ABDOMINAL TRAUMA
Evaluation and management of complex abdominal injury can challenge even the most seasoned surgeon, and missed abdominal injury remains a common cause of preventable morbidity and mortality. In the trauma patient, the abdomen is defined as the area between the nipples and the symphysis pubis. Because of the wide excursion of the diaphragm, penetrating injury at or below the nipple line (4th intercostal space) may involve the abdominal viscera. Understanding injury patterns increases the likelihood of timely diagnosis and treatment of injuries. The broad categories of abdominal injury are blunt injury and penetrating injury, with different injury patterns and different management and treatment. The paradigm shift to nonoperative management for blunt abdominal injury, and occasionally penetrating injury, has been made possible by the wide availability of computed tomography (CT) and a better understanding of the natural history of injury to the solid organs; spleen, liver, and kidney, specifically.1
INITIAL ASSESSMENT
The initial assessment of the trauma patient begins with the assessment of airway, breathing, and circulation. Based upon patient physiology on emergency department presentation, different levels of detailed examination and attention to life-threatening and non-life-threatening injuries may have occurred. Initial resuscitation of the trauma patient is discussed in Chapter 21, but for the purpose of this chapter, it is imperative to know that in a patient with potential abdominal injury, intravenous access should be supradiaphragmatic (i.e., subclavian, internal jugular, or antecubital fossa). In the patient with femoral intravenous access, potential abdominal vascular injuries such as liver, inferior vena cava (IVC), iliac arteries/veins, or aorta will result in fluid/colloid infusion into the abdominal compartment, creating further complications and ineffective resuscitation. In the patient with multiple gunshot wound (GSW) or stab wound (SWs) injuries, and high likelihood of both chest and abdominal injuries, intravenous access must be secured above and below the diaphragm.
Critically ill trauma patients are typically cold, coagulopathic, and acidotic; a lethal state referred to as the triad of death. Remember that trauma patients die from abnormal physiology. Do not focus on defining every anatomic injury in a critically ill trauma patient; the critical determination is the need for laparotomy.
BLUNT TRAUMA
Blunt mechanisms of injury include motor vehicle collision (MVC), motorcycle collision, pedestrians being struck, blast and crush injury, and fall from a height. Intra-abdominal injury in these scenarios results from compression and shearing forces on the tissues. Solid organ injury is most common: liver, spleen, kidney, or mesentery. Remember that seat belts not only save lives, but change injury patterns. Intestinal perforation or mesenteric injury is common in the restrained victim of MVC; present in 25% of patients with a lap belt mark.
The trauma patient is initially triaged by EMS along with a physician by protocol or over radio communication, which then alerts the trauma team of the mechanism and suspected injuries. The trauma team prepares accordingly to perform a number of procedures starting always with the ABCs. In hemodynamically unstable patients, focused abdominal sonography for trauma (FAST) is utilized in the trauma bay to detect hemoperitoneum (Fig. 28.1). A positive initial FAST (~80% accuracy) in an unstable patient mandates immediate exploration. Diagnostic peritoneal lavage (DPL) has largely been supplanted by FAST and CT due to its invasive nature and lack of specificity for organ injury. However, DPL is sensitive for hemoperitoneum and may be appropriate in the unstable patient with a negative or nondiagnostic FAST. Depending on the injuries suspected or identified, the disposition of the patient will be to the radiology suite for further imaging, the operating room (OR) for definitive repairs, or the ICU for further resuscitation and monitoring. Many hemodynamically normal patients following substantial blunt injury will complete CT from head to midthigh, including the neck, chest, abdomen, and pelvis, although individual institutions and patient management algorithms exist on imaging strategies. Imaging of the abdominal and pelvic cavities has multiple goals: identify solid organ injury and severity, active arterial extravasation, vascular injury, and the presence of free air or free fluid in the pelvis. For these reasons, CT is obtained in an arterial phase and a venous phase.
FIGURE 28.1. Focused abdominal sonography for trauma. (Redrawn from Rozycki GS, Ochsner MG, Schmidt JA, et al. A prospective study of surgeon-performed ultrasound as the primary adjuvant modality for injured patient assessment. J Trauma. 1995;39:493.)
