The postoperative planning begins with the preoperative evaluation and formation of an intraoperative anesthetic plan. The type of anesthetic (i.e., inhalation technique, total intravenous anesthetic, sedation, local, regional) influences the type and length of postanesthesia care unit (PACU) recovery.
 The level of PACU care depends on the type/approach of surgery, type of anesthetic, intraoperative course of events, as well as patient pre-existing and evolving comorbidities. Typical recovery settings include inpatient recovery, ambulatory recovery (phase 1 for more intensive needs and phase 2 for less intensive needs), short stay (23-hour admit), and recovery from specific procedures (i.e., computed tomography, magnetic resonance imaging, invasive radiology, cardiac, pediatric, and radiation procedures).
The level of PACU care depends on the type/approach of surgery, type of anesthetic, intraoperative course of events, as well as patient pre-existing and evolving comorbidities. Typical recovery settings include inpatient recovery, ambulatory recovery (phase 1 for more intensive needs and phase 2 for less intensive needs), short stay (23-hour admit), and recovery from specific procedures (i.e., computed tomography, magnetic resonance imaging, invasive radiology, cardiac, pediatric, and radiation procedures).
 The transfer of care to a PACU nurse includes assuring that the patient has had appropriate monitoring applied, admission vital signs were taken, a direct and thorough report received that allows for rapid evaluation should complications arise, as well as a nurse capable of handling the acuity of the patient’s medical/surgical problems.
The transfer of care to a PACU nurse includes assuring that the patient has had appropriate monitoring applied, admission vital signs were taken, a direct and thorough report received that allows for rapid evaluation should complications arise, as well as a nurse capable of handling the acuity of the patient’s medical/surgical problems.
 Relative hypovolemia should be evaluated and managed in PACU based on the patient’s comorbidities, preoperative status (i.e., bowel preparation, postdialysis), type and duration of surgery, blood loss, and urine output.
Relative hypovolemia should be evaluated and managed in PACU based on the patient’s comorbidities, preoperative status (i.e., bowel preparation, postdialysis), type and duration of surgery, blood loss, and urine output.
 Postoperative analgesia should be individualized to requirements and expectations. A multimodal approach includes the appropriate use of nonsteroidal anti-inflammatory drugs, narcotics, adjuncts, regional and local anesthetics, as well as anxiety relief and appropriate emotional support.
Postoperative analgesia should be individualized to requirements and expectations. A multimodal approach includes the appropriate use of nonsteroidal anti-inflammatory drugs, narcotics, adjuncts, regional and local anesthetics, as well as anxiety relief and appropriate emotional support.
 Discharge criteria should be tailored to the individual patient’s underlying disease, recovery course, and postdischarge level of care.
Discharge criteria should be tailored to the individual patient’s underlying disease, recovery course, and postdischarge level of care.
 The cardiac risks during the postoperative stay include myocardial ischemia, which may be minimized with continued use of β-blockers, analgesia, nitrates, supplemental oxygen, adequate circulating volume, oxygen-carrying capacity, heart rate control, and an understanding of hypercoagulable states.
The cardiac risks during the postoperative stay include myocardial ischemia, which may be minimized with continued use of β-blockers, analgesia, nitrates, supplemental oxygen, adequate circulating volume, oxygen-carrying capacity, heart rate control, and an understanding of hypercoagulable states.
 The respiratory risks of a patient must take into account the preoperative respiratory disease status. Residual anesthetics, opioids, and sedatives all impair responsiveness to increasing CO2 and decreasing O2 levels. Pain itself can decrease respiration/minute ventilation, leading to CO2 retention and hypoxia. Supplemental O2 application alone does not guarantee hypoxemia will not occur.
The respiratory risks of a patient must take into account the preoperative respiratory disease status. Residual anesthetics, opioids, and sedatives all impair responsiveness to increasing CO2 and decreasing O2 levels. Pain itself can decrease respiration/minute ventilation, leading to CO2 retention and hypoxia. Supplemental O2 application alone does not guarantee hypoxemia will not occur.
 The evaluation of a patient’s ability to void may be affected by the type of surgery (i.e., genitourinary surgery, hernia repairs) or the type of anesthetic (i.e., regional, neuraxial, or opioids).
The evaluation of a patient’s ability to void may be affected by the type of surgery (i.e., genitourinary surgery, hernia repairs) or the type of anesthetic (i.e., regional, neuraxial, or opioids).
 Glycemic monitoring and control should persist as a continuum from intraoperative management. Good glycemic control may help with fighting infection, improve wound healing, which can result in better surgical outcomes. Hypoglycemia occurs because of nothing by mouth status, intraoperative administration of insulin, as well as the patient using programmable insulin pumps.
Glycemic monitoring and control should persist as a continuum from intraoperative management. Good glycemic control may help with fighting infection, improve wound healing, which can result in better surgical outcomes. Hypoglycemia occurs because of nothing by mouth status, intraoperative administration of insulin, as well as the patient using programmable insulin pumps.
 Hypothermia can lead to an increased length of stay in PACU, lethargy, decreased minute ventilation, decreased strength, and increased cardiac demand. It is important to assure that the patient is dry and insulated. The use of air warming blankets, warming mats, and intravenous fluid warmers all minimize hypothermia.
Hypothermia can lead to an increased length of stay in PACU, lethargy, decreased minute ventilation, decreased strength, and increased cardiac demand. It is important to assure that the patient is dry and insulated. The use of air warming blankets, warming mats, and intravenous fluid warmers all minimize hypothermia.
 Many elderly patients experience a varied degree of postoperative confusion, delirium, or cognitive dysfunction in the PACU. Many pediatric patients also experience postemergence delirium leading to increased length of stay in the PACU.
Many elderly patients experience a varied degree of postoperative confusion, delirium, or cognitive dysfunction in the PACU. Many pediatric patients also experience postemergence delirium leading to increased length of stay in the PACU.
 Postoperative nausea and vomiting is a major cause of patient discomfort and dissatisfaction, as well as an aspiration risk and causes prolonged PACU stay.
Postoperative nausea and vomiting is a major cause of patient discomfort and dissatisfaction, as well as an aspiration risk and causes prolonged PACU stay.
Multimedia
 Tracheostomy
Tracheostomy
 Aspiration
Aspiration
POSTANESTHESIA RECOVERY
Each patient recovering from an anesthetic has circumstances that require an individualized problem-oriented approach. Postoperative planning begins with the preoperative evaluation and formation of an intraoperative anesthetic plan. Postanesthesia recovery must continue to adapt to meet the needs of the changing perioperative landscape, advances in technology, changing surgical techniques; and to respond to improved evidence-based research. Dissemination of anesthesia services beyond the perisurgical arena has brought changes and greater demands on recovery units.
Postanesthesia recovery must continue to adapt to meet the needs of the changing perioperative landscape, advances in technology, changing surgical techniques; and to respond to improved evidence-based research. Dissemination of anesthesia services beyond the perisurgical arena has brought changes and greater demands on recovery units.
Standards for Postanesthesia Care
The ASA House of Delegates approved Standards for Postanesthesia Care on October 12, 1988. These standards were last amended on October 27, 2004.1
VALUE AND ECONOMICS OF POSTANESTHESIA CARE UNIT
The quality of postanesthesia care is composed of many variables such as tracking of complications, time per patient spent in recovery, overall clinical outcomes, and patient satisfaction. The value of postanesthesia care is a measure of the quality of care provided compared with the amount of resources spent per patient outcome. The postanesthesia care unit (PACU) helps to use resources efficiently by having trained staff that routinely care for postsurgical patients, thereby recognizing/preventing complications, and by having physicians instituting appropriate and timely therapies.
The actual cost of PACU care incorporates costs of staffing, space, and hardware (resource utilization). Triage and discharge policies affect both how many admissions occur and what resources each admission consumes. Nurse staffing continues to be the largest direct cost in the PACU. The mix of nursing staff, experience of nurses, staffing ratios, and the complexity and duration of PACU stay affect the overall personnel cost per admission. The level of monitoring provided affects the capital expenditure for equipment, and disposable items account for operating expenditures. The patient acuity mix also determines needs for staffing and equipment such as ventilators, additional monitors, intravenous pumps, and patient-controlled analgesia pumps. The type of physician coverage—such as dedicated coverage versus on-demand coverage—can affect response time, efficiency of care, costs, and patient outcomes. The use of routine postoperative diagnostic testing and therapies without evidence-based need can lead to unnecessary treatments, increasing cost per patient and possible worse patient outcomes.
Cost comparisons between institutions are difficult because charges and cost factors vary widely across institutions, in different regions of the United States, and between countries. They constantly change over time. Regulatory requirements, standards of care, medical–legal climates, and institutional requirements vary greatly between regions and even between facilities in the same locale. It is difficult to establish cost-effectiveness goals of a single PACU because of the differing requirements of individual patients having the same procedures. This difference can be the result of levels of patient comorbidities, level of procedure complexity, surgeon, type of anesthetic, as well as patient perception and expectations. These are just some of the factors that can determine the type of care needed postoperatively. Continued pressures from many fronts to contain costs and maximize cost-effectiveness force each surgical facility to continually evaluate the value of its PACU care to each individual patient.
