The goals of a preoperative evaluation are to reduce patient risk and morbidity associated with surgery and coexisting diseases, promote efficiency and reduce costs, as well as to prepare the patient medically and psychologically for surgery and anesthesia.
 The Joint Commission requires that all patients receive a preoperative anesthetic evaluation, and the American Society of Anesthesiologists (ASA) published a Practice Advisory for Preanesthesia Evaluation in 2002 and Approved Basic Standards for Preoperative Care, which were reaffirmed in 2010.
The Joint Commission requires that all patients receive a preoperative anesthetic evaluation, and the American Society of Anesthesiologists (ASA) published a Practice Advisory for Preanesthesia Evaluation in 2002 and Approved Basic Standards for Preoperative Care, which were reaffirmed in 2010.
 It is important for the evaluation to be complete, accurate, and clear, not only to allow the information to be relayed to others who may care for the patient perioperatively, but also for medicolegal purposes.
It is important for the evaluation to be complete, accurate, and clear, not only to allow the information to be relayed to others who may care for the patient perioperatively, but also for medicolegal purposes.
 The preoperative evaluation serves as a screening tool to anticipate and avoid airway difficulties or problems with anesthetic drugs. In addition to the history and physical examination, previous anesthesia records should be reviewed. Contraindications to specific drugs, such as succinylcholine, nitrous oxide, or volatile agents, should be sought.
The preoperative evaluation serves as a screening tool to anticipate and avoid airway difficulties or problems with anesthetic drugs. In addition to the history and physical examination, previous anesthesia records should be reviewed. Contraindications to specific drugs, such as succinylcholine, nitrous oxide, or volatile agents, should be sought.
 Review of the patient’s allergies and medication list, including over-the-counter and herbal medications, should specifically screen for latex allergy and potential drug interactions. It should also alert the anesthesiologist to the need for steroid coverage.
Review of the patient’s allergies and medication list, including over-the-counter and herbal medications, should specifically screen for latex allergy and potential drug interactions. It should also alert the anesthesiologist to the need for steroid coverage.
 When evaluating the patient with hypertension, diabetes mellitus, or obesity it is important to determine the presence of end-organ damage such as cardiovascular disease.
When evaluating the patient with hypertension, diabetes mellitus, or obesity it is important to determine the presence of end-organ damage such as cardiovascular disease.
 Exercise tolerance is the most important determinant of cardiac risk. Algorithms exist for preoperative evaluation of cardiac patients undergoing noncardiac surgery and are useful guides for further testing and evaluation.
Exercise tolerance is the most important determinant of cardiac risk. Algorithms exist for preoperative evaluation of cardiac patients undergoing noncardiac surgery and are useful guides for further testing and evaluation.
 Preoperative laboratory tests should be ordered on the basis of positive findings from the history and physical examination or anticipated physiologic disturbances during surgery such as blood loss.
Preoperative laboratory tests should be ordered on the basis of positive findings from the history and physical examination or anticipated physiologic disturbances during surgery such as blood loss.
 Optimization of the patient’s health status prior to surgery includes clear instruction regarding nothing by mouth times as well as which medications to administer immediately before surgery. In general, most medications for hypertension or cardiac disease should be continued, and consideration should be given to titrating an appropriate dose of beta-blocker in patients at risk. The need for subacute bacterial endocarditis prophylaxis should be anticipated. Likewise, drugs for asthma or chronic obstructive pulmonary disease should be continued or administered prophylactically. Medications taken for the treatment of reflux should be continued, or initiated for those patients with untreated symptoms. For diabetic patients, oral hypoglycemic agents should often be held, but patients requiring insulin will need to continue to take adjusted doses.
Optimization of the patient’s health status prior to surgery includes clear instruction regarding nothing by mouth times as well as which medications to administer immediately before surgery. In general, most medications for hypertension or cardiac disease should be continued, and consideration should be given to titrating an appropriate dose of beta-blocker in patients at risk. The need for subacute bacterial endocarditis prophylaxis should be anticipated. Likewise, drugs for asthma or chronic obstructive pulmonary disease should be continued or administered prophylactically. Medications taken for the treatment of reflux should be continued, or initiated for those patients with untreated symptoms. For diabetic patients, oral hypoglycemic agents should often be held, but patients requiring insulin will need to continue to take adjusted doses.
 Although preoperative sedation is generally limited to drugs given immediately prior to anesthesia, the timing of administration must be planned when oral sedation is needed in children to allow optimal effect and avoid operating room delays.
Although preoperative sedation is generally limited to drugs given immediately prior to anesthesia, the timing of administration must be planned when oral sedation is needed in children to allow optimal effect and avoid operating room delays.
Multimedia
 Airway Exam
Airway Exam
 The goals of preoperative evaluation are to reduce patient risk and the morbidity of surgery, as well as to promote efficiency and reduce costs.
The goals of preoperative evaluation are to reduce patient risk and the morbidity of surgery, as well as to promote efficiency and reduce costs.  The Joint Commission requires that all patients receive a preoperative anesthetic evaluation. The American Society of Anesthesiologists (ASA) published on their web site an updated Practice Advisory for Preanesthesia Evaluation in 2003 and also reaffirmed the ASA Basic Standards for Preanesthetic Care in 2010, which outline the minimum requirements for a preoperative evaluation. The most recent ASA Practice Guidelines can be found at http://www.asahq.org. Conducting a preoperative evaluation is based on the premise that it will modify patient care and improve outcome. There is evidence, although not entirely convincing in all instances, that the preoperative evaluation will increase patient safety. That is, armed with knowledge preoperatively, the anesthesiologist can formulate and conduct an anesthetic plan that avoids dangers inherent in patient disease states. Furthermore, preoperative evaluations may reduce costs and cancellation rates, increasing resource utilization in the operating room (OR). This notion assumes that evaluations are done by anesthesiologists or other health-care providers familiar with anesthesia, surgery, and perioperative events.
The Joint Commission requires that all patients receive a preoperative anesthetic evaluation. The American Society of Anesthesiologists (ASA) published on their web site an updated Practice Advisory for Preanesthesia Evaluation in 2003 and also reaffirmed the ASA Basic Standards for Preanesthetic Care in 2010, which outline the minimum requirements for a preoperative evaluation. The most recent ASA Practice Guidelines can be found at http://www.asahq.org. Conducting a preoperative evaluation is based on the premise that it will modify patient care and improve outcome. There is evidence, although not entirely convincing in all instances, that the preoperative evaluation will increase patient safety. That is, armed with knowledge preoperatively, the anesthesiologist can formulate and conduct an anesthetic plan that avoids dangers inherent in patient disease states. Furthermore, preoperative evaluations may reduce costs and cancellation rates, increasing resource utilization in the operating room (OR). This notion assumes that evaluations are done by anesthesiologists or other health-care providers familiar with anesthesia, surgery, and perioperative events.
The preoperative evaluation has several components and goals. One should review the available medical record, obtain a history, and perform a physical examination pertinent to the patient and contemplated surgery. On the basis of the history and physical examination, the appropriate laboratory tests and preoperative consultations should be obtained. Through these, one needs to determine whether the patient’s preoperative condition may be improved prior to surgery. Guided by these factors, the anesthesiologist should choose the appropriate anesthetic and care plan. Finally, the process should be used to educate the patient about anesthesia and the perioperative period, answer all questions, and obtain informed consent.
The first part of this chapter outlines clinical risk factors pertinent to patients scheduled for anesthesia and surgery and the use of tests to confirm diagnoses. The second part discusses preoperative medication. The chapter provides only an overview of the preoperative management process; for more details, the reader is referred to chapters focusing on specific organ systems.
CHANGING CONCEPTS IN PREOPERATIVE EVALUATION
In the past, patients were admitted to the hospital at least a day prior to surgery. Currently, more and more patients are admitted to the hospital from the postanesthesia care unit. Older patients are scheduled for more complex procedures, and there is more pressure on the anesthesiologist to reduce the time between cases. The first time that the anesthesiologist performing the anesthetic sees the patient may be just prior to anesthesia and surgery. Others may have seen the patient previously in a preoperative evaluation clinic. Only a short time exists to engender trust and answer last-minute questions. It is often impossible to alter medical therapy immediately preoperatively. However, preoperative screening clinics are becoming more effective and clinical practice guidelines are becoming more prevalent. Information technology has helped the anesthesiologist in previewing the upcoming patients who will be anesthetized. Preoperative questionnaires and computer-driven programs have become alternatives to traditional information gathering. Finally, when anesthesiologists are responsible for ordering preoperative laboratory tests, cost saving occurs and cancellations of planned surgical procedures become less likely. In this setting it is important that there is communication between the preoperative evaluation clinic and the anesthesiologist performing the anesthetic.