Abdominal solid organs include the liver, spleen, pancreas, and kidneys. Over the last two decades, a marked paradigm shift toward nonoperative management of most solid organ injuries in hemodynamically stable patients has occurred (with the pancreas as the major exception).2 Nonoperative management of solid organ injury at a minimum involves observation with frequent abdominal exams and serial hemoglobin checks. A major reason for the success of nonoperative management of blunt solid organ injury is the infrequent occurrence of hollow viscus injury. The Eastern Association for the Surgery of Trauma (EAST) multicenter study documented small bowel injury in only 0.3% of 275,000 patients.3,4 On the other hand, the authors also documented that delay of only 8 hours in the treatment of an intestinal perforation increased mortality fourfold (from 2% to 9%).3,4 Thus, we must be aware of clinical situations in which hollow viscus injury is likely, as its diagnosis remains difficult. Associated injuries on physical exam include seat belt or tire marks across the chest or abdomen, abdominal wall contusion, or truncal degloving injury. A Chance fracture (lumbar flexion/compression fracture) secondary to a lap belt has associated intestinal injury in as many as 25%-30% of patients. CT (with or without oral contrast) is a poor predictor of small bowel injury with a false-negative rate of 15%-30%. Findings suggestive of small bowel injury on CT include free fluid, free air, and bowel wall thickening. Eighty-four percent of patients with free fluid in the abdomen after blunt trauma on CT without solid organ injury have an intestinal injury or mesenteric injury, but only 30% of those have full thickness perforation. Moreover, a negative CT scan does not exclude intestinal perforation. Thus, when there is free fluid in the abdomen without solid organ injury, intestinal/mesenteric injury is present until proven otherwise by surgical exploration. Nance et al.5 demonstrated that increasing number of solid organ injuries increased the likelihood of hollow viscus injury (most commonly small intestine). A single solid organ injury (spleen, liver, pancreas, or kidney) seen on CT had an associated hollow viscus injury in 7.3%, irrespective of the grade of solid organ injury. With additive solid organ injury, the incidence of hollow viscus injury increased; 15.4% with two solid organ injuries and 34.4% with three solid organ injuries. As mentioned, delay to operative intervention for hollow viscus perforation leads to greater mortality, sepsis, and wound dehiscence. Early exploration with control of intestinal contamination reduces morbidity and mortality considerably. The stomach and colon/rectum are injured less commonly than small bowel in blunt trauma, but require prompt diagnosis and exploration as well.
PENETRATING TRAUMA
Penetrating trauma mechanisms include GSWs, knife or SWs, and less commonly, impalement. The likelihood of injury requiring operative repair is higher for abdominal GSW (80%-95%) than for SWs (25%-33%), and the management algorithms differ. Abdominal organs commonly injured with penetrating wounds include the small bowel, liver, stomach, colon, and vascular structures. Any penetrating wound from the nipple line anteriorly (4th intercostal space) or scapular tip posteriorly to the buttocks inferiorly can produce an intraperitoneal injury.
GUNSHOT WOUNDS
In most instances, patients sustaining GSWs to the abdomen require laparotomy as their diagnostic and therapeutic modality, because of the high incidence of injury requiring repair.
PHYSICAL EXAMINATION
Carefully inspect the patient to avoid missing wounds. Bullets that do not strike bone or other solid objects generally travel in a straight line. Trajectory determination is the key to injury identification. Based on your estimate of the path of the bullet(s), determine which body cavities were violated and if so, which structures are at greatest risk of injury. With multiple GSWs, multiple cavity violation, this may be difficult. Nonetheless, hemodynamically unstable patients with abdominal GSWs should not have extensive evaluation before laparotomy. Carefully examine the patient, paying special attention to the body creases, perineum, ears, eyes, and rectum. Bullet wounds should be counted and assessed. An odd number of wounds suggests a retained bullet; elongated wounds without penetration typify graze injuries. Do not describe bullet wounds as entry or exit wounds; physical examination findings are unreliable in this regard. Describe in detail all missile wounds: site, size and configuration, presence of stippling or powder burns. Palpate the abdomen for signs of tenderness. A neurologic examination should be performed to exclude spinal cord injury.