PACU directors are challenged to optimize clinical results while minimizing expenditures. Innovative PACU practices should guarantee safe care, minimize cost, and fulfill regulatory and institutional requirements. Medical professionals (physicians, nursing, and support staff) must work in concert to identify practices that are wasteful versus those that have proven yield/benefit. The impact of many PACU-proposed interventions on clinical outcomes are not easily substantiated by controlled scientific analysis. Useless testing, unnecessary or unjustifiable therapy, and inappropriate PACU admissions should be eliminated. However, using a more expensive therapy may generate real savings by decreasing additional therapies, testing, admissions, or length of stay. Another important element essential for patient safety and efficiency in the PACU is communication with the intraoperative anesthesiology service. Communication is perhaps the least expensive tool in medicine and the one most universally proven to be involved in human error events. Utilization of PACU resources is directly related to anesthetic duration and technique. In one study, 22.1% of 37,000 patients had a minor anesthesia-related event or complication that prolonged PACU stays and consumed PACU resources.2 Another study showed how postoperative adverse events increase the amount of nursing resources needed in the PACU.3 Close coordination between the PACU and the anesthesiology service should reduce the frequency and impact of such events.
Improvements in surgical and anesthetic techniques might create an opportunity to shorten the length of stay in the PACU, but realized change is frequently reduced by transportation delays, persistence of pain or nausea, waiting for space, or surgeon discharge delays.4 Cost-saving measures in other areas may also increase the cost of PACU care; for example, fast-tracking to discharge to home rather than to a hospital bed. The cost savings of not occupying a hospital bed is offset by an increase in PACU stay and therefore greater consumption of PACU resources.5 The savings may be cost savings for the patient and beneficial for the facility as a whole but at a greater expense to the PACU. True savings are only realized when operational changes yield a decrease in expenditures for staff, supplies, or equipment. For example, patients who are able to bypass the PACU creates a savings opportunity only if paid nursing hours are reduced or if more surgical cases are covered with the same hours. With the use of less-invasive surgical techniques combined with innovative anesthetic techniques, such as regional anesthetics, shorter PACU stays can result in real savings opportunities. However, the areas of scheduling, clerical, or maintenance tasks must not consume excess staffing hours, without savings realized. Finally, trimming costs could entail an increase in unwanted risk to patients. Differentiating between cost-effective postanesthesia care and unsafe practice remains a matter of constant professional judgment and debate daily in most PACUs.
LEVELS OF POSTOPERATIVE/POSTANESTHESIA CARE
With continued demand to increase overall healthcare efficiency, caution must be taken to provide the most appropriate care for each patient. As anesthesia services expand to cover a variety of patient types in ever-increasing areas outside the operating room, selecting the correct type of recovery is essential. For the many differing anesthesia areas ranging from inpatient surgery, ambulatory surgery, to off-site procedures, the level of postoperative care that a patient requires is determined by the degree of underlying illness, comorbidities, and the duration as well as the type of anesthesia and surgery. These factors are used to assess the risk of postoperative complications. Less-invasive surgeries or procedures combined with shorter-duration anesthetic regimens facilitate high levels of arousal and minimal cardiovascular or respiratory depression at the end of surgery.
Using a less intensive postanesthesia setting for selected patients can reduce costs for a surgical procedure and allow the facility to divert scarce PACU resources to patients with greater needs. Alert patients are more satisfied when spared the unnecessary assessments in interventions of PACU care. Amenities such as recliners, reading material, television, music, and food improve perceptions (emotional satisfaction) without affecting quality or safety. Earlier reunion with family or visitors in the low-intensity setting is desirable assuming that postoperative care is safe and appropriate.
Creation of separate PACUs for inpatients, ambulatory, or off-site patients is one possible way to streamline PACU care for appropriately triaged patients. Phase I recovery would be reserved for more intense recovery and would require more one-on-one care for staff. Phase II recovery should be less intensive and is appropriate for patients after less-invasive procedures requiring less attention from nursing while recovering. If separation of different phases of care is not possible, then providing the appropriate level of monitoring and coverage to the degree of postoperative impairment achieves similar results in a single PACU area. However, care equal to a full-intensity PACU must always be available, given the incidence of complications after anesthesia and surgery .6 As the aging population generates an increase in the complexity of surgical care in the face of tighter control of resources, maintaining appropriate PACU capacity and safety by observing applicable PACU guidelines and standards will be increasingly important.7,8
.6 As the aging population generates an increase in the complexity of surgical care in the face of tighter control of resources, maintaining appropriate PACU capacity and safety by observing applicable PACU guidelines and standards will be increasingly important.7,8
POSTANESTHETIC TRIAGE
Patients must be carefully evaluated to determine which level of care is appropriate. Triage should be based on clinical condition, length/type of procedure and anesthetic, and the potential for complications that require intervention. Alternatives to PACU care must be used in a nondiscriminatory fashion. Arbitrary criteria based on age, American Society of Anesthesiologists (ASA) classification, ambulatory versus inpatient versus off-site procedure status, or type of insurance should not be used for determining the level of recovery care. An individual patient undergoing a specific procedure or anesthetic should receive the same appropriate level of postoperative care whether the procedure is performed in a hospital operating room, an ambulatory surgical center, an endoscopy room, an invasive radiology suite, or an outpatient office. If doubt exists about a patient’s safety in a lower-intensity setting, the patient should be admitted to a higher level of care for recovery. Patient safety should always be favored regardless of the cost.
After superficial procedures using local infiltration, minor blocks, or sedation, patients can almost always recover with less intensive monitoring and coverage. Healthy patients undergoing more extensive procedures (e.g., hernia repairs, arthroscopic procedures, minor orthopedic procedures) under local, plexus, or peripheral nerve blockade might also bypass phase I recovery and go directly to phase II. The increasing use of continuous peripheral nerve catheters for surgery has shortened PACU time and can eliminate many hospital admissions.9 Innovative anesthetic techniques, advanced surgical techniques, and use of bispectral index monitoring help facilitate fast-track postoperative care.10
For more intensive procedures and patients with greater acuity, bypassing the PACU and direct admission to intensive care units (ICUs) can reduce demands on the PACU as well as reduce errors with decreased number of hand offs. This still requires proper postoperative reporting to the accepting unit including how to communicate with the surgical service and anesthesiologist. These ICUs must be trained and prepared to receive immediate postoperative patients as well as meet the standards of the PACU.
SAFETY IN THE POSTANESTHESIA CARE UNIT
The PACU medical director (every PACU should have medical oversight) must ensure that the PACU environment is as safe as possible for both patients and staff. Beyond usual safety policies, maintain staffing and training to ensure appropriate coverage and skill mix are available to deal with unforeseen crises. Incidence of adverse events in the PACU correlates with nursing workload and staff availability.3 Ideally, all staff should have PACU certification, and staffing ratios should never fall below acceptable standards.8 Less skilled or training staff must be appropriately supervised, and a sufficient number of certified personnel must always be available to handle worst-case scenarios.
The PACU staff protects patients who are temporarily incompetent and preserves patients’ rights to observance of advanced directives and to informed consent for additional procedures. The staff is obligated to optimize each patient’s privacy, dignity, and to minimize the psychological impact of unpleasant or frightening events. Observance of procedures for hand-washing, sterility, and infection control should be strictly enforced.11 Medical directors must safeguard against potential for personal assault of patients during recovery such as unwarranted restraints and procedures without consent. Access to the PACU should be strictly controlled. With increasing acceptance of reuniting patients with family/friends, safety and privacy need to be continually addressed.
The PACU environment must also be safe for professionals. Air handling should guarantee that personnel are not exposed to unacceptable levels of trace anesthetic gases, although trace gas monitoring is not necessary. Ensure that staff members receive appropriate vaccinations, including that for hepatitis B. Practitioners must adhere to policies for radiation safety, infection control, disposal of sharps, universal precautions for blood-borne diseases, and safeguarding against exposure to pathogens such as methicillin-resistant Staphylococcus, vancomycin-resistant Enterococcus, Clostridium difficile, or tuberculosis. Always keep masks, gloves, gowns, eye protection, and appropriate particulate respiratory equipment easily accessible. Following current infection control policies and guidelines are essential for patient and staff safety. Ensure that sufficient help is available to avoid injury while lifting and positioning patients or while dealing with emergence situations. Precise documentation and clear delineation of responsibility is essential for proper care of patients and can protect staff against unnecessary medicolegal exposure.