APPROACH TO THE HEALTHY PATIENT
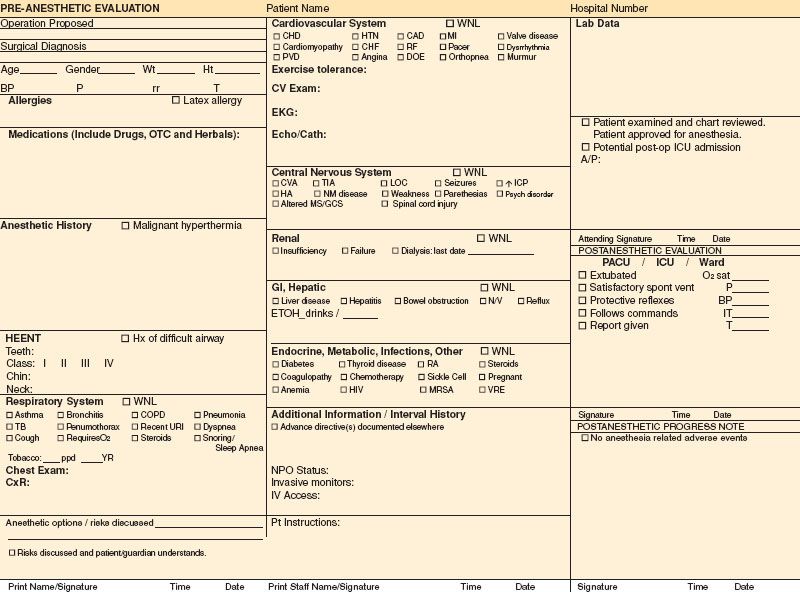
Standardization of best clinical practices may be enhanced by process control practices. In this regard, the preoperative evaluation form can serve as the basis for formulating the best anesthetic plan tailored to the patient.  It should aid the anesthesiologist in identifying potential complications, increase consistency in best care practices, as well as serve as a medicolegal document. The importance of the design has increased as it is more common today for the evaluation to be completed in a preoperative clinic by another physician or health professional who may not personally perform the anesthetic, but also because regulatory agencies such as Joint Commission on Accreditation of Healthcare Organizations (JCAHO) demand better documentation. Therefore, the information obtained needs to be complete, concise, and legible. In those hospitals that have electronic medical records, legibility is no longer an issue. A group from University of California, San Diego, studied the quality of preoperative evaluation forms across the United States and rated them in three categories: informational content, ease of use, and ease of reading.1 Their results revealed that a surprisingly high percentage of forms are missing important information. While most anesthesiology departments will transition to electronic records, Figure 22-1 is an example of a preoperative evaluation form in paper format, which gives a concise view of the subjects that should be covered, with pertinent information listed in check boxes.
It should aid the anesthesiologist in identifying potential complications, increase consistency in best care practices, as well as serve as a medicolegal document. The importance of the design has increased as it is more common today for the evaluation to be completed in a preoperative clinic by another physician or health professional who may not personally perform the anesthetic, but also because regulatory agencies such as Joint Commission on Accreditation of Healthcare Organizations (JCAHO) demand better documentation. Therefore, the information obtained needs to be complete, concise, and legible. In those hospitals that have electronic medical records, legibility is no longer an issue. A group from University of California, San Diego, studied the quality of preoperative evaluation forms across the United States and rated them in three categories: informational content, ease of use, and ease of reading.1 Their results revealed that a surprisingly high percentage of forms are missing important information. While most anesthesiology departments will transition to electronic records, Figure 22-1 is an example of a preoperative evaluation form in paper format, which gives a concise view of the subjects that should be covered, with pertinent information listed in check boxes.
FIGURE 22-1. Example of preanesthetic evaluation form.
The approach to the patient should always begin with a thorough history and physical examination. These two evaluations alone may be sufficient (without additional routine laboratory tests).
Indication for the Surgical Procedure. This is part of the preoperative history because it will help determine the urgency of the surgery. True emergency procedures, which are associated with a recognized higher anesthetic morbidity and mortality, require a more abbreviated evaluation. A less defined area is the approach to urgent procedures. For example, ischemic limbs require surgery soon after presentation, but can usually be delayed for 24 hours for further evaluation. The indication for the surgical procedure may also have implications on other aspects of perioperative management. For example, the presence of a small bowel obstruction has implications regarding the risk of aspiration and the need for a rapid sequence induction. The extent of a lung resection will dictate the need for further pulmonary testing and perioperative monitoring. Patients undergoing carotid endarterectomy may require a more extensive neurologic examination, as well as testing to rule out coronary artery disease (CAD). The planned procedure also dictates patient positioning and often whether blood products will be necessary. Frequently, further information will be required that necessitates contacting the surgeon. Perioperative care of the patient, as well as efficiency in the OR, is always enhanced by close communication with the surgeons.
Response to Previous Anesthetics.  The ability to review previous anesthetic records is helpful in detecting the presence of a difficult airway, a history of malignant hyperthermia (MH), and the individual’s response to surgical stress and specific anesthetics. The patient should be questioned regarding any previous difficulty with anesthesia or other family members having difficulty with anesthesia. A patient history relating an “allergy” to anesthesia should make one suspicious for MH. In those patients diagnosed with MH or as MH susceptible, not only will it affect the anesthetic regimen, but it should also bring into question the appropriateness of outpatient surgery.
The ability to review previous anesthetic records is helpful in detecting the presence of a difficult airway, a history of malignant hyperthermia (MH), and the individual’s response to surgical stress and specific anesthetics. The patient should be questioned regarding any previous difficulty with anesthesia or other family members having difficulty with anesthesia. A patient history relating an “allergy” to anesthesia should make one suspicious for MH. In those patients diagnosed with MH or as MH susceptible, not only will it affect the anesthetic regimen, but it should also bring into question the appropriateness of outpatient surgery.
Perhaps not life threatening, but persistent nausea and vomiting after a previous surgery may be the patient’s most negative and lasting memory. There are multiple predictors for postoperative nausea and vomiting, including the type of surgical procedure, the anesthetic agents, as well as patient factors. A risk score for predicting postoperative nausea and vomiting after inhalation anesthesia identified four risk factors: female gender, prior history of motion sickness or postoperative nausea, nonsmoking, and the use of postoperative opioids. The investigators suggested prophylactic antiemetic therapy when two or more of the risk factors were present when using volatile anesthetics.2,3 However, armed with this knowledge preoperatively, the anesthesiologist is able to tailor the anesthetic or possibly avoid general anesthesia and opioids altogether.
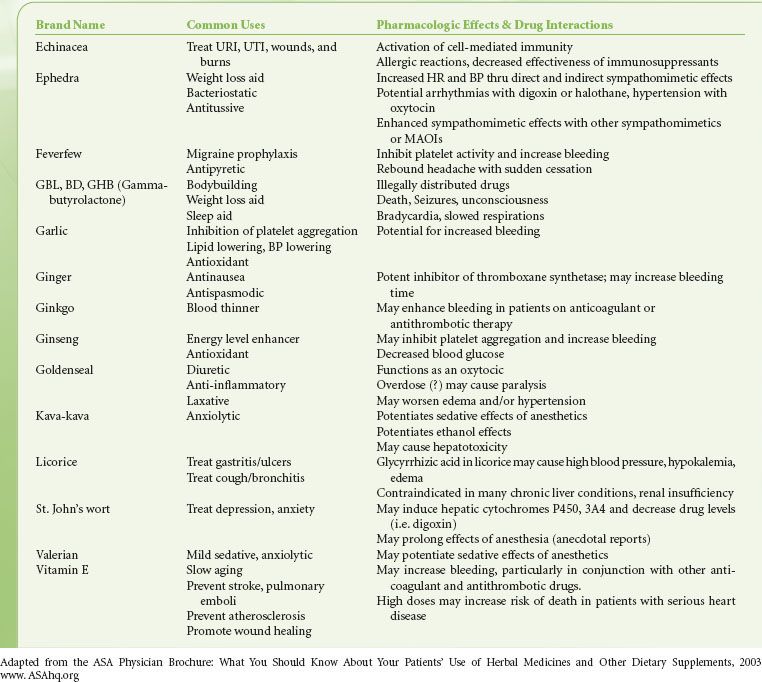
Medications/Allergies. The  history should include a complete list of medications, including over-the-counter and herbal products (Table 22-1), to define a preoperative medication regimen, anticipate potential drug interactions, and provide clues to underlying disease. A complete list of drug allergies, including previous reactions, should be obtained, as well as an inquiry concerning reaction to latex.
history should include a complete list of medications, including over-the-counter and herbal products (Table 22-1), to define a preoperative medication regimen, anticipate potential drug interactions, and provide clues to underlying disease. A complete list of drug allergies, including previous reactions, should be obtained, as well as an inquiry concerning reaction to latex.