Plain radiographs are essential in the determination of bullet trajectory. This is facilitated by marking cutaneous bullet wounds with radiopaque markers. In addition, pneumoperitoneum, spinal fractures, pneumothorax, or hemothorax may be found. CT has a limited role in the evaluation of patients with abdominal GSW. However, in hemodynamically stable patients in whom peritoneal penetration is questioned, the extraperitoneal path may be documented on CT. On the other hand, high-velocity weapons can produce full-thickness bowel perforation or thrombosis of major vessels without peritoneal penetration. If any doubt exists, laparotomy is mandatory. In addition, selected patients with right upper quadrant GSW isolated to the liver may be candidates for nonoperative management. Similarly, FAST has a limited role in the evaluation of patients with abdominal GSW. It may be useful in operative planning of hypotensive patients with multi-cavity wounds or documenting cardiac effusion/tamponade. Laparoscopy can be useful in the assessment of peritoneal violation or diaphragmatic injury in hemodynamically stable patients with tangential GSW, particularly in the left upper abdomen.
STAB WOUNDS
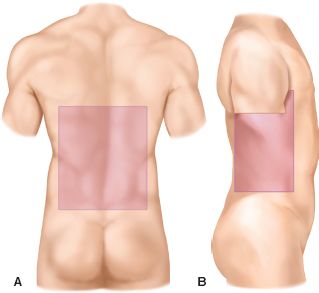
Indications for immediate exploration of abdominal SWs include hypotension, peritoneal signs, and evisceration. If these signs are not present, a selective management approach is justified. Although several articles have advocated observation of the stable patient with omental evisceration after a SW, we believe the incidence of visceral injury makes routine exploration the safest approach in these patients as well.6,7 Anterior SWs refer to those in front of the anterior axillary line. One-third of SWs fail to violate the peritoneal cavity. Thus, two-thirds of anterior SWs enter the peritoneal cavity. Of these, less than half produce visceral injury, which requires operative repair. Thus, of patients with anterior SWs, only one-fourth to one-third require laparotomy. The clinical challenge is to safely select patients who require laparotomy and avoid nontherapeutic laparotomy in patients without major injury. Abdominal organs are at risk with thoracic wounds inferior to the nipple line anteriorly (4th intercostal space) and scapular tip posteriorly (Fig. 28.2). Flank SWs lie between the anterior and posterior axillary lines from the scapular tip to the iliac crest (Fig. 28.2b). Back (posterior) SWs are posterior to the posterior axillary line. Flank and posterior SWs have a lower incidence of visceral injury than anterior SWs (Fig. 28.2a).
FIGURE 28.2. A. The posterior zone is from scapular tip to iliac crest and between each posterior axillary line. B. The flank zone is from 4th intercostal space to iliac crest between anterior and posterior axillary lines. (Redrawn from Boyle EM Jr, Maier RV, Salazar JD, et al. Diagnosis of injuries after stab wounds to the back and flank. J Trauma. 1997;42:261.)
Selective management (serial examination) can be used to detect the development of peritoneal signs in a hemodynamically stable patient. The same surgeon should repeat abdominal examinations documenting subtle change in abdominal findings, temperature, pulse rate, and white blood count. With this evaluation method, the delayed laparotomy rate is 40% with <3% mortality.6–9
Local wound exploration can be performed in the trauma resuscitation area on patients without indications for operation after anterior abdominal stab. The skin is prepared and anesthetized and the original wound is enlarged. Exploration is considered positive if fascial penetration is found. Most series define this as violation of the anterior fascia, as assessment of posterior fascial penetration is more difficult and less reliable.8,9 Patients with positive local wound exploration progress to laparotomy.
CT scan with triple contrast (oral, IV, and rectal) can be used to evaluate back and flank SW. Although a popular tool in the past, triple-contrast CT is not often utilized nowadays. CT is not helpful in the evaluation of anterior abdominal SWs, especially in thin patients with slight abdominal musculature.
DPL has been used to evaluate abdominal SWs. The criteria for red blood cell (RBC) counts are generally lower than that for patients with blunt injury, but the range for positive results is from 1,000 to 100,000/mm3.10,11 Lower threshold values will improve the sensitivity of the modality, but increase the negative or nontherapeutic laparotomy rate.
Shotgun wounds. Close-range shotgun wounds are high-energy injuries. As such, they can result in blast and penetrating abdominal wounds. Shotgun wounds with peritoneal penetration mandate laparotomy. Those delivered from a distance, and thus lower velocity injury, can be evaluated with CT to determine peritoneal penetration by the pellets. The wider the area of scatter by the pellets, the greater the distance from the shotgun to the victim, with less tissue penetration.
Impalement injury. The impaled object is secured in place and removed in the OR under direct visualization with the abdomen open.
THE EXPLORATORY LAPAROTOMY
Refinements in diagnostic capabilities have allowed a more selective application of laparotomy, reducing the number of nontherapeutic laparotomies.