ADMISSION TO THE POSTANESTHESIA CARE UNIT
Every patient admitted to a PACU should have heart rate, rhythm, systemic blood pressure, airway patency, peripheral oxygen saturation, ventilatory rate/character, and level of pain recorded and periodically monitored.7 Assessment with periodic recording every 5 minutes for the first 15 minutes and every 15 minutes thereafter is a minimum. Document temperature, level of consciousness, mental status, neuromuscular function, hydration status, degree of nausea on admission/discharge, and more frequently if appropriate, are also minimum standards of care. Every patient should be continuously monitored with a pulse oximeter and at least a single-lead electrocardiogram (ECG). Extra leads, particularly precordial V3 to V6, are appropriate if left ventricular ischemia is likely. Capnography is necessary for patients receiving mechanical ventilation or those at risk for compromised ventilatory function. Transduction and recorded output from invasive monitors such as central venous, systemic, or pulmonary arterial catheters must be accomplished. Diagnostic (laboratory) testing should be ordered only for specific indications.
Anesthesiology personnel should manage the patient until a PACU nurse secures admission vital signs, attaches appropriate monitors, and care is transferred with a complete report to the nursing staff. A succinct but thorough report that includes sufficient information to allow rapid evaluation and intervention for postoperative complications must be legibly recorded using a standardized format printed on the PACU record (Table 54-1). This report should be similar to the OR timeout, providing patient identification, procedure performed, anesthetic type and continuing therapies. Documentation of the time and amount of all neuromuscular relaxants, respiratory depressant medications, and reversal agents should be standard. Outlined orders, specific therapeutic end points, and, most importantly, how to contact the responsible anesthesiologist all must be transmitted. The anesthesiologist should never transfer responsibility to PACU personnel until the patient’s airway status, ventilation, and hemodynamics are appropriate for the caregivers to whom he or she entrusts the patient’s care. Leaving a patient in the hands of someone unfamiliar or incapable of adequately handling the acuity of the medical situation in a rush to perform “the next case” may constitute abandonment of care. Check the function of indwelling cannulae, intravenous catheters, monitors and verify medication type and rates of any intravenous infusions before leaving.
The anesthesiologist should never transfer responsibility to PACU personnel until the patient’s airway status, ventilation, and hemodynamics are appropriate for the caregivers to whom he or she entrusts the patient’s care. Leaving a patient in the hands of someone unfamiliar or incapable of adequately handling the acuity of the medical situation in a rush to perform “the next case” may constitute abandonment of care. Check the function of indwelling cannulae, intravenous catheters, monitors and verify medication type and rates of any intravenous infusions before leaving.
POSTOPERATIVE PAIN MANAGEMENT
Relief of surgical pain with minimal side effects is a major goal during PACU care and a top priority for patients.7,12–14 Periodically assess and document level of pain throughout recovery. The Joint Commission for Accreditation of Health Organizations has mandated that a numerical pain scale be used with periodic recording and an acceptable score for discharge. Inadequate postoperative analgesia is a major source of preoperative fear/dissatisfaction for surgical patients. In addition to improving comfort, analgesia reduces sympathetic nervous system response, thereby avoiding hypertension, tachycardia, and dysrhythmias. In hypovolemic patients, the sympathetic nervous system activity may well mask relative hypovolemia. Administration of analgesics can precipitate hypotension in an apparently stable patient, especially if direct or histamine-induced vasodilation occurs. It is important to assess a tachycardic patient with low or normal blood pressure who complains of pain carefully before giving analgesics that might precipitate or accentuate hypotension.
The actual degree of postoperative pain can be difficult to establish. Severity of pain varies among surgical procedures and anesthetic techniques. Staff members are relatively ineffective at quantifying level of discomfort. Patients are able to communicate despite having received sedative hypnotic drugs. Furthermore, patients may be impaired in their communication abilities coming into the hospital or may be affected by the entire medical experience, and thereby may be afraid to express their needs. Inexperienced nurses overestimate a patient’s pain, whereas more experienced nurses tend to underestimate the pain.15 Either error can lead to inappropriate treatment. Use of a numeric pain scale yields more reliable results but requires that a patient be willing to communicate. A wide divergence can exist between a patient’s cognitive perception of pain and sympathetic nervous system response, related to psychological, cultural, and cardiovascular differences among individuals. Some patients perceive severe pain with minimal sympathetic nervous system activity, whereas others exhibit hypertension and tachycardia with minimal complaint of discomfort. The best measure of analgesia is the patient’s perception. Heart rate, respiratory rate and depth, sweating, nausea, and vomiting all may be signs of pain but their absence or presence is not in itself reliable as a measure of the presence of pain.
TABLE 54-1. COMPONENTS OF A POSTANESTHESIA CARE UNIT ADMISSION REPORT

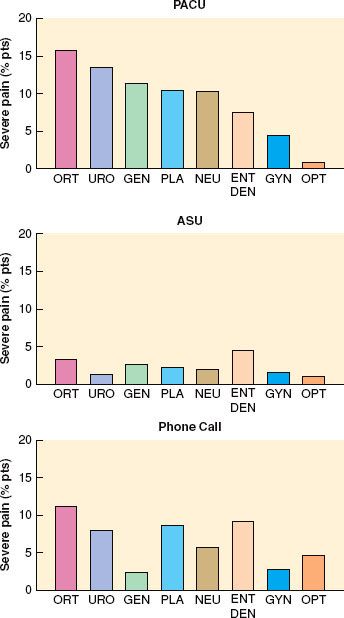
Careful identification of patient subgroups, assessment of individual analgesic requirements, and implementation of a planned, multimodal approach will provide seamless pain control through and beyond the PACU interval .16 In a study of postoperative pain in 10,008 ambulatory patients, only 5.3% related severe pain in the PACU and 1.7% in the discharge area (Fig. 54-1). However, a much higher percentage of patients relate that moderate-to-severe pain recurs after discharge.17,18 To avoid masking signs of an unrelated condition or a surgical complication, ascertain that the nature and intensity of pain are appropriate for the surgical procedure. The central nervous system (CNS) signs of hypoxemia, acidemia, or cerebral hypoperfusion often mimic those of pain, especially during emergence. Administration of parenteral analgesics or sedatives can acutely worsen hypoventilation, airway obstruction, or hypotension, causing sudden deterioration. Evaluating orientation, the level of arousal, and cardiovascular or pulmonary status usually identifies such patients.
.16 In a study of postoperative pain in 10,008 ambulatory patients, only 5.3% related severe pain in the PACU and 1.7% in the discharge area (Fig. 54-1). However, a much higher percentage of patients relate that moderate-to-severe pain recurs after discharge.17,18 To avoid masking signs of an unrelated condition or a surgical complication, ascertain that the nature and intensity of pain are appropriate for the surgical procedure. The central nervous system (CNS) signs of hypoxemia, acidemia, or cerebral hypoperfusion often mimic those of pain, especially during emergence. Administration of parenteral analgesics or sedatives can acutely worsen hypoventilation, airway obstruction, or hypotension, causing sudden deterioration. Evaluating orientation, the level of arousal, and cardiovascular or pulmonary status usually identifies such patients.
FIGURE 54-1. Percentage of patients experiencing severe pain in the postanesthesia care unit (PACU), the ambulatory surgery unit (ASU), and during phone call follow-up at 24 hours. ORT, orthopedics; URO, urology; GEN, general; PLA, plastics; NEU, neurology; ENT, ear, nose, throat; DEN, dental; GYN, gynecology; OPT, ophthalmology. (Reprinted from: Chung F, Ritchie E, Su J. Postoperative pain in ambulatory surgery. Anesth Analg. 1997;85:808, with permission.)
Surgical pain can be effectively treated with intravenous opioids as part of a planned analgesic continuum that begins prior to the induction of surgical anesthesia and continues throughout the postoperative course. Sufficient analgesia is the end point, even if large doses of opioids are necessary in tolerant patients. Short-acting opioids are useful to expedite discharge and minimize nausea in ambulatory settings,19 although duration of analgesia can be a problem. During intravenous titration of opioids, assess for incremental respiratory or cardiovascular depression. Disadvantages of intramuscular administration include larger dose requirements, delayed onset, and unpredictable uptake in hypothermic patients. Oral and transdermal analgesics have a limited role in the PACU but are helpful for ambulatory patients after PACU discharge. Rectal analgesics are sometimes useful in small children.
Perioperative use of cyclooxygenase-2 inhibitors has decreased because of adverse cardiovascular events. These events have led to the withdrawal of most of this class of drug with the exception of celecoxib, which has shown to reduce opioid requirements and the incidence of opioid adverse events.20 The concerns surrounding the negative cardiac side effects have made the overall appropriateness of this therapy more complicated. Nonselective nonsteroidal anti-inflammatory drugs such as ibuprofen or acetaminophen are also effective, especially when administered orally before surgery. Intravenous acetaminophen, now available in the United States can also lower opioid requirements. Preoperative administration likely augments the overall level of analgesia rather than offering a substantial preemptive advantage.21 Ketorolac is an effective analgesic and anti-inflammatory that lowers opioid requirements, although the possibility of hemorrhage due to its antiplatelet properties can limit its use. Although intraoperative use has not been associated with increased bleeding, preoperative oral administration has some evidence of postoperative hemorrhage. Ketorolac might also decrease ischemic events in patients with coronary artery disease through analgesic and antiplatelet actions. Use of clonidine to supplement analgesia is effective but can cause hypotension. Agonist–antagonist analgesics offer little advantage. Interventions such as repositioning, reassurance, or extubation also help minimize discomfort.