TABLE 22-1. HERBAL/DIETARY SUPPLEMENTS AND DRUG INTERACTIONS
If the patient presents on the day of surgery, the anesthesiologist should determine when the patient last ate, as well as note the sites of preexisting intravenous cannulae and invasive monitors. Once the general issues are completed, the preoperative history and physical examination can focus on specific systems.
Screening Patients Using a Systems Approach
Airway
At the forefront of every anesthesiologist’s mind is the concern about the patient’s airway. Questions to address include whether there is potential for difficulty in maintaining a patent airway with a mask and a laryngeal mask airway or in the ability to place an endotracheal tube when the patient is under general anesthesia. The ability to review previous anesthetic records is especially useful in uncovering an unsuspected “difficult airway” or to confirm previous uneventful intubations, noting whether the patient’s body habitus has changed in the interim. Patients should be questioned about their ability to breathe through their nose, whether there is suspected or diagnosed obstructive sleep apnea (OSA), and whether they have orthopnea. Evaluation of the airway involves examination of the oral cavity including dentition, determination of the thyromental distance, assessment of the size of the patient’s neck and scanning for tracheal deviation or masses, as well as evaluation of their ability to flex the base of the neck and extend the head. For trauma patients or patients with severe rheumatoid arthritis or Down syndrome, assessment of the cervical spine is critical. The presence of symptoms or signs of cervical cord compression should be assessed. In some instances, radiographic examination may also be required.
The Mallampati classification has become the standard for assessing the relationship of the tongue size relative to the oral cavity (Table 22-2),4 although by itself the Mallampati classification has a low positive predictive value in identifying patients who are difficult to intubate.5,6 Intubation involves multiple steps: Flexion of the neck, extension of the head, opening the mouth to insert the laryngoscope, and displacing the tongue forward and down into the submandibular space to expose the glottis. Therefore, a multifactorial approach to predict intubation difficulty as shown in Table 22-3 has proven more helpful. One must keep in mind that factors that predict a difficult intubation are not necessarily the same factors that predict a difficult mask airway. For example, the absence of teeth clearly makes laryngoscopy less difficult, but at the same time can make maintaining a mask airway more challenging.
TABLE 22-2. MODIFIED MALLAMPATI AIRWAY CLASSIFICATION SYSTEM

TABLE 22-3. COMPONENTS OF THE AIRWAY EXAMINATION WHICH SUGGEST DIFFICULTY WITH INTUBATION

Pulmonary System
A screening evaluation should include questions regarding the history of tobacco use, dyspnea, exercise tolerance, cough, wheezing, inhaler use, recent upper respiratory tract infection, stridor, and snoring or sleep apnea. Physical examination should assess the respiratory rate as well as the chest excursion, use of accessory muscles, nail color, and the patient’s ability to carry on a conversation or to walk without dyspnea. Auscultation should be used to detect decreased breath sounds, wheezing, stridor, or rales. For the patient with positive findings, see the section on the preoperative evaluation of the patient with pulmonary disease.
Cardiovascular System
 When screening a patient for cardiovascular disease prior to surgery, the anesthesiologist is most interested in recognizing the signs and symptoms of uncontrolled hypertension and unstable cardiac disease such as myocardial ischemia, congestive heart failure, valvular heart disease, and significant cardiac dysrhythmias. Symptoms of cardiovascular disease should be carefully determined, especially the characteristics of chest pain, if present. Certain populations of patients, such as the elderly, women, or diabetics, may present with more atypical features. The presence of unstable angina has been associated with a high perioperative risk of myocardial infarction (MI).7 The perioperative period is associated with a hypercoagulable state and surges in endogenous catecholamines, both of which may exacerbate the underlying process in unstable angina, increasing the risk of acute infarction. The preoperative evaluation can affect a patient’s short- and long-term health by instituting treatment of unstable angina. Symptoms of clinically important valvular disease should be sought, such as angina, dyspnea, syncope, or congestive heart failure that would require further evaluation. Importantly, the anesthesiologist must identify patients who have undergone coronary artery stent or cardiac implantable device placement to be able to coordinate perioperative management with the cardiologist (see section on patient with cardiovascular disease).
When screening a patient for cardiovascular disease prior to surgery, the anesthesiologist is most interested in recognizing the signs and symptoms of uncontrolled hypertension and unstable cardiac disease such as myocardial ischemia, congestive heart failure, valvular heart disease, and significant cardiac dysrhythmias. Symptoms of cardiovascular disease should be carefully determined, especially the characteristics of chest pain, if present. Certain populations of patients, such as the elderly, women, or diabetics, may present with more atypical features. The presence of unstable angina has been associated with a high perioperative risk of myocardial infarction (MI).7 The perioperative period is associated with a hypercoagulable state and surges in endogenous catecholamines, both of which may exacerbate the underlying process in unstable angina, increasing the risk of acute infarction. The preoperative evaluation can affect a patient’s short- and long-term health by instituting treatment of unstable angina. Symptoms of clinically important valvular disease should be sought, such as angina, dyspnea, syncope, or congestive heart failure that would require further evaluation. Importantly, the anesthesiologist must identify patients who have undergone coronary artery stent or cardiac implantable device placement to be able to coordinate perioperative management with the cardiologist (see section on patient with cardiovascular disease).
The anesthesiologist should also be familiar with the American Heart Association (AHA) web site (http://www.heart.org/), which has links to the latest AHA statements and guidelines for health professionals. Here, one can find the most recent recommendations regarding which patients and procedures require subacute bacterial endocarditis prophylaxis.8
The examination of the cardiovascular system should include blood pressure evaluation, measuring both arms when appropriate. The anesthesiologist should take into account the effects of preoperative anxiety and may want a record of resting blood pressure measurements. However, Bedford and Feinstein9 reported that the admission blood pressure was the best predictor of HR and BP response to laryngoscopy. Auscultation of the heart is performed, specifically listening for a murmur radiating to the carotids suggestive of aortic stenosis, abnormal rhythms, or a gallop suggestive of heart failure. The presence of bruits over the carotid arteries would warrant further workup to determine the risk of stroke. The extremities should be examined for the presence of peripheral pulses to exclude peripheral vascular disease or congenital cardiovascular disease.
Neurologic System
A screening of the neurologic system in the apparently healthy patient can be accomplished through simple observation. The patient’s ability to answer health history questions practically ensures a normal mental status. Questions can be directed regarding a history of stroke and to exclude the presence of cerebrovascular disease, seizure history, preexisting neuromuscular disease, or nerve injuries. The neurologic examination may be cursory in healthy patients or extensive in patients with coexisting disease. Testing of strength, reflexes, and sensation may be important in patients if the anesthetic plan or surgical procedure may result in a change in the condition.
Endocrine System
Each patient should be questioned for symptoms that suggest endocrine diseases that may affect the perioperative course: diabetes mellitus, thyroid disease, parathyroid disease, endocrine-secreting tumors, and adrenal cortical suppression.
EVALUATION OF THE PATIENT WITH KNOWN SYSTEMIC DISEASE
Cardiovascular Disease
The preoperative evaluation of the patient with suspected cardiovascular disease has been approached in two ways: Clinical risk indices and preoperative cardiac testing. The goals are to define risk, determine which patients will benefit from further testing, form an appropriate anesthetic plan, and identify patients who will benefit from perioperative beta-blockade, interventional therapy, or even surgery. Clinical risk indices range from the physical status index of the ASA (Table 22-4) to the Goldman Cardiac Risk Index, which has recently been updated.
TABLE 22-4. AMERICAN SOCIETY OF ANESTHESIOLOGISTS (ASA) PHYSICAL STATUS (PS) CLASSIFICATION

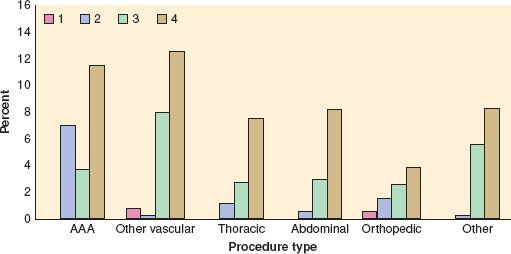
In an update of the Goldman Cardiac Risk Index, the investigators studied 4,315 patients aged 50 years and older who were undergoing elective, major noncardiac procedures.10 Six independent predictors of complications were identified and included in a revised risk index: high-risk type of surgery, history of ischemic heart disease, history of congestive heart failure, history of cerebrovascular disease, preoperative treatment with insulin, and preoperative serum creatinine >2.0 mg/dL. Cardiac complications rose with an increase in the number of risk factors present. Rates of major cardiac complications with 0, 1, 2, or 3 of these factors were 0.5%, 1.3%, 4%, and 9%, respectively, in the derivation cohort and 0.4%, 0.9%, 7%, and 11%, respectively, among 1,422 patients in the validation cohort (Fig. 22-2).
