed on initial evaluation.29 A protocol with a dedicated internal rotation AP radiograph of the hip, fine-cut CT scan through the femoral neck, intraoperative fluoroscopic lateral radiograph of the hip, and postoperative AP and lateral radiographs of the proximal femur has been recommended to better detect and treat associated femoral neck fractures.30
Acute intramedullary nailing is the treatment choice for most diaphyseal fractures. Fat embolism syndrome occurs when marrow fat macroglobules are released from the intramedullary canal following a long-bone fracture, especially in the femoral shaft. The lack of venous valves in the femur allows canal contents to drain into the bloodstream. After the fat emboli are released to the bloodstream, the emboli are surrounded by activated platelets that together cause an intense inflammatory response in the lung. The inflammatory response results in increased vasoconstriction and increased pulmonary artery pressures that can exacerbate pulmonary problems in a multiply injured patient.31 Urgent stabilization of long-bone fractures has been recommended to decrease fat embolization, which is primarily accomplished with intramedullary nailing. The use of early external fixation followed by delayed intramedullary nailing has been shown to have decreased systemic inflammation when compared to early intramedullary nailing.32 The degree of fat embolization has been shown to be greatest during nailing insertion with only minor differences during reaming.27
Open reduction and internal fixation (ORIF) with plates and screws is infrequently used to treat diaphyseal fractures since IMN minimizes soft-tissue stripping, devascularization of bone fragments, and infection rates. ORIF does have a role in the treatment of proximal and distal metadiaphyseal fractures with articular extension, ipsilateral femoral neck fracture, the presence of a total joint arthroplasty, extremely small intramedullary canals, and when there is a preexisting deformity that prevents the use of IMN.
Periarticular Fractures
The treatment of periarticular fractures varies from the management of diaphyseal fractures in that the articular surface must be anatomically reduced in addition to restoring alignment, length, and rotation. Inadequate restoration of the articular surface predisposes the patient to post-traumatic arthritis. Surgery must frequently be delayed to allow for soft-tissue swelling to decrease as well fracture blisters to resolve prior to definitive treatment. This is most evident in the treatment of tibial plateau and distal tibial pilon fractures.33,34 The extensive surgical approaches that must be made to reduce and stabilize periarticular fractures may lead to further soft-tissue insult and failure to allow for the resolution of the initial swelling can result in postoperative wound complications. Patients must be closely monitored for signs of compartment syndrome and if fasciotomies are performed, the incisions must be placed to allow for subsequent surgical approaches to be made during definitive fixation.
A two-staged approach is frequently utilized in treating tibial plateau and pilon fractures when significant soft-tissue injury is present.33 Initial management requires reduction of the fracture with realignment of the articular segment through ligamentotaxis. Acute application of a spanning external fixator allows for realignment of the joint, restoration of length, and immobilization to facilitate soft-tissue healing. In pilon fractures, the fibula can be acutely plated to assist in restoration of length and to maintain the talus reduced in the mortise if, soft tissues allow. Plain radiographs and CT scans are required to adequately visualize the fracture. CT scans should be obtained after the fracture has been reduced and length restored. CT scans allow for evaluation of intra-articular depression that must be addressed during definitive fixation.
When soft-tissue swelling has decreased, fracture blisters have reepithelialized, and skin wrinkles have returned definitive fixation can be performed. The surgical principles of ORIF of periarticular fractures are restoration of length, anatomic reduction of the articular surface, and bone grafting of metaphyseal defects. Nonsurgical management of periarticular fractures is generally reserved for patients that are nonambulatory, a poor surgical candidate secondary to comorbidities, or significant soft-tissue injury that precludes surgery.35
PELVIC FRACTURES
Incidence, Mechanism, and Associated Injuries
Pelvic fractures can result from low-energy trauma, such as a ground-level fall in the elderly, or from high-energy trauma, such as motor vehicle and motorcycles crashes, pedestrians struck by motor vehicles, and falls from heights. Stable fracture patterns generally result from low-energy trauma and can be managed nonoperatively. Surgery is generally required for the treatment of the unstable fractures that are caused by high-energy trauma.