Other analgesic modalities provide pain relief in and beyond the PACU.22 Intravenous opioid loading in the PACU is important for smooth transition to intravenous patient-controlled analgesia. Injection of opioids into the epidural or subarachnoid space during anesthesia or in the PACU yields prolonged postoperative analgesia in selected patients.23,24 Nausea and pruritus are troubling side effects, and immediate or delayed ventilatory depression can occur related to vascular uptake and cephalad spread in cerebrospinal fluid. Nausea should resolve with antiemetics, whereas pruritus and ventilatory depression often respond to naloxone infusion. Addition of local anesthetic or clonidine enhances analgesia and decreases the risk of side effects from epidural opioids, although local anesthetics add risk of hypotension or motor blockade. Epidural analgesia is effective after thoracic and upper abdominal procedures and helps wean obese patients or those with chronic obstructive pulmonary disease (COPD) from mechanical ventilation. Whether epidural analgesia improves surgical outcomes remains debatable.
Continuous flow catheters with pressure delivery systems of local anesthetics have been used intrawound to reduce pain and opioid requirements, increase patient satisfaction, and reduce length of hospital stay.25 These same delivery systems have been safely used with continuous peripheral nerve catheters for in-hospital as well as outpatient use.26,27 With the use of ultrasound-guided techniques for catheter placement, appropriately selected outpatients can safely receive the pain control benefits of regional anesthesia.9 However, extensive written and oral postoperative instructions must be provided, with systems in place for 24-hour access by patients for catheter-related complications.
Placement of long-acting regional analgesic blocks reduces pain, controls sympathetic nervous system activity, and often improves ventilation.22 After shoulder procedures, interscalene block yields almost complete pain relief with only moderate inconvenience from motor impairment. Paralysis of the ipsilateral diaphragm can impair postoperative ventilation in patients with marginal reserve, although the impact is small in most patients.28 Suprascapular nerve block might be an alternative to avoid this potentially serious side effect. Percutaneous intercostal or paravertebral blocks reduce analgesic requirements after thoracic, breast, or high abdominal incision, although beneficial effects on postoperative pulmonary function are questionable. Transversus abdominis plane (TAP) blocks are effective for lower abdominal surgeries as well as those innervated by the ilioinguinal and iliohypogastric nerves. Caudal analgesia or paravertebral blocks can also be effective in children after inguinal or genital procedures, whereas infiltration of local anesthetic into joints, soft tissues, or incisions decreases the intensity of pain. Other modalities, such as guided imagery, hypnosis, transcutaneous nerve stimulation, music, massage, or acupuncture, have limited utility for surgical pain but may provide a positive patient experience.
Use of patient-controlled analgesia, spinal opioids, or neural blockade mandates anticipation of risk beyond the PACU. The plan for extended postoperative analgesia should be prepared before induction of surgical anesthesia, and then orient the anesthetic and PACU care toward that plan. These plans should be in agreement with the patient, surgeon, and anesthesiologist. If one analgesic modality proves inadequate, take particular care when implementing a second technique.
Fear, anxiety, and confusion often accentuate postoperative pain during recovery, especially after general anesthesia. Titration of an intravenous sedative such as midazolam attenuates this psychogenic component, although analgesic requirements may increase slightly because benzodiazepines interact with γ-aminobutyric acid receptors. It is important to distinguish between requirements for analgesia and for anxiolysis. Opioids are poor sedatives and anxiolytics, whereas benzodiazepines are poor analgesics. However, when opioid dose appears larger than what might be anticipated as what the patient should require, one should consider the possibility that anxiety is playing a large role in the dysphoric event in the PACU.
DISCHARGE CRITERIA
When possible before discharge from postoperative care, each patient should be sufficiently oriented to assess his or her physical condition and be able to summon assistance. Airway reflexes and motor function must be adequate to maintain patency and prevent aspiration. One should ensure that ventilation and oxygenation are acceptable, with sufficient reserve to cover minor deterioration in unmonitored settings. Blood pressure, heart rate, and indices of peripheral perfusion should be relatively constant for at least 15 minutes and appropriately near baseline. Achieving normal body temperature is not an absolute requirement, but there should be resolution of shivering. Acceptable analgesia must be achieved and vomiting appropriately controlled. Patients should be observed for at least 15 minutes after the last intravenous opioid or sedative is administered to assess peak effects and side effects. If regional anesthetics have been administered, longer observation could be appropriate. One should monitor oxygen saturation for 15 minutes after discontinuation of supplemental oxygen to detect hypoxemia and then assess likely complications of surgery (e.g., bleeding, vascular compromise, pneumothorax) or of underlying conditions (e.g., hypertension, myocardial ischemia, hyperglycemia, bronchospasm). One should also document a brief neurologic assessment of orientation, eye signs, facial symmetry, and extremity movement and review results of diagnostic tests. If these generic criteria cannot be met, postponement of discharge or transfer to a specialized unit is advisable. There is no demonstrable benefit from a mandatory minimum duration of PACU care.
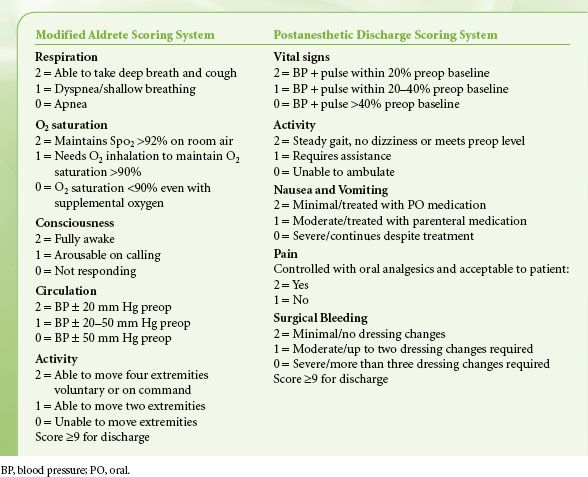
Scoring systems such as the Modified Aldrete Score or Postanesthesia Discharge Scoring System (Table 54-2) are two commonly used systems for patient assessment and attempt to simplify and standardize patient discharge criteria. Fixed PACU discharge criteria must be used with caution because variability among patients is tremendous. Scoring systems that quantify physical status or establish thresholds for vital signs are useful for assessment but cannot replace individual evaluation.29,30 Ideally, each patient should be evaluated for discharge by an anesthesiologist using a consistent set of criteria, considering the severity of underlying disease, the anesthetic and recovery course, and the level of care at the destination  (Table 54-2). A plan for the continued management of likely postdischarge symptoms such as pain, nausea, headache, dizziness, drowsiness, and fatigue must be made prior to discharge.18
(Table 54-2). A plan for the continued management of likely postdischarge symptoms such as pain, nausea, headache, dizziness, drowsiness, and fatigue must be made prior to discharge.18
TABLE 54-2. TWO MOST COMMONLY USED POSTANESTHESIA CARE UNIT DISCHARGE CRITERIA SYSTEMS
POSTOPERATIVE EVALUATION
The Centers for Medicare and Medicaid Services (CMS) have instituted compliance policies for those entities that participate in the Medicare and Medicaid programs. The policy for postanesthesia follow-up requires a written documentation that is performed by an individual that is qualified to administer anesthesia no later than 48 hours postprocedure. The time frame starts as soon as the patient arrives to the recovery area or ICU. The evaluation should be performed only after the patient has sufficiently recovered from anesthesia to be able to participate such as answer questions or perform simple tasks. The postanesthesia evaluation must contain the following elements:
 Respiratory function, including respiratory rate, airway patency, and oxygen saturation
Respiratory function, including respiratory rate, airway patency, and oxygen saturation
 Cardiovascular function, including pulse rate and blood pressure
Cardiovascular function, including pulse rate and blood pressure
 Mental status
Mental status
 Temperature
Temperature
 Pain
Pain
 Nausea and vomiting
Nausea and vomiting
 Postoperative hydration.
Postoperative hydration.