FIGURE 22-2. Cardiac risk index (CRI). Bars represent rate of major cardiac complications in entire patient population (both derivation and validation cohorts combined) for patients in revised CRI classes according to the type of procedure performed. Note that, by definition, patients undergoing abdominal aortic aneurysm (AAA), thoracic, and abdominal procedures were excluded from class I. In all subsets except patients undergoing AAA, there was a statistically significant trend toward greater risk with higher-risk class. See text for details. (Reproduced from Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043, with permission.)
While all of these indices provide information to assess the probability of complications and provide an estimate of risk, they do not prescribe perioperative management. In contrast, the anesthesiologist is most concerned with forming an anesthetic plan after defining the cardiovascular risk factors.
In patients with symptomatic coronary disease, the preoperative evaluation may reveal a change in the frequency or pattern of anginal symptoms. Certain populations of patients—for example, the elderly, women, or diabetics—may present with more atypical features. The presence of unstable angina has been associated with a high perioperative risk of MI.7
In virtually all studies, the presence of active congestive heart failure preoperatively has been associated with an increased incidence of perioperative cardiac morbidity.11,12 Stabilization of ventricular function and treatment for pulmonary edema are important prior to elective surgery. Because the type of perioperative monitoring and treatments would be different, clarifying the cause of heart failure is important. Congestive symptoms may be a result of nonischemic cardiomyopathy or cardiac valvular insufficiency and/or stenosis.
Adults with a prior MI almost always have CAD. Traditionally, risk assessment for noncardiac surgery was based on the time interval between the MI and surgery. Multiple older studies have demonstrated an increased incidence of reinfarction if the MI was within 6 months of surgery.13–15 With improvements in perioperative care, this difference has decreased. Therefore, the importance of the intervening time interval may no longer be valid in the current era of interventional therapy and risk stratification after an acute MI. Although many patients with an MI may continue to have myocardium at risk for subsequent ischemia and infarction, other patients may have their critical coronary stenoses either totally occluded or widely patent. For example, the use of percutaneous transluminal coronary angioplasty, thrombolysis, and early coronary artery bypass grafting (CABG) has changed the natural history of the disease.16,17 Therefore, patients should be evaluated from the perspective of their risk for ongoing ischemia. The American Heart Association (AHA)/American College of Cardiology (ACC) Task Force on Perioperative Evaluation of the Cardiac Patient Undergoing Noncardiac Surgery has defined patient risk groups—based on clinical predictors (Table 22-5).18
TABLE 22-5. CLINICAL PREDICTORS OF INCREASED PERIOPERATIVE CARDIOVASCULAR RISK (MYOCARDIAL INFARCTION, CONGESTIVE HEART FAILURE, DEATH)

Identifying Patients at Risk for Cardiac Disease
For those patients without overt symptoms or history, the probability of CAD varies with the type and number of atherosclerotic risk factors present. Peripheral arterial disease has been shown to be associated with CAD in multiple studies.19
Diabetes Mellitus
Diabetes mellitus is a common disease with a pathophysiology that affects multiple organ systems. Complications of diabetes mellitus are frequently the cause of urgent or emergent surgery, especially in the elderly. Diabetes accelerates the progression of atherosclerosis, so it is not surprising that diabetics have a higher incidence of CAD than nondiabetics. There is a high incidence of both silent MI and myocardial ischemia.20 Eagle et al.21 demonstrated that diabetes is an independent risk factor for perioperative cardiac morbidity. The duration of the disease and other associated end-organ dysfunction may alter the overall cardiac risk. Autonomic neuropathy has been reported as the best predictor of silent CAD.22 Because these patients are at very high risk for a silent MI, an electrocardiogram (ECG) should be obtained to examine for the presence of Q waves.
Hypertension
Hypertension has also been associated with an increased incidence of silent myocardial ischemia and infarction.20 Hypertensive patients who have left ventricular hypertrophy and are undergoing noncardiac surgery are at a higher perioperative risk than nonhypertensive patients.23
Investigators have suggested that the presence of a strain pattern on ECG suggests a chronic ischemic state.24 Therefore, these patients should also be considered to have an increased probability of CAD and for perioperative morbidity.
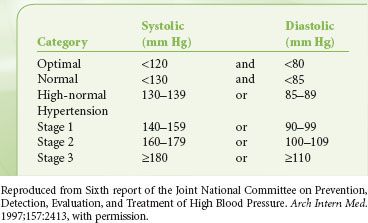
There is controversy regarding a trigger to delay or cancel a surgical procedure in a patient with untreated or inadequately treated hypertension. Hypertension has been divided into three stages, with stage 3 denoting that which might be used as a cutoff (Table 22-6).25 Aggressive treatment of blood pressure is associated with increased reduction in long-term risk, although the effect diminishes in all but diabetic patients as diastolic blood pressure is reduced below 90 mm Hg. Although there has been a suggestion in the literature that a case should be delayed if the diastolic pressure is >110 mm Hg, the study often quoted as the basis for this determination demonstrated no major morbidity in that small group of patients.26 Other authors state that there is little association between blood pressures of <180 mm Hg systolic or 110 mm Hg diastolic and postoperative outcomes. However, such patients are prone to perioperative myocardial ischemia, ventricular dysrhythmias, and lability in blood pressure. It is less clear in patients with blood pressures above 180/110 mm Hg, although no absolute evidence exists that postponing surgery will reduce risk.27,28 In the absence of end-organ changes, such as renal insufficiency or left ventricular hypertrophy with strain, the benefits of optimizing blood pressure must be weighed against the risks of delaying surgery.
TABLE 22-6. BLOOD PRESSURE
Other Risk Factors
Several other factors associated with atherosclerosis have been used to suggest an increased probability of CAD. These include tobacco use and hypercholesterolemia. Although these risk factors increase the probability of developing CAD, they have not been shown to increase perioperative cardiac risk. When attempting to determine the overall probability of disease, the number of risk factors and severity of each are important.
Importance of Surgical Procedure
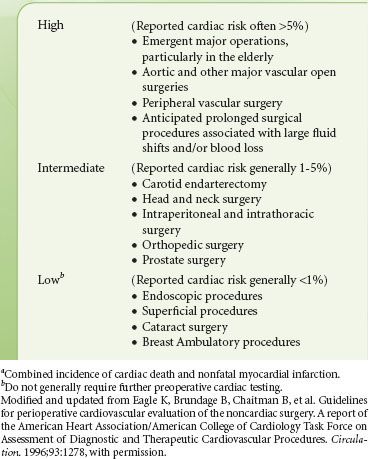
The surgical procedure influences the scope of preoperative evaluation required by determining the potential range of physiologic flux during the perioperative period. Few data exist defining the surgery-specific incidence of complications. Peripheral procedures, such as those included in a study of ambulatory surgery completed at the Mayo Clinic, are associated with an extremely low incidence of morbidity and mortality,29 while major vascular procedures are associated with the highest incidence of complications. The AHA/American College of Cardiology guidelines describe risk stratification for noncardiac surgery as shown in Table 22-7.18 Eagle et al.30 published data on the incidence of perioperative MI and mortality by procedure for patients enrolled in the Coronary Artery Surgery Study. They determined the overall risk of perioperative morbidity in patients with known CAD treated either medically or with prior CABG. Their data differed slightly and found that high-risk procedures include major vascular, abdominal, thoracic, and orthopedic surgery.
TABLE 22-7. CARDIAC RISKa STRATIFICATION BASED ON THE SURGICAL PROCEDURE IN PATIENTS WITH KNOWN CORONARY ARTERY DISEASE
Importance of Exercise Tolerance
 Exercise tolerance is one of the most
Exercise tolerance is one of the most  important determinants of perioperative risk and the need for further testing and invasive monitoring. An excellent exercise tolerance, even in patients with stable angina, suggests that the myocardium can be stressed without failing. If a patient can walk a mile without becoming short of breath, the probability of extensive CAD is small. Alternatively, if patients experience dyspnea associated with chest pain during minimal exertion, the probability of extensive CAD is high, which has been associated with greater perioperative risk. In addition, these patients are at risk for developing hypotension with ischemia and therefore may benefit from more extensive monitoring, coronary intervention therapy, or revascularization. Exercise tolerance can be assessed with formal treadmill testing or with a questionnaire that assesses activities of daily living (Table 22-8).18
important determinants of perioperative risk and the need for further testing and invasive monitoring. An excellent exercise tolerance, even in patients with stable angina, suggests that the myocardium can be stressed without failing. If a patient can walk a mile without becoming short of breath, the probability of extensive CAD is small. Alternatively, if patients experience dyspnea associated with chest pain during minimal exertion, the probability of extensive CAD is high, which has been associated with greater perioperative risk. In addition, these patients are at risk for developing hypotension with ischemia and therefore may benefit from more extensive monitoring, coronary intervention therapy, or revascularization. Exercise tolerance can be assessed with formal treadmill testing or with a questionnaire that assesses activities of daily living (Table 22-8).18
TABLE 22-8. ESTIMATED ENERGY REQUIREMENT FOR VARIOUS ACTIVITIES

Reilly et al.31 have evaluated the predictive value of self-reported exercise tolerance for serious perioperative complications and demonstrated that a poor exercise tolerance (could not walk four blocks and climb two flights of stairs) independently predicted complications. The likelihood of a serious adverse event was inversely related to the number of blocks that could be walked. Therefore, there is good evidence to suggest that minimal additional testing is necessary if the patient is able to describe a good exercise tolerance.