Indications for Exploratory Laparotomy
Laparotomy for trauma is performed on the basis of physical examination findings alone or on the basis of results of further diagnostic tests. Remember that physical examination alone with blunt injury will miss up to 45% of abdominal injuries, generally in patients with altered mental status or distracting injuries. Indications for laparotomy based on physical finding include obvious peritoneal signs on physical examination, hypotension with a distended abdomen on physical examination, abdominal GSW with peritoneal penetration, or abdominal SW with evisceration, hypotension, or peritonitis.
Findings on diagnostic tests, which mandate laparotomy include positive FAST (in the unstable patient), grossly positive DPL or peritoneal aspiration, or findings with any other diagnostic intervention (e.g., chest x-ray with ruptured diaphragm or pneumoperitoneum, abdominal CT, or laparoscopy suggestive of an intra-abdominal injury requiring repair).
An OR appropriately stocked with appropriate anesthetics and nursing and support should be immediately available 24 hours a day. Once the decision is made to operate, the patient must be rapidly transported directly to the OR with appropriate airway support personnel and the trauma team in attendance. Informed consent is obtained from the patient or relative before laparotomy if possible. This is not always possible or practical, depending on the injuries involved and the clinical state of the patient. In such cases, the operation should proceed without delay to obtain consent.
The patient should already have at least two large-bore intravenous lines placed; other intravenous and arterial access can be placed as necessary in the OR. Control of cavitary bleeding should not be delayed by fluid resuscitation; the primary goal in the hemorrhaging patient is control of the bleeding. Administer broad-spectrum, gram-negative, and anaerobic antibiotic coverage (e.g., an extended-spectrum penicillin or a third-generation cephalosporin). Attach chest tubes to underwater seal, do not clamp them, during transport and immediately to suction drainage on arrival in the OR. Make certain that anesthesia and the operating team can visualize the drainage systems of the chest tubes to monitor output during the operation.
Place nasogastric or orogastric tubes and a bladder catheter before laparotomy. However, no procedure should be performed in such a way, which delays control of bleeding and contamination. Sequential compression devices can be used for hemodynamically stable patients. Move the patient onto the operating table with appropriate cervical spine and thoracolumbar spine precautions. If the patient is still immobilized on a backboard, log roll the patient and remove the board before beginning the operation. Occult penetrating wounds must be sought before beginning laparotomy. Be certain to check all creases and folds where penetrating injury is likely to be missed.
Prime the infusion system to infuse blood products and “cell-saved blood” quickly via large-bore lines before the incision releases the tamponade. Ascertain that packed RBCs are in the OR and plasma and platelets are available for the patient with active hemorrhage. Activate the massive transfusion protocol if indicated.
Preparation of the Patient
The patient is shaved only if time allows. The entire anterolateral neck (remove anterior portion of cervical collar and then sandbag to maintain cervical spine immobilization), chest to the table bilaterally, abdomen, groin, and thigh regions (to the knees bilaterally) are prepared and draped in sterile fashion (Fig. 28.3).
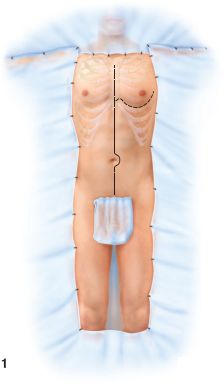
FIGURE 28.3. Full prep of the trauma patient for laparotomy. (Redrawn from Champion HR, Robbs JV, Trunkey DD. Trauma surgery. In: Dudley H, Carter D, Russell RCG, eds. Rob and Smith’s Operative Surgery. Boston, MA: Butterworth; 1989:540, Figure 1).
CONDUCT OF THE TRAUMA LAPAROTOMY
Initial Goals
Stop bleeding and control gastrointestinal contamination. The exploratory laparotomy for trauma is a sequential, consistently conducted, operation. For urgent laparotomy, a generous midline incision is made. Adequate exposure is critical. Self-retaining retractor systems and headlights are useful. Control active bleeding first. Scoop free blood and rapidly pack all four quadrants to control bleeding as a first step. With blunt injury, the likely sources of bleeding are the liver, spleen, and mesentery. Pack the liver and spleen, and quickly clamp the mesenteric bleeders. With penetrating injury, the likely sources of bleeding are the liver, retroperitoneal vascular structures, and mesentery. Pack the liver and retroperitoneum, and quickly clamp bleeding mesenteric vessels. If packing does not control a bleeding site, this source of hemorrhage must be controlled as the first priority.