CARDIOVASCULAR COMPLICATIONS
The purpose of this section is not to entirely review all the possible cardiovascular events that might beset a patient in the PACU, rather it is to help the reader decide what events might be unique to the PACU. The cardiac risks during the postoperative stay include myocardial ischemia, which may be minimized with continued use of β-blockers, analgesia, nitrates, supplemental oxygen, adequate circulating volume, oxygen-carrying capacity, heart rate control, and an understanding of hypercoagulable states. In the PACU, some reflexes previously blunted by general anesthetics, sedatives, and opioids return toward baseline revealing an unexpected cardiovascular compromise. Perhaps the two most common types of patients to encounter troubles will be the patient with coronary artery disease and the patient with congestive heart failure. In the PACU it is a rare event for a patient to complain, de novo, of anginal type chest pain. The patients have significant blood opioid levels, and endorphins may be high because of the operation. The anesthetic makes the sensorium dulled and dysfunctional. The first sign of myocardial ischemia may well be hypotension, and the use of newer sedation techniques using drugs like dexmedetomidine can lead to hypotension postoperatively which can cloud the picture of a patient’s cardiac disease. The most common sign of myocardial ischemia is tachycardia. Tachycardia is very often a reaction to, not the cause of, myocardial ischemia. That does not mean that all tachycardia heralds myocardial ischemia, but in a patient who seems at risk for coronary artery disease, new-onset tachycardia that is not caused by pain should be taken seriously. The ECG may show classic ST–T wave elevation or depression depending on lead placement and area of ischemia. But the lack of ST–T wave elevation does not rule out coronary artery disease. Transmural myocardial infarctions outside the PACU show no ECG diagnostic changes 10% to 30% of the time. So the clinician must be especially suspicious of a series of hemodynamic changes in a person at risk for coronary artery disease. Early intervention with nitrates, opioids, β-blockers, and even anticoagulants may save a life. Cardiology should be involved to gain immediate and timely access to the cardiac catheterization laboratory or for angiolytic drug therapy. Involvement and communication with the surgical service must be immediate and decisions especially as to anticoagulation and lytic therapy should be made among several services in consultation. Thus, cardiac ischemia in the PACU may manifest subtly! With increasing use of bare metal and drug-eluting stents (DES), recognition of those patients who have stopped antiplatelet therapy and are in postoperative hypercoagulable states can quickly occlude these stents. This situation requires quick recognition and response for intervention in the cath lab.
In the PACU, some reflexes previously blunted by general anesthetics, sedatives, and opioids return toward baseline revealing an unexpected cardiovascular compromise. Perhaps the two most common types of patients to encounter troubles will be the patient with coronary artery disease and the patient with congestive heart failure. In the PACU it is a rare event for a patient to complain, de novo, of anginal type chest pain. The patients have significant blood opioid levels, and endorphins may be high because of the operation. The anesthetic makes the sensorium dulled and dysfunctional. The first sign of myocardial ischemia may well be hypotension, and the use of newer sedation techniques using drugs like dexmedetomidine can lead to hypotension postoperatively which can cloud the picture of a patient’s cardiac disease. The most common sign of myocardial ischemia is tachycardia. Tachycardia is very often a reaction to, not the cause of, myocardial ischemia. That does not mean that all tachycardia heralds myocardial ischemia, but in a patient who seems at risk for coronary artery disease, new-onset tachycardia that is not caused by pain should be taken seriously. The ECG may show classic ST–T wave elevation or depression depending on lead placement and area of ischemia. But the lack of ST–T wave elevation does not rule out coronary artery disease. Transmural myocardial infarctions outside the PACU show no ECG diagnostic changes 10% to 30% of the time. So the clinician must be especially suspicious of a series of hemodynamic changes in a person at risk for coronary artery disease. Early intervention with nitrates, opioids, β-blockers, and even anticoagulants may save a life. Cardiology should be involved to gain immediate and timely access to the cardiac catheterization laboratory or for angiolytic drug therapy. Involvement and communication with the surgical service must be immediate and decisions especially as to anticoagulation and lytic therapy should be made among several services in consultation. Thus, cardiac ischemia in the PACU may manifest subtly! With increasing use of bare metal and drug-eluting stents (DES), recognition of those patients who have stopped antiplatelet therapy and are in postoperative hypercoagulable states can quickly occlude these stents. This situation requires quick recognition and response for intervention in the cath lab.
Congestive heart failure is epidemic in our ever-aging population. The outpatient cardiology services have an expanding armamentarium of new inotropic/vasodilator therapy, devices, and interventions that allow patients to compensate for their congestive heart failure. One should know not only the ejection fraction but the activities of daily living, exercise tolerance, and other risk indices. The ejection fraction is only an estimate of the fractional shortening of the myocardial actin and myosin fibrils. Although it is a useful estimate of severity of impairment, one is struck by how stable some patients may be with a large dilated heart contracting at a 15% ejection fraction. They are compensated but have little reserve. The potential problems of bleeding, volume shifts, and respiratory compromise in the PACU could quickly cause decompensation. There are also no absolute numbers with regard to fluid restriction. The usage of transesophageal echocardiography revolutionized cardiac anesthesia. It, along with transthoracic echo, may be of great use in the PACU. Within a very few minutes a puzzling hypotensive situation might be explained by an echocardiogram. In the fast-paced dynamic environment of the PACU, placing a pulmonary artery (PA) catheter may give useful information, but may also take valuable time away from patient triage and treatment. The echocardiogram allows rapid viewing of myocardial contractility, regional wall motion, volume status, and valvular dysfunction.
The PACU has in the recent history taken on a new role in some hospitals. Cardiac surgical care is pushing toward “early extubation” or “fast-tracking.” In years past, especially when a “cardiac anesthetic” involved very large dosages of semisynthetic opioids that obligated patients to ≥24 hours of ventilation, the ICU was the standard place for all postoperative heart patients. Today, there is no such entity as a cardiac anesthetic. Balanced anesthetic techniques are used most often. Those who write about early extubation have pushed the limits from 24 hours all the way to extubation of patients on the operating table. Series are available with few if any reintubation catastrophes or events when this technique is practiced with good teams. The natural extension is to establish some highly specialized PACUs that function as step-down or short-term ICUs. In a study of 85 prospective patients31 undergoing “off-pump” coronary artery bypass graft procedures, the patients were extubated in 12 ± 2 minutes after the chest was closed. They were then taken to a special part of the PACU where they were monitored for a number of hours (up to 480 minutes in some situations). Patients were then either discharged to the cardiac floors or sent to an ICU. Of the 85 patients in this study, only 4 failed the PACU stay and had to be admitted to an ICU. Bradycardia was the cause for failure in three cases and one there was one case of myocardial infarction. Two patients later returned to the ICU from the cardiac ward; there was one case of atrial fibrillation and another case of myocardial infarction. During the same time 304 patients who were not undergoing off-pump coronary artery bypass graft surgery were admitted to the cardiac ICU. The cost for PACU stay was $5,140.00 less than for an ICU-admitted patient. Although this study seems quite favorable, the two groups of patients were not comparable.
Studies from the mid-to-late 1990s looking at high-risk vascular and thoracic surgery patients showed that they could each be adequately cared for in an adequately staffed and prepared PACU.32 The conclusion was that a hospital could well improve its patient throughput by putting more resources into expanded PACU care and not so much into ICU services. Several nursing reviews are available to give input as to how to structure such new units.33,34
Anesthesiology services are in increased demand throughout most hospitals. The PACU will likely need to prepare to care for those patients or to staff “ectopic” sites. In the evoked potential laboratories, for example, ablation procedures for dysrhythmias and the newer “mini-Maze” procedures may require care in the PACU. Automated implantable defibrillators are placed in hybrid suites, operating rooms, or catheterization laboratories. Now there is the possibility of percutaneous valve replacements as well as some hybrid and percutaneous coronary revascularization procedures. If these patients require deep sedation or general anesthesia, the patient will also require PACU care.
The cardiac patient is the common patient today. The new procedures and pressure to ever streamline operating room care is pressuring the PACU to become more and more a cardiac mini-ICU. The smart PACU medical director and hospital administrator will see that with targeted resources, patients may well be safely cared for in a more cost-effective manner with quicker throughput by using a PACU approach.
POSTOPERATIVE PULMONARY DYSFUNCTION
Mechanical, hemodynamic, and pharmacologic factors related to surgery and anesthesia impair ventilation, oxygenation, and airway maintenance.35 Heavy smoking, obesity, sleep apnea, severe asthma, and COPD increase the risk of postoperative ventilatory events.36 Preoperative pulmonary function testing has limited predictive value for postoperative complications,37 perhaps with the exception of postoperative bronchospasm in smokers.38
Inadequate Postoperative Ventilation
In PACU patients, mild respiratory acidemia due to atelectasis and decreased minute ventilation is expected; thus, elevated PaCO2 does not necessarily indicate inadequate postoperative ventilation. Inadequate ventilation should be suspected when (1) respiratory acidemia occurs coincident with tachypnea, anxiety, dyspnea, labored ventilation, or increased sympathetic nervous system activity; (2) hypercarbia reduces the arterial pH below 7.30; or (3) PaCO2 progressively increases with a progressive decrease in arterial pH.
Inadequate Respiratory Drive
During early recovery from anesthesia, residual effects of intravenous and inhalation anesthetics blunt the ventilatory responses to both hypercarbia and hypoxemia. Sedatives augment depression from opioids or anesthetics and reduce the conscious desire to ventilate (a significant component of ventilatory drive).