Indications for Further Cardiac Testing
Multiple algorithms have been proposed to determine which patients require further testing. As described previously, the risk associated with the proposed surgical procedure influences the decision to perform further diagnostic testing and interventions. With the reduction in perioperative morbidity, it has been suggested that extensive cardiovascular testing is not necessary. However, until these findings can be confirmed, further testing may be warranted.
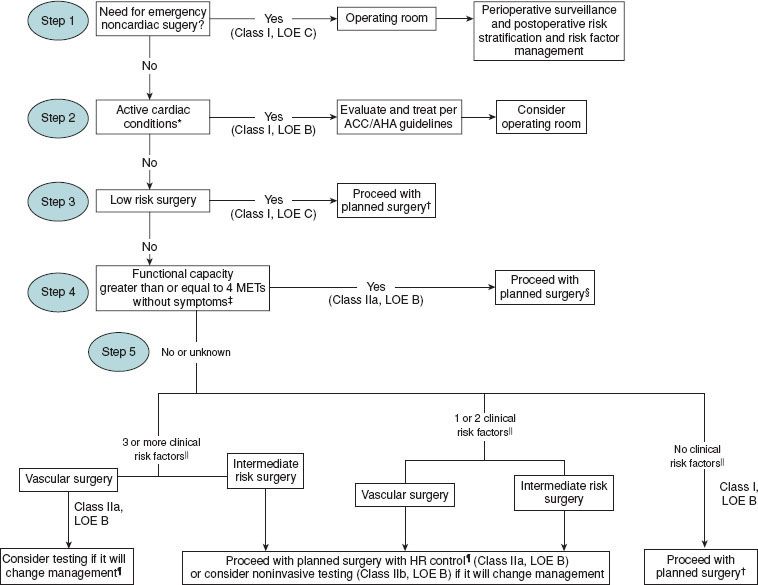
The algorithm to determine the need for testing proposed by the American College of Cardiology/American Heart Association Task Force updated in 2002 and again in 200732 is based on the available evidence and expert opinion that integrates clinical history, surgery-specific risk, and exercise tolerance. In the first step, the clinician evaluates the urgency of the surgery and the appropriateness of a formal preoperative assessment. Next, one should determine if the patient has undergone a recent revascularization procedure or coronary evaluation. Those patients with unstable coronary syndromes should be identified, and appropriate treatment instituted. Finally, the decision to undergo further testing depends on the interaction of the clinical risk factors, surgery-specific risk, and functional capacity. For patients at intermediate clinical risk, both exercise tolerance and the extent of the surgery are taken into account to determine the need for further testing. Importantly, no preoperative cardiovascular testing should be performed if the results will not change perioperative management.
Electrocardiogram
Preoperative 12-lead ECG can provide important information on the state of the patient’s myocardium and coronary circulation. Abnormal Q waves in high-risk patients are highly suggestive of a past MI. Confirmation of active ischemia usually requires changes in at least two leads. It has been estimated that approximately 30% of MIs occur without symptoms (“silent infarctions”) and can only be detected on routine ECGs, with the highest incidence occurring in patients with either diabetes or hypertension. The Framingham study showed that long-term prognosis is not improved by lack of symptoms.20 The absence of Q waves on the ECG does not exclude the occurrence of a Q-wave MI in the past. Between 5% and 27% of Q waves disappeared over the 10-year period following an infarction during the 1970s.33 Those patients in whom the ECG reverts to normal have improved survival compared with those with consistent abnormalities, with or without Q waves. The presence of Q waves on a preoperative ECG in a high-risk patient, regardless of symptoms, should alert the anesthesiologist to the increased perioperative risk and the possibility of active ischemia.
It has not been established that information obtained from the preoperative ECG affects clinical care. A review of clinical studies on the matter is inconclusive. In one retrospective review of adult patients undergoing ambulatory surgery, the preoperative ECG was not predictive of perioperative risk.34 Although controversy exists, there are current recommendations for the need for a preoperative ECG. A preoperative resting 12-lead ECG is recommended for patients with at least one clinical risk factor who are undergoing vascular surgical procedures and for patients with known CAD, peripheral arterial disease, or cerebrovascular disease who are undergoing intermediate-risk surgical procedures. A perioperative ECG is reasonable in persons with no clinical risk factors who are about to undergo vascular surgical procedures and may be reasonable in patients with at least one clinical risk factor who are undergoing intermediate-risk operative procedures.32
Noninvasive Cardiovascular Testing
The exercise ECG has been the traditional method in the past for evaluating patients with suspected CAD. It represents the most cost-effective and least invasive method for detecting ischemia, with a sensitivity of 70% to 80% and a specificity of 60% to 75% for identifying CAD. A positive exercise stress test alerts the anesthesiologist that the patient is at risk for ischemia associated with increased heart rate, with the greatest risk in those who develop ischemia only after mild exercise. However, as discussed previously, the ability to exercise suggests that no further testing is necessary, and therefore stress electrocardiography is infrequently indicated.
A number of high-risk patients are either unable to exercise or have contraindications to exercise, for example, those with claudication. Therefore, pharmacologic stress testing and ambulatory electrocardiography have come into vogue, particularly as preoperative cardiovascular tests in patients scheduled for vascular surgery. Pharmacologic stress thallium imaging is useful in those patients who are unable to exercise. Dipyridamole or adenosine is administered as a coronary vasodilator to assess flow heterogeneity. The presence of a redistribution defect is predictive of postoperative cardiac events, especially in patients undergoing peripheral vascular surgery. Similarly, dobutamine can be used to increase myocardial oxygen demand, by increasing heart rate and blood pressure, in those patients who cannot exercise.
The ambulatory ECG (e.g., Holter monitoring) provides a means of continuously monitoring the ECG for significant ST segment changes preoperatively. One study demonstrated that the presence of silent ischemia is a strong predictor of outcome, while its absence is associated with a favorable outcome in 99% of the patients studied.35 Other investigators have demonstrated the value of ambulatory ECG monitoring, although the negative predictive values have not been as high as reported by some.
Stress echocardiography is another preoperative test that may be of value in evaluating patients with suspected CAD. The appearance of either new or more severe regional wall motion abnormalities with exercise is considered a positive test. Either represents areas at risk for myocardial ischemia. The advantage of stress echocardiogram is that it is a dynamic assessment of ventricular function. Dobutamine echocardiography has also been studied and found to have among the best predictive values. It is generally accepted that the group at risk is composed of those who demonstrate regional wall motion abnormalities at low heart rates.
Several groups have published meta-analyses of preoperative diagnostic tests. One group of investigators demonstrated good predictive values using ambulatory ECG monitoring, radionuclide angiography, dipyridamole thallium imaging, or dobutamine stress echocardiography.36 Shaw et al.37 also demonstrated good predictive values of dipyridamole thallium imaging and dobutamine stress echocardiography. Both of these studies demonstrated the superior value of dobutamine stress echocardiography; however, there was significant overlap of the confidence intervals with other tests. The most important determinant with respect to the choice of preoperative testing is the expertise of the local institution.
Current recommendations are that patients with active cardiac conditions such as unstable angina, congestive heart failure, significant dysrhythmias, and severe valvular disease should undergo noninvasive stress testing before noncardiac surgery. Noninvasive stress testing for patients with multiple clinical risk factors and poor functional capacity (less than four metabolic equivalents) who require vascular surgery is reasonable if it will change management. Noninvasive testing in other patients about to go under intermediate-risk noncardiac surgery or vascular surgery is less clear.32
Assessment of Ventricular and Valvular Function
Both echocardiography and radionuclide angiography can assess cardiac ejection fraction at rest and under stress. Echocardiography is less invasive and able to assess regional wall motion abnormalities, wall thickness, valvular function, and valve area. Pulse-wave Doppler can be used to determine the velocity time integral. Ejection fraction can then be calculated by determining the cross-sectional area of the ventricle. Conflicting results exist with regard to the predictive value of ejection fraction using either echocardiographic or radionuclide measurements. It is reasonable for those with dyspnea of unknown origin and for those with current or prior heart failure with worsening dyspnea or other change in clinical status to have preoperative evaluations of left ventricular function. The benefits of reassessment of left ventricular function in clinically stable patients with previous cardiomyopathy are unknown.32 Echocardiography can provide important information regarding valvular function, which may have important implications for either cardiac or noncardiac surgery, and is discussed more fully later in this text. Aortic stenosis has been associated with a poor prognosis in noncardiac surgical patients, and knowledge of valvular lesions may modify perioperative hemodynamic therapy.11
Coronary Angiography
Coronary angiography is currently the best method for defining coronary anatomy. In addition, information regarding ventricular and valvular function can also be assessed. Hemodynamic indices can be determined such as ventricular pressures and pressure gradients across valves. This information is routinely available in patients scheduled for CABG. Narrowing of the left main coronary artery and certain other lesions may be associated with a greater perioperative risk. Diffuse atherosclerosis in small vessels, as seen in diabetics, may lead to incomplete revascularization and a risk of developing ischemia despite CABG. Coronary angiography is used by cardiologists to determine whether coronary vascularization is an option.