Once active bleeding is stopped, control of gastrointestinal contamination must be achieved next. Quickly control bowel content spillage using Babcock clamps, Allis clamps, a stapler, rapid temporary sutures, or ligatures. Only when hemorrhage and GI contamination are controlled, should the surgeon proceed to full exploration of the abdomen. Systematically explore the entire abdomen, giving priority to areas of ongoing hemorrhage to definitively control bleeding: liver, spleen, stomach, right colon, transverse colon, descending colon, sigmoid colon, rectum, and small bowel, from ligament of Treitz to terminal ileum, carefully inspecting the entire bowel wall and the mesentery. Next, open the lesser sac and explore the pancreas (visualize and palpate). A Kocher maneuver may be needed to visualize the duodenum, with evidence of possible injury. Finally, inspect the left and right hemidiaphragms and retroperitoneum, pelvic structures, including the bladder and rectum. Note the size of any retroperitoneal hematoma. With penetrating injury, exploration should focus on following the path of the weapon or missile. Retroperitoneal violation by a penetrating wound requires exploration of the retroperitoneum. The abdomen may be closed with running nonabsorbable or absorbable monofilament suture (e.g., No. 1 nylon or No. 1 looped slowly absorbable suture). Leave skin open with delayed secondary closure if there is contamination or prolonged hypoperfusion. If gross edema of abdominal contents precludes closure, absorbable mesh, sterile intravenous bags (Bogota bag), or intestinal bags can be used with moist gauze and an impermeable dressing, or a vacuum-assisted closure (VAC) dressing to prevent possible abdominal compartment syndrome (ACS) (see Chapter 6). Recognize the combination of complex injuries and physiologic signs (hypothermia, acidosis, and coagulopathy) that dictate abbreviated laparotomy (damage control).
SPECIFIC ORGAN INJURY
Diaphragmatic Injury
Anatomy and Physiology.
The diaphragm is an arched muscular tendinous structure that separates the thorax from the abdomen. It is formed of a central tendon, which receives muscle fibers in a radial fashion. These fibers originate from the xiphoid and sternum anteriorly, the inferior border of the cartilage of the 9th-10th rib, and portions of the tips of the 11th and 12th ribs. Posteriorly, the diaphragm originates from the first and second lumbar vertebrae.
The aorta, thoracic duct, and azygos vein course through the aortic hiatus at the level of the 12th thoracic vertebra. The esophagus and the vagus nerves course through the esophageal hiatus at the level of the tenth thoracic vertebra and the vena cava is the only anatomic structure coursing through the caval hiatus, at the level of the eighth thoracic vertebra (Fig. 28.4).
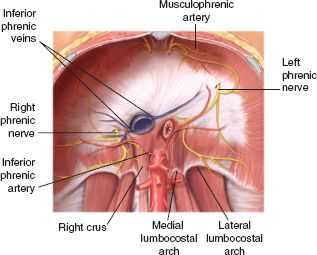
FIGURE 28.4. The origins and neurovascular supply of the diaphragm. (From Valentine RJ, Wind SS. Anatomic exposures in vascular surgery. Philadelphia, PA: Lippincott Williams and Wilkins; 2003:236, Figure 9-7).
The diaphragm has a fundamental function in the respiratory process, when it flattens, increasing the size of the thoracic cavity, thus creating a tidal volume. To accomplish this function, the diaphragm moves 3–5 cm in either direction. The simultaneous contraction of the abdominal muscles creates a gradient between the abdominal and thoracic cavity. When the patient is in the supine position, the gradient between the abdominal and thoracic pleural cavity fluctuates from 7 to 20 cm of water. During maximal inspiration, this gradient can be higher than 100 cm of water. It has been postulated, that the sudden increase of the intra-abdominal pressure (IAP), as in the case of blunt compressive mechanisms, elevates the gradient to levels responsible for the disruption of the diaphragm. Furthermore, this increase in the thoracoabdominal gradient may facilitate the migration of abdominal organs into the thoracic cavity.
Incidence. From the National Trauma Data Base, the incidence of diaphragmatic injury is 35% for blunt trauma and 65% for penetrating injury.12 The most common location of diaphragmatic injury is the left side; in blunt trauma, 80% on the left and 20% on the right.13 In penetrating trauma, diaphragmatic injury is more common on the left due to the preponderance of right hand-handed assailants and the fact that the liver protects the right hemidiaphragm, preventing abdominal viscera from herniation into the thoracic cavity. The organs more frequently involved in herniation in order of frequency are the stomach, colon, spleen, and small bowel.