Hypoventilation and hypercarbia can evolve insidiously during transfer and admission to the PACU. Although effects of intraoperative medications are usually waning, the peak depressant effect of an intravenous opioid given just before transfer occurs in the PACU. Coincident depression of medullary centers that regulate the sympathetic nervous system can blunt signs of acidemia or hypoxemia such as hypertension, tachycardia, and agitation, concealing hypoventilation. Patients might communicate lucidly and even complain of pain while experiencing significant opioid-induced hypoventilation. A balance must be struck between an acceptable level of postoperative ventilatory depression and a tolerable level of pain or agitation. Patients with abnormal CO2/pH responses from morbid obesity, chronic airway obstruction, or sleep apnea are more sensitive to respiratory depressants.39 Risk for apnea after anesthesia in preterm infants depends on type of anesthetic, postconceptual age, and preoperative hematocrit. Preterm infants should be monitored for at least 12 hours (see Chapter 41). Children with active or recent upper respiratory infection are more prone to breath-holding, severe cough, and arterial desaturations below 90% during recovery, especially if they have a history of reactive airway disease or secondhand smoke exposure or have undergone intubation and/or airway surgery.40 If hypoventilation from opioids is excessive, forced arousal and careful titration (20 to 40 μg at a time) of intravenous naloxone reverses respiratory depression without affecting analgesia. Flumazenil (0.1 mg titrated to effect up to 1.5 mg) directly reverses depressant effects of benzodiazepines on ventilatory drive but is usually unnecessary.
The abrupt diminution of a noxious stimulus (e.g., tracheal extubation, placement of a postoperative block) may promote hypoventilation or airway obstruction by altering the balance between arousal from discomfort and depression from medication. Intracranial hemorrhage or edema sometimes presents with hypoventilation, especially after posterior fossa craniotomy. Bilateral carotid body injury after endarterectomy can ablate peripheral hypoxic drive. Chronic respiratory acidemia from COPD alters CNS sensitivity to pH and makes hypoxic drive dominant, but hypoventilation from supplemental oxygen rarely occurs.
Increased Airway Resistance
High resistance to gas flow through airways increases work of breathing and CO2 production. If inspiratory muscles cannot generate sufficient pressure gradients to overcome resistance, alveolar ventilation fails to match CO2 production and progressive respiratory acidemia occurs.
In postoperative patients, increased upper airway resistance is caused by obstruction in the pharynx (posterior tongue displacement, change in anteroposterior and lateral dimensions from soft-tissue collapse), in the larynx (laryngospasm, laryngeal edema), or in the large airways (extrinsic compression from hematoma, tumor, or tracheal stenosis). Weakness from residual neuromuscular relaxation,41 myasthenia gravis or myasthenic syndromes can contribute, but it is seldom the primary cause of airway compromise. If the airway is clear of vomitus or foreign bodies, simple maneuvers such as improving the level of consciousness, lateral positioning, chin lift, mandible elevation, or placement of an oropharyngeal or nasopharyngeal airway usually relieve obstruction. A nasopharyngeal airway is better tolerated when the patient has functional gag reflexes. Acute extrinsic upper airway compression (e.g., an expanding neck hematoma) must be relieved.
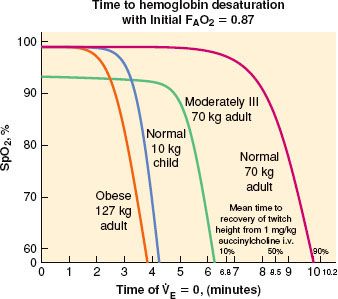
During emergence, stimulation of the pharynx or vocal cords by secretions, blood, foreign matter, or extubation can generate laryngospasm.42 Laryngeal constrictor muscles occlude the tracheal inlet and reduce gas flow. Patients who smoke or are chronically exposed to smoke have irritable airway conditions, have copious secretions, or have undergone upper airway surgery are at higher risk.35,40 Laryngospasm can usually be overcome by providing gentle positive pressure (10 to 20 mm Hg continuous) in the oropharynx by mask with 100% O2. Prolonged laryngospasm is relieved with a small dose of succinylcholine (e.g., 0.1 mg/kg) or deepening sedation with propofol. An intubating dosage of succinylcholine should not be used to break postoperative laryngospasm, especially if the alveolar partial pressure of oxygen (PAO2) is decreased by hypoventilation. As little as 5 to 10 mg of succinylcholine can break the laryngospasm. Unless assisted ventilation is provided, declining PAO2 causes serious hypoxemia before spontaneous ventilation resumes43 (Fig. 54-2). If the functional residual capacity (FRC) is abnormally reduced, the decreased volume of O2 available in the lungs accelerates the development of hypoxemia. Severe laryngeal obstruction can occur secondarily because of hypocalcemia after parathyroid excision.
FIGURE 54-2. Rate of SpO2 decline after onset of apnea. (Reprinted from: Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology. 1997;87:979, with permission.)
Soft-tissue edema worsens airway obstruction, especially in children and adults recovering from procedures on the neck. Nebulized vasoconstrictors like epinepherine help somewhat, but steroids have little effect acutely. Patients with C1 esterase inhibitor deficiency can develop severe angioneurotic edema after even slight trauma to the airway. Pathologic airway obstruction (e.g., severe edema, epiglottitis, retropharyngeal abscess, encroaching tumors) might require emergency tracheal intubation, but airway manipulation is dangerous because minor trauma from intubation attempts can convert a marginal airway into a total obstruction. Judgment by the individual anesthesiologist regarding timing, patient status, available equipment along with airway management skills all play a part of the decision as to where, when, and how to intubate. Sedatives or muscle relaxants used to facilitate intubation can worsen obstruction by compromising the patient’s volitional efforts to maintain the airway and by eliminating spontaneous ventilation. Equipment and personnel necessary for emergency cricothyroidotomy or  tracheostomy should be available. Needle cricothyroidotomy using a 14-gauge intravenous catheter or a commercially available kit permits oxygenation and marginal ventilation until the airway is secured, especially if jet ventilation with 100% oxygen is used.
tracheostomy should be available. Needle cricothyroidotomy using a 14-gauge intravenous catheter or a commercially available kit permits oxygenation and marginal ventilation until the airway is secured, especially if jet ventilation with 100% oxygen is used.
Reduction of cross-sectional area in small airways increases overall airway resistance because resistance varies inversely with the fourth power of radius during laminar flow and with the fifth power during turbulent flow. Pharyngeal or tracheal stimulation from secretions, suctioning, aspiration, or a tracheal tube can trigger a reflex constriction of bronchial smooth muscle in emerging patients with reactive airways. Histamine release precipitated by medication or allergic reactions also increases airway smooth muscle tone. Decreased radial traction on small airways reduces cross-sectional area in patients with COPD or with decreased lung volume secondary to obesity, surgical manipulation, excessive lung water, or splinting. Preoperative spirometric evidence of increased airway resistance predicts an increased risk of postoperative bronchospasm.38 Smokers and patients with bronchospastic conditions are at highest risk.44 If ventilatory requirements are increased by warming, hyperthermia, or work of breathing, high flow rates convert laminar flow to higher-resistance turbulent flow. Prolonged expiratory time or audible turbulent air flow (wheezing) during forced vital capacity expiration often unmasks subclinical airway resistance. (Resistance is higher during expiration because intermediate-diameter airways are compressed by positive intrathoracic pressure.) High airway resistance does not always cause wheezing because flow might be so impeded that no sound is produced. Signs of increased resistance mimic those of decreased pulmonary compliance. Spontaneously breathing patients exhibit accessory muscle recruitment, labored ventilation, and increased work of breathing with either condition. Mechanically ventilated patients exhibit high peak inspiratory pressures.
The treatment of small airway resistance is directed at an underlying etiology. One must eliminate laryngeal or airway stimulation. Patients often respond to their pre-existing regimen of albuterol, pirbuterol, or salmeterol inhalers. Levalbuterol or metaproterenol nebulized in oxygen resolves postoperative bronchospasm with minimal tachycardia. Nebulized racemic epinephrine effectively relaxes smooth muscle, but side effects of tachycardia and flushing can be seen. Isoproterenol has also been nebulized with good results. Intramuscular or sublingual terbutaline can be added. Administration of steroid therapy offers little acute improvement, but may prevent later recurrence. Bronchospasm that is resistant to β2-sympathomimetic medication may improve with an anticholinergic medication such as atropine or ipratropium. If bronchospasm is life-threatening, an intravenous epinephrine infusion yields profound bronchodilation. Increased small airway resistance caused by mechanical factors (e.g., loss of lung volume, retained secretions, pulmonary edema) usually does not resolve with bronchodilators. Restoration of lung volume with incentive spirometry or deep tidal ventilation increases radial traction on small airways. Reducing left ventricular filling pressures might relieve airway resistance caused by increased lung water, although interstitial fluid accumulation can persist. Also, extended contraction of airway smooth muscle obstructs venous and lymphatic flow, leading to airway wall edema that resolves slowly.
Decreased Compliance
Reduced pulmonary compliance accentuates the work of breathing. In the extreme, low compliance causes progressive respiratory muscle fatigue, hypoventilation, and respiratory acidemia. Parenchymal changes also affect compliance. Reduction of FRC leads to small airway closure and distal lung collapse, requiring greater energy expenditure to re-expand the lung. Pulmonary edema increases the lung’s weight and inertia and elevates surface tension by interfering with surfactant activity, making expansion more difficult. Pulmonary contusion or hemorrhage interferes with lung expansion, as do restrictive lung diseases, skeletal abnormalities, intrathoracic lesions, hemothorax, pneumothorax, or cardiomegaly. Obesity affects pulmonary compliance, especially when adipose tissue compresses the thoracic cage or increases intra-abdominal pressure in supine or lateral positions. Extrathoracic factors such as tight muscles of the chest or abdominal dressings and gas in the stomach or bowel reduce compliance. Most notably after intra-abdominal laparoscopic procedures, retained CO2 may impair diaphragm movement. The CO2 has the capability to dissecting into the thorax creating either a pneumothorax or pneumomediastinum, which is usually a self-limited event as the CO2 is relatively rapidly absorbed. There is usually no need for chest tube intervention. An intra-abdominal tumor, hemorrhage, ascites, bowel obstruction, or pregnancy impairs diaphragmatic excursion and reduces compliance.
Work of breathing is improved by resolving problems that reduce compliance. Allowing patients to recover in a semisitting (semi-Fowler) position reduces work of breathing. Incentive spirometry and chest physiotherapy help restore lung volume, as does positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP). In patients with COPD and highly compliant lungs, positive airway pressure might force the rib cage and diaphragms toward their excursion limits, accentuating inspiratory muscular effort.
Neuromuscular and Skeletal Problems
Postoperative airway obstruction and hypoventilation are accentuated by incomplete reversal of neuromuscular relaxation. Residual paralysis compromises airway patency, ability to overcome airway resistance, airway protection, and ability to clear secretions.45 In the extreme, paralysis precludes effective spontaneous ventilation. Intraoperative use of shorter-acting relaxants might decrease the incidence of residual paralysis but does not eliminate the problem. Marginal reversal can be more dangerous than near-total paralysis because a weak, agitated patient exhibiting uncoordinated movements and airway obstruction is more easily identified. A somnolent patient exhibiting mild stridor and shallow ventilation from marginal neuromuscular function might be overlooked, allowing insidious hypoventilation and respiratory acidemia or regurgitation with aspiration to occur. PACU staff should be aware of patients who have received nondepolarizing muscle relaxants but no reversal agents because they often exhibit low levels of residual paralysis.46 Safety of techniques designed to avoid reversal of short- and intermediate-duration relaxants has not been substantiated, and reversal of nondepolarizing relaxants is recommended.7 The selective relaxant binding agent used widely in Europe but currently unavailable in the United States, γ-cyclodextrins (i.e., Sugammadex), is a promising reversal drug that can avoid the side effects of other anticholinesterases and anticholinergics.47 Patients with neuromuscular abnormalities such as myasthenia gravis, Eaton–Lambert syndrome, periodic paralysis, or muscular dystrophies exhibit exaggerated or prolonged responses to muscle relaxants. Even without relaxant administration, these patients can exhibit postoperative ventilatory insufficiency. Medications potentiate neuromuscular relaxation (e.g., antibiotics, furosemide, propranolol, phenytoin), as does hypocalcemia or hypermagnesemia.
Diaphragmatic contraction is compromised in some postoperative patients, forcing more reliance on intercostal muscles and reducing the ability to overcome decreased compliance or increased ventilatory demands. Impairment of phrenic nerve function from interscalene block, trauma, or thoracic and neck operations can “paralyze” one or rarely both diaphragms.28 Adequate ventilation will normally be maintained with only one diaphragm, and marginal ventilation by external intercostal muscles alone. However, with high work of breathing, muscle weakness, or increased ventilatory demands, a nonfunctional diaphragm impairs minute ventilation. Thoracic spinal or epidural blockade interferes with intercostal muscle function and reduces ventilatory reserve, especially in patients with COPD. Abnormal motor neuron function (e.g., Guillain–Barré syndrome, cervical spinal cord trauma), flail chest, or severe kyphosis or scoliosis can cause postoperative ventilatory insufficiency.
Simple tests help assess mechanical ability to ventilate. The ability to sustain head elevation in a supine position, a forced vital capacity of 10 to 12 mL/kg, an inspiratory pressure more negative than −25 cm H2O, and tactile train-of-four assessment imply that strength of ventilatory muscles is adequate to sustain ventilation and to take a large enough breath to cough. However, none of these clinical end points reliably predicts recovery of airway protective reflexes,46 and failure on these tests does not necessarily indicate the need for assisted ventilation.
The use of noninvasive mechanical ventilation techniques such as continuous positive airway pressure or bilevel ventilation can help safely extubate some patients earlier or prevent reintubating others. By using these noninvasive airway techniques, patients can often overcome some of the above discussed issues interfering with normal respiration. Thus reducing the risk of remaining intubated or reintubation. Units other than ICUs are able to manage these patients therefore offloading the burden on the ICU.
Occasionally, a clinical picture suggests ventilatory insufficiency when ventilation is adequate. Voluntary limitation of chest expansion to avoid pain (splinting) causes labored, rapid, shallow breathing characteristic of inadequate ventilation. Splinting seldom causes actual hypoventilation and usually improves with analgesia and repositioning. Ventilation with small tidal volumes due to thoracic restriction or reduced compliance seems to generate afferent input from pulmonary stretch receptors, leading to dyspnea, labored breathing, and accessory muscle recruitment in spite of appropriate minute ventilation. Occasional large, “satisfying” lung expansions often relieve these symptoms. Finally, spontaneous hyperventilation to compensate for a metabolic acidemia might generate tachypnea or labored breathing, which is mistaken for ventilatory insufficiency.
Increased Dead Space
Ventilation of unperfused dead space or of poorly perfused alveoli with high ventilation/perfusion (V/Q) ratios is less effective in removing CO2. Expansion of dead space volume or reduction of tidal volume increases the fraction of each breath wasted in dead space VD/VT and the amount of CO2 from the previous exhalation that is rebreathed. A proportionally larger increase in total minute ventilation is required to meet any increase in CO2 production. Patients with high VD/VT are at greater risk for postoperative ventilatory failure.
Occasionally, an acute increase in dead space contributes to respiratory acidemia in postoperative patients. Although upper airway dead space is reduced after tracheal intubation and tracheostomy, excessive tubing volume or valve reversal in breathing circuits promotes rebreathing of CO2. PEEP or CPAP elevates physiologic dead space, especially in patients with high pulmonary compliance. Pulmonary embolization with air, thrombus, or cellular debris increases physiologic dead space, although impact on CO2 excretion is often compensated by accelerated minute ventilation from hypercarbic and hypoxic drives or reflex responses. Decreased cardiac output can transiently increase VD/VT by decreasing perfusion to well-ventilated, nondependent lung. Irreversible increases in dead space occur if adult respiratory distress syndrome (ARDS) related to sepsis, transfusion-related acute lung injury (TRALI), or hypoxia destroys pulmonary microvasculature. Dead space may appear high if an inhalation interrupts the previous exhalation and the spent alveolar gas is retained. This “gas trapping” occurs when high airway resistance lengthens the time required to exhale completely, or if improper inspiration/expiration ratios or high ventilatory rates are used during mechanical ventilation.
Increased Carbon Dioxide Production
Carbon dioxide production varies directly with metabolic rate, body temperature, and substrate availability. During anesthesia, CO2 production falls to approximately 60% of the normal 2 to 3 mL/kg/min as hypothermia lowers metabolic activity and neuromuscular relaxation reduces tonic muscle contraction. Therefore, during recovery, metabolic rate and CO2 production can increase by 40%. Shivering, high work of breathing, infection, sympathetic nervous system activity, or rapid carbohydrate metabolism during intravenous hyperalimentation accelerates CO2 production. Malignant hyperthermia generates CO2 production many times greater than normal, which rapidly exceeds ventilatory reserve and causes severe respiratory and metabolic acidemia. Even mild increases of CO2 production can precipitate respiratory acidemia if low compliance, airway resistance, or neuromuscular paralysis interferes with ventilation. With the exception of adjusting hyperalimentation, improving work of breathing, reducing shivering, or treating hyperthermia, there is little yield from addressing CO2 production in PACU patients.
Inadequate Postoperative Oxygenation
Systemic arterial partial pressure of oxygen (PaO2) is the best indicator of pulmonary oxygen transfer from alveolar gas to pulmonary capillary blood. Arterial hemoglobin saturation monitored by pulse oximetry yields less information on alveolar–arterial gradients and is not helpful in assessing impact of hemoglobin dissociation curve shifts or carboxyhemoglobin.48 Evaluation of metabolic acidemia or mixed venous oxygen content yields insight into peripheral oxygen delivery and utilization. Adequate arterial oxygenation does not mean that cardiac output, arterial perfusion pressure, or distribution of blood flow will maintain tissue oxygenation. Sepsis, hypotension, anemia, or hemoglobin dissociation abnormalities can generate tissue ischemia despite adequate oxygenation.
In postoperative patients, the acceptable lower limit for PaO2 varies with individual patient characteristics. A PaO2 below 65 to 70 mm Hg causes significant hemoglobin desaturation, although tissue oxygen delivery might be maintained at lower levels. Maintaining PaO2 between 80 and 100 mm Hg (saturation: 93% to 97%) ensures adequate oxygen availability. Little benefit is derived from elevating PaO2 above 110 mm Hg because hemoglobin is saturated and the amount of additional oxygen dissolved in plasma is negligible. During mechanical ventilation, a PaO2 above 80 mm Hg with 0.4 FIO2 and 5 cm H2O PEEP,48 CPAP or spontaneous breathing trial usually predicts sustained adequate oxygenation after tracheal extubation.
Distribution of Ventilation
Loss of dependent lung volume commonly causes V/Q mismatching and hypoxemia. A reduction in FRC decreases radial traction on small airways, leading to collapse and distal atelectasis that can worsen for 36 hours after surgery.49 Reduced ventilation in dependent lung is particularly damaging because gravity directs pulmonary blood flow to dependent areas. Obese patients sustain large decreases in FRC during surgery. Older patients normally exhibit some airway closure at end expiration, and those with COPD have more severe closure that is exacerbated by small reductions in FRC. Retraction, packing, manipulation, or peritoneal insufflation during upper abdominal surgery reduces FRC, as does compression from leaning surgical assistants.50 Prone, lithotomy, or Trendelenburg positions are disadvantageous, especially in obese patients. Right upper lobe collapse secondary to partial right main stem intubation is a frequently overlooked cause. During one-lung anesthesia, the weight of unsupported mediastinal contents, pressure from abdominal contents on the dependent diaphragm, and lung compression all reduce dependent lung volume. Gravity and lymphatic obstruction promote interstitial fluid accumulation and further V/Q mismatching. This “down lung syndrome” may appear as unilateral pulmonary edema on the chest film.
Postoperatively, acute pulmonary edema from overhydration, ventricular dysfunction, airway obstruction, or increased capillary permeability (e.g., including transfusion-related acute lung injury, drug reactions) leads to hypoxemia by interfering with both V/Q matching and diffusion of oxygen. Strong inspiratory efforts against an obstructed airway decrease FRC and promote negative-pressure pulmonary edema. Small airway occlusion from compression, retained secretions, or aspiration leads to distal hypoventilation and hypoxemia, as does main stem intubation. Pneumothorax or hemothorax also reduce lung volume.
Conservative measures that restore lung volume often improve oxygenation. If possible, patients should recover in a semisitting or reverse Trendelenberg position to reduce abdominal pressure on the diaphragms. Pain with ventilation encourages shallow breathing, so analgesia helps maintain FRC, especially with upper abdominal or chest wall incisions. Deep ventilation, cough, chest physiotherapy, and incentive spirometry seem to help expand FRC, mobilize secretions, and accustom a patient to incisional discomfort, but actual efficacy is debated.51,52 For serious postoperative reduction of FRC, positive pressure is effective. CPAP (5 to 7 cm H2O) or bilevel can be delivered by face mask for several hours until factors promoting loss of lung volume resolve. If hypoxemia is severe or patient acceptance of CPAP or the often more tolerable bilevel mask is poor, tracheal intubation is usually required. Intubation for delivery of noninvasive ventilation does not mandate positive-pressure ventilation. Ventilatory requirements should be assessed independently, considering PaCO2, arterial pH, and work of breathing. Usually, 5 to 10 cm H2O of CPAP or PEEP improves PaO2 without risking hypotension, increased intracranial pressure, or barotrauma. If PaO2 does not improve, one must re-evaluate the etiology. An occasional patient with ARDS or pulmonary contusion might require expiratory pressures in excess of 10 cm H2O for improved oxygenation.
Tracheal intubation eliminates normal expiratory resistance and the “physiologic PEEP” (2 to 5 cm H2O) that helps maintain lung volume during spontaneous ventilation. Exposing an intubated trachea to ambient pressure may cause a gradual reduction in FRC. Healthy, slender patients will often tolerate short periods of intubation without positive pressure, but generally it is prudent to use 5 cm H2O CPAP for intubated postoperative patients.
Distribution of Perfusion
Poor distribution of pulmonary blood flow also interferes with V/Q matching and oxygenation. Flow distribution is primarily determined by hydrodynamic factors (PA and venous pressures, vascular resistance), which are affected by gravity, airway pressure, lung volume, and cardiac dynamics. Flow distribution is modulated by hypoxic pulmonary vasoconstriction (HPV), which diverts flow from air spaces that exhibit low PaO2. In postoperative patients, position affects oxygenation if gravity forces blood flow to areas with reduced ventilation. For example, placing a poorly ventilated lung in a dependent position can reduce PaO2. Postoperative changes in PA pressure, airway pressure, and lung volume also have complex effects on blood flow distribution that can adversely affect V/Q matching. Residual inhalation anesthetics, vasodilators, and sympathomimetics directly affect vascular tone and HPV, partially explaining larger alveolar–arterial oxygen gradients after general anesthesia. (Changes in distribution of ventilation also contribute.) Patients with liver cirrhosis exhibit poor V/Q matching caused by small arteriovenous shunts that form throughout their lungs. Circulating endotoxin impairs HPV, contributing to hypoxemia in septic patients.
In the PACU, few interventions are useful to improve V/Q matching by changing the distributions pulmonary blood flow. Maintain PA and airway pressures within an acceptable range. When possible, avoid placing an atelectatic or diseased lung in a dependent position. Placing poorly ventilated parenchyma in a nondependent position could improve V/Q matching, but positioning a diseased lung in an “up” position may promote drainage of purulent material into the unaffected lung. Avoiding vasodilatory medications may improve PaO2 but benefits from the medication usually outweigh drawbacks of impaired HPV.
Inadequate Alveolar PAO2
Postoperative hypoxemia is occasionally caused by a global reduction of PAO2, usually from inadequate ventilation, and marked increase in PACO2 (see the alveolar gas equation in Chapter 11). Hypoventilation must be severe to cause hypoxemia based on the alveolar gas equation. Complete apnea or airway obstruction by a foreign body, soft-tissue edema, or laryngospasm as well as very high small airway resistance all lead to rapid depletion of alveolar oxygen, and precludes effective ventilation. If cessation of ventilation does occur, the rate of PAO2 decline varies with age, body habitus, degree of underlying illness, and initial PAO243 (Fig. 54-3). Hypoxemia might also occur if opioids or residual anesthetic levels severely depress ventilatory drives.  Partial airway obstruction does not usually reduce PAO2, especially when patients are receiving supplemental oxygen. Increasing the oxygen content of the FRC with supplemental oxygen safeguards against hypoxemia from hypoventilation or airway obstruction, and eliminates the use of the pulse oximeter as a monitor of hypoventilation. Rarely, excessive concentrations of other gases reduce PAO2. After general anesthesia, rapid outpouring of nitrous oxide displaces alveolar gas and can lower PAO2 if a patient is hypoventilating or breathing ambient air, but this “diffusion hypoxia” would usually occur before PACU admission. Volume displacement of oxygen could also occur during severe hypercarbia in a patient breathing ambient air, although acidemia is often a greater problem.
Partial airway obstruction does not usually reduce PAO2, especially when patients are receiving supplemental oxygen. Increasing the oxygen content of the FRC with supplemental oxygen safeguards against hypoxemia from hypoventilation or airway obstruction, and eliminates the use of the pulse oximeter as a monitor of hypoventilation. Rarely, excessive concentrations of other gases reduce PAO2. After general anesthesia, rapid outpouring of nitrous oxide displaces alveolar gas and can lower PAO2 if a patient is hypoventilating or breathing ambient air, but this “diffusion hypoxia” would usually occur before PACU admission. Volume displacement of oxygen could also occur during severe hypercarbia in a patient breathing ambient air, although acidemia is often a greater problem.
FIGURE 54-3. SpO2 versus postanesthesia care unit time in patients spontaneously breathing room air after general anesthesia (group 1, 0 to 1 year of age; group 2, 1 to 3 years; group 3, 3 to 14 years; group 4, 14 to 58 years). (Reprinted from: Xue FS, Huang YG, Tong SY, et al. A comparative study of early postoperative hypoxemia in infants, children, and adults undergoing elective plastic surgery. Anesth Analg. 1996;83:709, with permission.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