Unlike the exercise or pharmacologic stress tests discussed earlier, coronary angiography provides anatomic, not functional, information. Although a critical coronary stenosis delineates an area of risk for developing myocardial ischemia, the functional response of that ischemia cannot be assessed by angiography alone. A critical stenosis may or may not be the underlying cause for a perioperative MI that occurs. In the ambulatory population, many infarctions are the result of acute thrombosis of a noncritical stenosis. Therefore, the value of routine angiography prior to noncardiac surgery depends on the identification of lesions that will cause morbidity and mortality.
Patients with restricted physical activity in whom functional capacity is difficult to determine may benefit from sophisticated imaging techniques such as cardiac computed tomography.38
Perioperative Coronary Interventions
Guidelines to reduce the perioperative risk of noncardiac surgery have recently been reviewed. There are several large studies that suggest that in patients who survive CABG, the risk of subsequent noncardiac surgery is low.7,10 Although there is little data to support the notion of coronary revascularization solely for the purpose of improving perioperative outcome, it is true that for some patients scheduled for high-risk surgery, long-term survival may be enhanced by revascularization. Two studies used the Coronary Artery Surgery Study database and found that CABG significantly improved survival in those patients with both peripheral vascular disease and triple-vessel coronary disease, especially the group with depressed ventricular function.39 After reviewing all available data, most clinicians believe the indication for CABG prior to noncardiac surgery remains the same as in other settings and is independent of the proposed noncardiac surgery.
The value of percutaneous transluminal coronary angioplasty is less well established. The current evidence does not support the use of percutaneous transluminal coronary angioplasty beyond established indications for nonoperative patients.
Patients with Coronary Artery Stents
Early surgery after coronary stent placement has been associated with adverse cardiac events. A significant incidence of perioperative death and of hemorrhage in patients after stent placement has been reported. The waiting period for surgery after bare metal stent placement is generally recognized as 1 month as a minimum, while the waiting period for drug-eluting stents is 12 months. This difference is because the incidence of stent thrombosis for the drug-eluting stents has been found to be similar to the bare metal stents in the early phase after placement, but less well defined over a longer period of time. Currently, patients are invariably taking aspirin and clopidogrel as antiplatelet therapy after stent placement. A thienopyridine (ticlopidine or clopidogrel) is generally continued with aspirin for 1 month after bare metal stenting and for 12 months after drug-eluting stent placement (Fig. 22-3). Perioperative management weighs the risk of bleeding versus a stent thrombosis. The decision must involve anesthesiologist, surgeons, cardiologists, and intensivists. For those patients who have a high risk for stent thrombosis, many advocate that at least aspirin be continued in the perioperative period. Also, the anesthesiologist must weigh the risk of regional versus general anesthesia when these patients are taking antiplatelet therapy. Surgery in patients with recent stent placement should probably only be considered in centers where 24-hour interventional cardiologists are available.40–42
FIGURE 22-3. Cardiac evaluation algorithm for patients at least 50 years with cardiac risk factors undergoing non-cardiac surgery. || Clinical risk factors include ischemic heart disease, diabetes mellitus, cerebrovascular disease, renal insufficiency, compensated or history of congestive heart failure. AHA: American Heart Association; ACC: American College of Cardiology; HR; heart rate; LOE, level of evidence; MET: metabolic equivalent. (Used with permission from ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. Circulation 2007;116:e418–e500.
Patients with Cardiovascular Implantable Electronic Devices
With the increasing prevalence of patients treated with pacemakers and implantable defibrillators, the preoperative evaluation must address their management during the perioperative period. The function of these devices can be impaired by electromagnetic interference during surgery. Current guidelines for the management of these devices have been recently published.43
Pulmonary Disease
Pulmonary complications occur more frequently than cardiac complications, with an incidence of 5% to 10% in those having major noncardiac procedures. Perioperative pulmonary complications include aspiration, atelectasis, pneumonia, bronchitis, bronchospasm, hypoxemia, exacerbation of chronic obstructive pulmonary disease, and respiratory failure requiring mechanical ventilation.44
Postoperative respiratory failure is a major cause of morbidity and mortality, contributing to increased length of hospital stay and substantial economic cost. The risk of mortality with the development of respiratory failure is substantial, similar to perioperative MI. Understanding of clinical risk factors has increased substantially through the use of epidemiologic analyses of large clinical databases. Recent epidemiologic studies support that preoperative covariates can be used in models to predict patient groups at increased risk for respiratory failure.44,45 Clinical guidelines from the American College of Physicians have been developed to assess both the preoperative risk and prevention strategies to limit the risk of respiratory failure.46 Preoperative testing, such as pulmonary function testing and chest x-rays, is not recommended on a routine basis, as it appears to have limited benefit in predicting perioperative respiratory failure and complication rate. Although preoperative chest x-rays can identify structural lung abnormalities, these are not frequently associated with significant changes in clinical management for the general population. In contrast, laboratory studies identifying a reduction in serum albumin levels and increased levels of blood urea nitrogen (BUN) appear associated with an increased risk of perioperative pulmonary morbidity.44
Epidemiologic studies significantly support the relationship of the anatomic location of the surgery and pulmonary risk.44 With regard to the surgical site, thoracic, open aortic, or upper abdominal surgery has been associated with the highest risk for postoperative pulmonary problems. Risk increases as the incision approaches the diaphragm.44,47–49 Decreases in postoperative vital capacity, functional residual capacity, as well as diaphragmatic dysfunction can contribute to hypoxemia and atelectasis.50 Functional residual capacity may take up to 2 weeks to return to baseline. Diaphragmatic dysfunction occurs despite adequate analgesia and is theorized to be caused by phrenic nerve inhibition.51 Neurosurgery and neck surgery may be associated with perioperative aspiration pneumonia.
The need for emergency surgery and the need for general anesthesia are also associated with increased risk. Not only can the surgery affect pulmonary function, but general anesthesia also results in mechanical changes such as a decrease in the functional residual capacity and altered diaphragmatic motion leading to ventilation/perfusion abnormalities. General anesthesia also induces negative changes at the microscopic level causing inhibition of mucociliary clearance, increased alveolar–capillary permeability, inhibition of surfactant production, increased nitric oxide synthetase, and increased sensitivity of the pulmonary vasculature to neurohumoral mediators. Subanesthetic levels of intravenous or volatile agents have the ability to blunt the ventilatory response to hypoxemia and hypercarbia. Duration of anesthesia is a well-established risk factor for postoperative pulmonary complications, with morbidity rates increasing after 2 to 3 hours.52 However, when considering laparoscopic surgery which is often longer in duration, the associated decrease in pulmonary complications compared with an open procedure usually outweighs the risk of increased anesthesia time.53
Atelectasis during the intraoperative and postoperative periods can contribute to the risk of perioperative respiratory failure. The preoperative assessment should address the following therapies: Epidural analgesia during the perioperative period, lung expansion methods, and deep venous thrombosis prophylaxis. Intraoperative measures to limit the risk of hospital-acquired pneumonia have been proposed, largely focused on reducing the risk of bacterial contamination of the lung during the perioperative period. For high-risk patient groups there are supportive studies of benefits of preoperative oral antiseptic decontamination before endotracheal intubation as well as the role of specialized endotracheal tubes to decrease the risk of nosocomial pneumonia.54–56
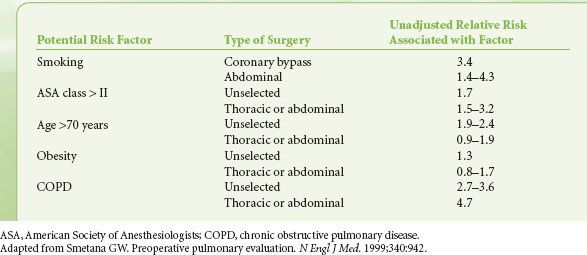
Preoperative evaluation of patients with preexisting pulmonary disease should include assessment of the type and severity of disease, as well as its reversibility (Table 22-9).