Diagnosis. The high incidence of associated injuries can overshadow the diagnosis of diaphragmatic injury in the acute phase. High index of suspicion should be exercised when a penetrating wound of the left chest occurs below the 4th interspace and in blunt trauma when there is a simultaneous hemoperitoneum and left hemopneumothorax. The clinician should also be cognizant of the location and trajectory of the penetrating object and the possibility of diaphragmatic involvement. Most diaphragmatic injuries from penetrating trauma are diagnosed intraoperatively. Blunt diaphragmatic injury is generally a larger tear. During the laparotomy, the diaphragm should be inspected carefully with the help of a good headlamp illumination and an extension of your hand using a sponge stick, which allows spreading this floppy muscular organ, to avoid missing other rents. Injury to the diaphragm may be missed at laparotomy (5%), usually a posterior laceration.
The symptoms and signs of diaphragmatic injury range from minimal dyspnea, chest pain, shoulder pain, bowel sounds in the chest, to severe respiratory distress and shock, when associated with massive viscus herniation or associated thoracoabdominal injuries. On occasion, a scaphoid abdomen is seen on physical examination, with extensive herniation of abdominal contents into the chest.
The chest radiograph is the best screening modality in the diagnosis of diaphragmatic rupture, and any lack of delineation of this organ, particularly if it is associated with pleural effusion, should raise the suspicion of diaphragmatic injury. The chest radiograph will be diagnostic for diaphragmatic rupture in 25% of cases with abdominal viscera (usually stomach or small bowel) seen in the chest and normal in 25% of cases of blunt diaphragmatic injury. In 50% of cases, the chest radiograph is abnormal but not diagnostic (Fig. 28.5). Findings include elevation of a hemidiaphragm, obscuring of the diaphragmatic border, loss of the costophrenic angles, or lower lobe collapse.
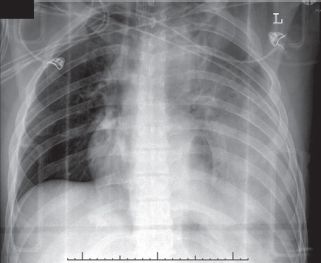
FIGURE 28.5. Chest radiograph of a patient with a ruptured left hemidiaphragm following a motor vehicle crash. A hemothorax is present and a hollow viscus as a result of the diaphragmatic tear is clearly seen in the chest.
Both DPL and FAST (focused assessment sonographic for trauma) will miss diaphragmatic injury.13 CT has become the most used tool in the evaluation of abdominal injury in the last two decades. Initially, CT reported a sensitivity of 14%-–61% and a specificity of 76%-99% in the diagnosis of diaphragmatic rupture. More modern CT technology has improved the accuracy to 80%-–100%.14,15 Magnetic resonance imaging may also be useful, particularly in cases of questionable CT or delayed diagnosis of diaphragmatic injury.16
Laparoscopy and thoracoscopy have become useful adjuncts in the diagnosis of diaphragmatic rupture.17 Laparoscopy perhaps is more useful in patients without other indication for laparotomy, and thoracoscopy in a subacute situation of diagnostic dilemma. Laparoscopy is ideal with penetrating thoracoabdominal wounds on the left side, where the presence of diaphragmatic or abdominal injury is unclear. Laparoscopy may be used as a therapeutic tool in this setting, allowing repair of the diaphragm.
Surgical Management of Acute Diaphragmatic Rupture. The surgical management of acute diaphragmatic rupture, once the abdominal cavity is open, should not preclude the meticulous inspection of the entire abdominal cavity and its contents (Table 28.1). Once other priorities such as control of bleeding and contamination are accomplished, the attention should be focused on the diaphragm. With herniation of abdominal structures, these organs should be carefully retracted back to the abdomen. Good illumination with head lamps, and good exposure, with the transection of the triangular and falciform ligaments if necessary, will provide excellent visualization of the area. Careful downward traction of the spleen and stomach on the left side and the liver on the right will allow inspection of the diaphragms.
TABLE 28.1
DIAPHRAGM INJURY SCALE
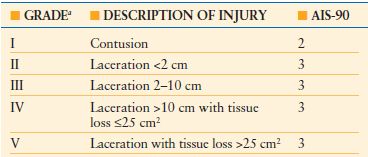
a Advance one grade for bilateral injuries up to grade III.