Between 3% and 5% of traffic accidents are associated with pelvic injuries and account for 74% of unstable pelvic ring disruptions.36,37 Among the unstable pelvic ring disruptions, more than 88% are associated with injuries to the head, chest, abdomen, spine, or long bones.38 Urogenital injury (bladder disruption, urethra disruption, and vaginal tear), vascular injury, or bowel injury are the most commonly associated intrapelvic injury.36 The mortality rate may be as high as 50% in complex pelvic ring disruptions.39
Clinical Evaluation
A trauma patient should be evaluated for a pelvic injury concurrently with resuscitation, especially in the setting of hypotension. The evaluation begins with obtaining a history and clinical examination. The involvement of the orthopedic team should occur as soon as a pelvic disruption is suspected. A communicative patient with a pelvic fracture may complain of pelvic pain and the inability to void if there is a urethral disruption.37
During the physical examination, it is critical to thoroughly examine the soft tissue around the pelvis for a laceration or degloving injury. While maintaining spine precautions, a patient can be logrolled to allow adequate evaluation of the posterior pelvis for lacerations or hematomas. The early identification of an open pelvic injury can facilitate an urgent exploratory laparotomy and a diverting colostomy. Approximately 4% of pelvic fractures are open and have a 50% mortality rate.37,40 Active bleeding through a soft-tissue defect in an open pelvic fracture poses a major challenge. The patient can rapidly exsanguinate since the fracture is no longer contained. Early blood transfusions, pressure tamponade of soft-tissue bleeding in the trauma room, urgent laparotomy for control of bleeding from extrapelvic sources, pelvic packing, and an arteriogram with embolization can assist with management of this problem.40,41 Only 1% of pelvic fractures have a major vascular disruption; however, these disruptions result in a 75% mortality rate.42
In males, the scrotum should be examined for testicular displacement and the urethral meatus for blood. A urethral injury cannot be excluded by the absence of blood at the urethral meatus and can be further evaluated with a retrograde urethrogram or cystogram. The pubic symphysis should be palpated for diastasis and the rami for crepitus. A visual and bimanual pelvic examination should be conducted in females to evaluate for lacerations of the urethra, vagina, or rectum. Vaginal lacerations with healthy tissue should be irrigated and closed, whereas perineal lacerations are generally managed with serial debridements and delayed closure.
Urethral injuries should be evaluated with a rectal examination before inserting a Foley catheter. Urethral disruptions are more common in males and commonly occur below the urogenital diaphragm. A displaced prostate is indicative of a urethral disruption. The presence of blood with a digital rectal examination requires further evaluation with anoscopy and proctoscopy. A rectal tear with a pelvic fracture warrants irrigation of the wound and a diverting colostomy. Proctoscopy should also be considered for all patients with significantly displaced pelvic fractures, which can perforate the rectum.
Pelvic instability can be difficult to assess manually. Rotational stability can be evaluated with direct pelvic compression and vertical stability with the push–pull maneuver of the lower leg. Once pelvic instability has been identified, further stressing should be avoided to prevent excessive bleeding and patient discomfort. A detailed motor and sensory examination of the lower extremities should also be performed to assess for a neurologic injury.
Radiographic Evaluation
An anteroposterior pelvis radiograph should be obtained on all patients involved in a high-energy trauma and those with pelvic pain following a low-energy trauma. Pelvic inlet and outlet views should be obtained to evaluate anteroposterior and vertical displacement of the hemipelvis, respectively. It is important to remember that the displacement observed on radiographs is a static representation of a dynamic process that could have recoiled following the initial injury.
A computed tomography (CT) scan of the pelvis with 3 mm contiguous cuts should be obtained to evaluate the magnitude of the fracture or joint displacement and compression of the sacral foramina. Displacement of the posterior pelvic complex by >5 mm indicates significant posterior or vertical instability. The CT defines the pelvic injury in greater detail than radiographs and allows for more accurate preoperative planning.
Classification
There are various classification systems used to describe pelvic injuries; however, Tile’s classification system based on the instability of the bony injury is the most commonly used (Table 30.2). The force vectors involved during injury correlate with the associated injuries seen with pelvic fractures.44 Lateral compression fractures are associated with lung, spleen, liver, and closed head injuries, whereas anteroposterior compression fractures are associated with bladder, urethra, rectum, lower extremity, and chest injuries. Vertical shear fractures are associated with neurovascular injury, calcaneal fractures, and thoracolumbar fractures.
TABLE 30.2
TILE’S CLASSIFICATION SYSTEM FOR PELVIC RING INJURIES
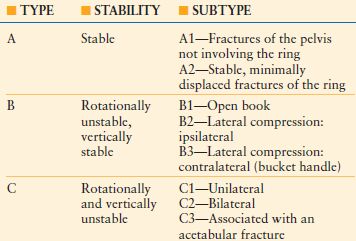
Ref. 43 (From Tile M. Pelvic ring fractures: should they be fixed? J Bone Joint Surg. 1988;70: 1–12.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