TABLE 22-9. POTENTIAL PATIENT-RELATED RISK FACTORS FOR POSTOPERATIVE PULMONARY COMPLICATIONS
Tobacco Use
Smoking is an important risk factor, but one that usually cannot be influenced. Even among smokers who have not developed chronic lung disease, smoking is known to increase carboxyhemoglobin levels, decrease ciliary function, and increase sputum production, as well as cause stimulation of the cardiovascular system secondary to nicotine. While cessation of smoking for 2 days can decrease carboxyhemoglobin levels, abolish the nicotine effects, and improve mucous clearance, prospective studies showed that smoking cessation for at least 4 to 8 weeks was necessary to reduce the rate of postoperative pulmonary complications.57,58 Recent studies of nicotine transdermal patches used during the perioperative period have shown increased mortality and are best avoided.59 Patients who smoke often show increased airway reactivity under general anesthesia, and it may be useful to administer a bronchodilator such as albuterol preoperatively.
Asthma
Asthma is one of the most common coexisting diseases that confront the anesthesiologist. During the patient interview it is important to elicit information regarding inciting factors, severity, reversibility, and current status. Frequent use of bronchodilators, hospitalizations for asthma, and requirement for systemic steroids are all indicators of the severity of the disease. After an acute exacerbation of asthma, airway hyperreactivity may persist for several weeks.60 In addition to bronchodilators, perioperative steroids are worth considering as prophylaxis for the severe asthmatic, for example, hydrocortisone 100 mg intravenously every 8 hours on the day of surgery. The possibility of adrenal insufficiency is also a concern in those patients who have received more than a “burst and taper” of steroids in the previous 6 months. This group of patients should be administered “stress doses” of steroids perioperatively. Kabalin et al.61 found there was a low complication rate for asthmatics treated with short-term steroids undergoing surgery. Significantly, they found no association with impaired wound healing or infections. For patients using inhaled steroids, they should be administered regularly, starting at least 48 hours prior to surgery for optimal effectiveness.
Obstructive Sleep Apnea
OSA is a syndrome defined by periodic obstruction of the upper airway during sleep, leading to episodic oxygen desaturation and hypercarbia. This episodic desaturation, in turn, causes episodic arousal, leading to chronic sleep deprivation with daytime hypersomnolence and even behavioral changes in children. Depending on the frequency and severity of events, it may lead to other changes such as chronic pulmonary hypertension and right heart failure. It is estimated to be present in 9% of women and 24% of men, with the great majority of these being undiagnosed. Factors commonly associated with an increased risk of sleep apnea include obesity (body mass index >35 kg/m2 or 95th percentile for age), increased neck circumference, severe tonsillar hypertrophy, and anatomic abnormalities of the upper airway.
Because of their propensity for airway collapse and sleep deprivation, patients with OSA are especially susceptible to the respiratory depressant and airway obstructive effects of sedatives, narcotics, and inhaled anesthetics both intraoperatively and postoperatively. Preoperative identification of those patients at risk allows them to undergo a formal sleep study to determine the presence and severity of symptoms and also allows preoperative initiation of continuous positive airway pressure (CPAP). In 2006, the ASA published practice guidelines for the perioperative management of patients with OSA.62 During the preoperative evaluation, specific questions should be directed toward the patient and family regarding the presence of symptoms and signs of OSA:
 Does the patient snore loudly enough to be heard through a door or snore frequently?
Does the patient snore loudly enough to be heard through a door or snore frequently?
 Have you observed pauses in the patient’s breathing during sleep?
Have you observed pauses in the patient’s breathing during sleep?
 Does the patient have frequent arousals from sleep or awakenings with a choking sensation?
Does the patient have frequent arousals from sleep or awakenings with a choking sensation?
 Does the patient experience frequent daytime somnolence and fatigue or fall asleep easily in a non-stimulating environment?
Does the patient experience frequent daytime somnolence and fatigue or fall asleep easily in a non-stimulating environment?
 Does your child appear restless when sleeping or have difficulty with breathing?
Does your child appear restless when sleeping or have difficulty with breathing?
 Is your child overly aggressive or does he/she have trouble concentrating?
Is your child overly aggressive or does he/she have trouble concentrating?
If the patient has predisposing anatomy and/or signs or symptoms in two or more areas, he/she should be referred for a sleep study. If a sleep study is not possible, the patient should be managed as he/she has OSA. The risk of perioperative complications in patients with OSA increases with the severity of sleep apnea, the invasiveness of surgery, and the amount of postoperative opioids required. There is general consensus that preoperative initiation of nasal mask CPAP reduces perioperative risk, perhaps by decreasing the sleep deprivation and secondary hypersomnolence.62 Importantly, OSA is also associated with difficult airway management, making it even more important to examine previous anesthesia records and to perform a thorough airway examination. Emergency airway equipment should be readily available at the surgical center.
There are multiple management decisions to make in coordination with the surgeon with respect to the OSA patient:
 Determine whether there are noninvasive ways of performing the operation that would decrease the need for opioids postoperatively.
Determine whether there are noninvasive ways of performing the operation that would decrease the need for opioids postoperatively.
 Discuss whether it is feasible to perform surgery under neuraxial, regional, or local anesthesia, decreasing the total amount of anesthesia or opioids needed.
Discuss whether it is feasible to perform surgery under neuraxial, regional, or local anesthesia, decreasing the total amount of anesthesia or opioids needed.
 Determine whether nonsteroidal antiinflammatory agents are acceptable for postoperative analgesia.
Determine whether nonsteroidal antiinflammatory agents are acceptable for postoperative analgesia.
 Discuss whether outpatient surgery is a safe option.
Discuss whether outpatient surgery is a safe option.
 Determine whether the patient will be able to use CPAP postoperatively.
Determine whether the patient will be able to use CPAP postoperatively.
 Determine whether postoperative admission to an intensive care unit or monitored unit is required for the patient who is a first-time user of CPAP.
Determine whether postoperative admission to an intensive care unit or monitored unit is required for the patient who is a first-time user of CPAP.
The ASA practice guidelines for OSA recommend hospitalization after uvulopalatoplasty surgery and after tonsillectomy for OSA in children younger than 3 years. Postoperative hospitalization is also recommended for those OSA patients with other coexisting diseases. When procedures are performed on an outpatient basis, prolonged postoperative monitoring should be continued to ensure that the patient is able to maintain room air saturation without obstruction when left undisturbed in recovery. Recovery with the patient’s head and thorax elevated is also recommended to optimize airway patency. The task force recommends continuous pulse oximetry during hospitalization, as well as supplemental oxygen until the patient can maintain their baseline oxygen saturation on room air.
Endocrine Disease
Diabetes Mellitus
Diabetes mellitus is the most common endocrinopathy, with the incidence of type 1 diabetes at 0.4% of the population and type 2 diabetes affecting approximately 8% to 10% of Americans, but projected to develop in >30% of Americans born after 2000, largely because of the rise in obesity.63 Critical illness–induced hyperglycemia, defined as a blood glucose >200 mg/dL in the absence of known diabetes, occurs frequently, particularly in the elderly.64 Diabetes mellitus has acute and chronic disease manifestations, making it more likely for diabetics to require surgery. The majority of diabetics develop secondary disease in one or more organ systems, which must be identified preoperatively so that an appropriate plan can be developed for perioperative management. While long-term, close control of glucose may limit some of the microvascular effects of diabetes (retinopathy, neuropathy, and nephropathy), macrovascular events such as myocardial ischemia or infarction or stroke may not be decreased. Diabetics have an increased risk of CAD, hypertension, congestive heart failure, and perioperative MI. The 2002 American College of Cardiology/AHA guidelines on perioperative cardiac assessment of patients undergoing noncardiac surgery place diabetics, especially those receiving insulin, at a minimum of intermediate risk.65 They also state that most diabetic patients >65 years of age have significant CAD, with the incidence of silent ischemia increased due to associated diabetic autonomic neuropathy.
Diabetics are also more likely than the general population to have cerebral vascular, peripheral vascular, and renal vascular disease. Diabetes mellitus is the leading cause of renal failure requiring dialysis. Peripheral neuropathies and vascular disease make these patients more susceptible to positioning injuries during surgery as well as postoperatively. Autonomic neuropathy may predispose the patient to hemodynamic instability during anesthesia and theoretically increase the risk of pulmonary aspiration because of the associated gastroparesis. These deficits should be documented prior to anesthesia and the anesthetic plan adjusted accordingly. Stiff joint syndrome due to glycosylation of proteins and abnormal collagen cross-linking may significantly affect the temporomandibular, atlantooccipital, and cervical spine joints in patients with long-standing type 1 diabetes, resulting in difficulty with intubation.  A thorough airway examination should be performed prior to anesthesia and a high index of suspicion maintained for a potentially difficult airway. Some suggest using the “prayer sign” as an evaluation tool: Patients who are unable to completely oppose their hands (with no space between) should be suspected of also having changes in other joints potentially impacting airway manipulation.
A thorough airway examination should be performed prior to anesthesia and a high index of suspicion maintained for a potentially difficult airway. Some suggest using the “prayer sign” as an evaluation tool: Patients who are unable to completely oppose their hands (with no space between) should be suspected of also having changes in other joints potentially impacting airway manipulation.
Regimens for perioperative glycemic control vary enormously, not only between type 1 and type 2 diabetics, but also within each group. Patients with type 1 diabetes have an absolute insulin deficiency usually due to destruction of pancreatic beta cells. These patients must receive insulin to prevent diabetic ketoacidosis. Home glucose management most often relies on a combination of short- and intermediate- or long-acting insulin regimens. Insulin pumps are increasingly common and are used to administer a continuous subcutaneous infusion of short-acting insulin, supplemented by boluses dictated by glucose levels, diet, and exercise. Type 2 diabetes accounts for the great majority of diabetics and is defined by variable degrees of insulin deficiency and resistance. Although most commonly associated with obesity, it may also be induced by corticosteroids or pregnancy. Ketoacidosis is uncommon in type 2 diabetes, and the stress of severe infection or illness is more likely to provoke a nonketotic hyperosmolar state, which is characterized by severe dehydration, hyperglycemia, and hyperosmolarity. In type 2 diabetics, glucose control is most commonly achieved with diet, exercise, and/or oral hypoglycemic drugs. These agents primarily work by increasing endogenous insulin release, increasing insulin sensitivity, and/or decreasing hepatic gluconeogenesis. These drugs fall under the main categories of sulfonylureas, biguanides, thiazolidinediones, and meglitinides. If glycemic control is unsuccessful, then insulin is generally added to the regimen.
Ideally, both type 1 and 2 diabetic patients should be evaluated by the preoperative clinic as well as the patient’s endocrinologist 1 to 2 weeks before elective surgery. In addition to a thorough history and physical examination, a judicious laboratory investigation should include determination of blood glucose, hemoglobin A1c, serum electrolytes, creatinine, and an ECG. If the patient’s glycemic control is inadequate based on a hemoglobin A1c being outside of the target range (7% to 9% for <5 years old; 6% to 8% for >5 years old), abnormal electrolytes, or ketonuria, then elective surgery should be delayed to allow optimization of preoperative glycemic control. Administration of perioperative beta-blockers should be considered in diabetic patients with CAD in an attempt to limit perioperative myocardial ischemia, as there is no evidence of worsened glucose intolerance or masking of hypoglycemic symptoms. However, the physician should be attentive to the possibility of precipitating heart failure.
Perioperative Glucose Management
Anesthesia and surgery interrupt the regular meal schedule and insulin administration in patients with diabetes mellitus. Perioperative stress may increase serum glucose concentrations secondary to the release of cortisol and catecholamines. The majority of available literature suggests that better glycemic control may limit morbidity (length of hospital/intensive care unit stay, infection rate, wound healing, outcomes after strokes/MIs) and mortality particularly in cardiac surgery patients, carotid endarterectomy patients, and the critically ill.64,66–68 Although a randomized trial found an increase in the incidence of death and perioperative stroke in cardiac surgery patients where an attempt was made to maintain the glucose between 80 and 100 mg/dL,69 recent systematic reviews have found a reduction in morbidity and mortality associated with better glycemic control, but recognize the increased risk of hypoglycemia.70 More studies are needed to more closely define the target level for glucose control. There is general consensus that an attempt should be made to control the upper limit of glucose to <200 mg/dL, although some will argue that tighter control is warranted. Guidelines for ambulatory and hospitalized patients have been recently published.71,72 The following recommendations can serve as a general guide:
 Plan with the surgeon to schedule the surgery as the first case of the day to prevent prolonged fasting.
Plan with the surgeon to schedule the surgery as the first case of the day to prevent prolonged fasting.
 As a general rule, oral hypoglycemic agents are held on the day of surgery to avoid reactive hypoglycemia until oral intake is restarted.
As a general rule, oral hypoglycemic agents are held on the day of surgery to avoid reactive hypoglycemia until oral intake is restarted.
 Insulin therapy should balance adequate glucose control with the avoidance of hypoglycemia. Insulin is usually continued through the evening before surgery.
Insulin therapy should balance adequate glucose control with the avoidance of hypoglycemia. Insulin is usually continued through the evening before surgery.
 Schedule the patient to arrive in the early morning with an empty stomach and check blood glucose on arrival.
Schedule the patient to arrive in the early morning with an empty stomach and check blood glucose on arrival.
 If patients develop symptoms of or measurable hypoglycemia, they should be counseled to take a glucose tablet or clear juice.
If patients develop symptoms of or measurable hypoglycemia, they should be counseled to take a glucose tablet or clear juice.
 Type 1 diabetics should be continued on basal insulin administration even during preoperative fasting to prevent ketoacidosis. Administer half the usual morning dose of intermediate- or long-acting insulin after arrival to the surgery center where a maintenance IV can be started. Hold the usual dose of rapid- or short-acting insulin.
Type 1 diabetics should be continued on basal insulin administration even during preoperative fasting to prevent ketoacidosis. Administer half the usual morning dose of intermediate- or long-acting insulin after arrival to the surgery center where a maintenance IV can be started. Hold the usual dose of rapid- or short-acting insulin.
 Use the patient’s own sliding scale to administer short-acting insulin subcutaneously prior to the scheduled surgery and during short operations.
Use the patient’s own sliding scale to administer short-acting insulin subcutaneously prior to the scheduled surgery and during short operations.
 Patients on insulin pumps may be managed by continuing the pump for short operations or changing over to an intravenous insulin infusion for moderate or major operations.
Patients on insulin pumps may be managed by continuing the pump for short operations or changing over to an intravenous insulin infusion for moderate or major operations.
This strategy, along with blood glucose determinations every 1 to 2 hours, may be all that is necessary for well-controlled diabetics undergoing short, noninvasive outpatient operations. In addition, it is important to prevent postoperative nausea and vomiting and to encourage the early resumption of diet, allowing return to their previous insulin regimen. For type 1 or 2 diabetics undergoing moderate or major surgery, insulin is generally administered in the form of an intravenous infusion of regular insulin. Discontinuing the patient’s own insulin pump will avoid problems with insulin preparations and pump technology.
There are several methods of administering an insulin infusion, none of which has proved superior. Concurrent separate infusions of insulin and glucose are more easily adjusted and may provide better glycemic control than combined glucose/insulin/potassium infusions. To increase the safety, the insulin infusion (which is on a separate pump) is added via a side port to the same line delivering the glucose infusion. A separate nonglucose isotonic solution should be used to replace deficits and intraoperative fluid losses. All protocols rely on the frequent determination of a plasma glucose level at least every 1 to 2 hours to allow titration of insulin.73–75
Thyroid and Parathyroid Diseases
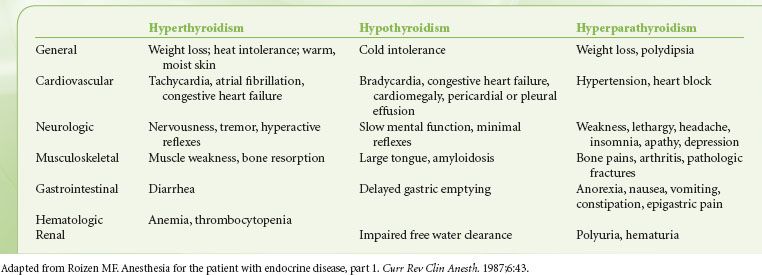
Thyroid and parathyroid diseases have clinical manifestations that are important to the preoperative evaluation (Table 22-10). Although thyroid function tests are more sensitive, thyroid disease is usually adequately evaluated by clinical history, and the evaluation should focus on evaluating for signs and symptoms of hypothyroidism and hyperthyroidism. Hypothyroidism can lead to the development of hypothermia, hypoglycemia, hypoventilation, and hyponatremia, as well as a susceptibility to depressant drugs. Anesthesiologists should be alert to the possibility of the hypermetabolic state of thyroid storm in patients with hyperthyroidism. A large thyroid mass may distort the upper airway, producing inspiratory stridor or wheezing, especially evident in the supine position. In these cases, a chest x-ray should be obtained looking for evidence of tracheal deviation or narrowing. A computed tomography scan of the upper airway and trachea will provide better detail of any airway compromise. Patients with hyperparathyroidism often have hypercalcemia, indicating the need for preoperative determination of a serum calcium level.
TABLE 22-10. CLINICAL MANIFESTATIONS OF THYROID AND PARATHYROID DISEASES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