AIS, abbreviated injury score.
From Moore EE, Malangoni MA, Cogbill TH, et al. Organ injury scaling IV: thoracic vascular, lung, cardiac and diaphragm. J Trauma. 1994;36:229–300.
The edges of the diaphragmatic tear should be grasped with long Allis clamps and then proceed with a careful inspection of any lesion in the intrathoracic organs, if the rent is big enough. If there is evidence of contamination of the pleural cavity, with gastroenteric contamination, extensive irrigation of the thoracic cavity should be performed at this time. If the irrigation of the thoracic cavity is considered inadequate, a subsequent thoracotomy, according to some authors,18 or a video-assisted thoracoscopy are options after completion of the laparotomy. In either case, large chest tubes should be placed as part of the procedure.
Multiple techniques are appropriate to suture the torn diaphragm. The diaphragm should be repaired with monofilament 0 or 1 (polypropylene) nonabsorbable material, with a one-layer or two-layer closure. Horizontal mattress sutures may serve as definitive closure or as the first of two layers of closure. This may be followed by a second layer of a running interlocking suture of the same material, superficial to the first layer. The function of this layer is, in theory, to prevent postoperative bleeding from the edges of this extremely vascularized structure. Central/pericardial tears occur in 5% of cases. The heart must be carefully inspected as well. With lacerations that extend to the esophageal hiatus, be certain not to compromise the orifice when closing; a large bougie may be helpful in this setting. On rare occasion, a detachment of lower insertion of the diaphragmatic fibers with reattachment of the diaphragm in a more cephalad manner is necessary. Mesh is rarely necessary in the acute phase. Diaphragmatic rupture has also been repaired successfully laparoscopically and thoracoscopically.
As a principle, an acute diaphragmatic disruption should be repaired through the abdomen, to address other intra-abdominal injury, which will be present in the majority of patients. In cases of subacute or delayed diagnosis of diaphragmatic injury, a thoracic approach may be easier, avoiding adhesions within the abdomen.
Stomach and Small Bowel Injury
Injury to the stomach or small intestine is more common after penetrating than blunt abdominal trauma. GSWs that violate the peritoneal cavity have a higher incidence of hollow viscus injury (70%) compared to SWs (30%). Thus, we have different strategies for the management of GSW versus SW of the abdomen; mandatory surgery for the GSWs and the possibility of a selective approach for the SWs. Blunt injuries of the stomach and small bowel are uncommon. The incidence found in a multicenter study of 275,000 blunt trauma victims by the EAST was 0.3%.3,4 In patients with any blunt abdominal injury, 4%–7% will have hollow viscus injury. The finding of solid organ injuries increases the likelihood of hollow viscus injury.5
Anatomy. The empty stomach is essentially intrathoracic and protected by the ribcage. When distended, the stomach is at risk for rupture because of direct compression or acute increase in intraluminal pressure. The arterial blood supply of the stomach is the left gastric artery, right gastric artery, left and right gastroepiploic arteries, and the short gastric arteries.
The small intestine distal to the ligament of Treitz is 5–6 m. The small intestine is suspended on its mesentery, with its blood supply originating from the superior mesenteric artery (SMA). The arterial supply to the small intestine has multiple arcades and thus is a well-vascularized structure.
Pathophysiology. Blunt trauma usually produces contusions and intramural hematomas on the stomach and small bowel; more serious injuries such as perforations and mesenteric avulsions are less common.19–21 Localized blows to the abdomen, by farm animals or handlebar injuries have been replaced by automobile crashes and sports injuries such as lacrosse sticks slashes to the abdomen, or football helmet collisions against the unprotected torso. Furthermore, the mandatory use of the seat belt has saved many lives but has increased the incidence of small bowel injury. The incidence of injury to the small intestine with the use of the three-point lap and shoulder restrain is increased 4.3-fold and increased 10-fold with the lap belt-only restraint, compared to the unrestrained victim.3 The association of small bowel perforation or mesenteric injury with Chance fractures of the lumbar spine, associated with the seat belt, has been emphasized earlier.
Blast injury presents different complexes of injury from contusions to perforation, depending whether from primary blast effect and depending on proximity of the patient to the center of the explosion. Secondary blast effect is caused by projectiles from the explosion, with a penetrating component. Tertiary and quaternary injuries are caused by the generation of “blast winds” and fire, respectively. The more serious concern with blast injuries is the possibility of delayed clinical presentation with serious adverse consequences.
Diagnosis. An accurate history of the mechanism of injury, penetrating or blunt, is essential. The location of entrance and trajectory of the penetrating weapons: thoracic–abdominal wall or anterior abdomen should raise the suspicion of gastric or small bowel injury.
The initial laboratory studies: hemoglobin, hematocrit, serum amylase, and white blood cell count are of little value in the initial diagnosis of gastric or small bowel injury. However, fever, leukocytosis, increase in the serum amylase, and metabolic acidosis in the next 24 hours postadmission may be the first sign of a missed hollow viscus injury
DPL has been supplanted by FAST and CT. However, DPL has its value in the detection of hemoperitoneum. In addition, when the white cell count in the DPL effluent is more than 500 per mm3, it may be considered positive for bowel injury. Nevertheless, Jacobs et al.22 demonstrated that even using an elevation of over 500 per mm3 white cells in the lavage, it should not be used a sole criterion in the diagnosis of bowel perforation. Elevation of the alkaline phosphatase >10 units in the DPL effluent or an elevation of the amylase >20 IU/L suggests bowel injury. More recently, authors23 have used ratios of red cells/white cells in the DPL effluent to make a more accurate diagnosis of bowel lesions, avoiding the “lag period” of appearance of the white cells after the injury occurs.
Despite its sensitivity of 84% and the specificity of 99% for detection of hemoperitoneum, Focused assessment by sonography for trauma (FAST) is unreliable in the diagnosis of bowel injury. CT findings of free intraperitoneal air, free peritoneal fluid, thickening of the bowel wall, and mesenteric fat streaking are important predictors of injury. The Elvis Presley trauma center23 found that the overall sensitivity and specificity of CT for bowel injury was 88.3% and 99.4%, respectively. The authors go further to recommend DPL if a single abnormal finding is found at CT and laparotomy or if several signs are found in the same diagnostic modality. The presence of intraperitoneal fluid on CT has different discriminatory values for different studies. Fakhry directed a multi-institutional study for EAST in 95 trauma centers and found that only 29% of patients with free fluid had full-thickness bowel injury. In the same series, 38% of patients with free fluid without solid viscus injury had bowel injury.3,4 Despite a plethora of publications on the subject, there is no consensus in regard to the best management of the patient with free abdominal fluid in the absence of solid viscus injury; DPL, serial abdominal physical examinations; “diet trials” in the absence of abdominal pain or diagnostic laparoscopy have been proposed. The EAST trial noted that 12% of patients with normal CT had bowel injury.3,4 Therefore, a prudent decision could be to keep the patient for observation.
Laparoscopy has been used for diagnosis and treatment of penetrating injury to the thoracoabdominal torso, to exclude penetration of the peritoneum and to assist in the diagnosis of diaphragmatic injuries. Its use in the diagnosis of gastric or small bowel lesions is limited at this point in time. However, it may have a role in the patient about whom there is a low level of suspicion to exclude bowel injury in the presence of fluid without a solid viscus injury.
Management of Gastric Injuries. Gastric injuries are managed with the help of a good illumination (head lights) and good exposure (Table 28.2). The gastroesophageal junction may be difficult to visualize. This area can be exposed better with the division of the left triangular ligament of the left lobe of the liver and reverse Trendelenburg maneuver. The posterior wall of the stomach should always be inspected, particularly in the presence of penetrating injuries of this organ, which have involved the anterior wall. The gastrocolic ligament should be opened carefully, trying to avoid injury to the middle colic artery.
TABLE 28.2
STOMACH INJURY SCALE
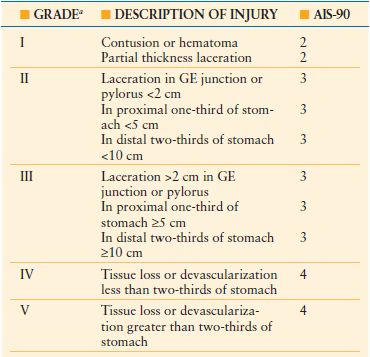
a Advance one grade for multiple lesions up to grade III.
GE, gastroesophageal.
From Moore EE, Jurkovich GJ, Knudson MM, et al. Organ injury scaling VI: extrahepatic biliary, esophagus, stomach, vulva, vagina, uterus, fallopian tube, ovary. J Trauma. 1995;39:1069–1070.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree