Amino Acid Solutions
Protein is provided as amino acid solutions that contain varying mixtures of essential (N=9), semi-essential (N=4), and nonessential (N=10) amino acids. These solutions are mixed with dextrose solutions in a 1:1 volume ratio. Examples of standard and “specialty” amino acid solutions are shown in Table 49.2.
Table 49.2 Standard and Specialty Amino Acid Solutions
Standard Solutions
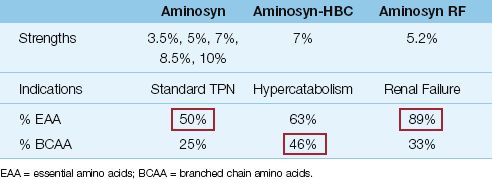
Standard amino acid solutions (e.g., Aminosyn in Table 49.2) are balanced mixtures of 50% essential amino acids and 50% nonessential and semi-essential amino acids. Available concentrations range from 3.5 % up to 10%, but 7% solutions (70 g/L) are used most often.
Specialty Solutions
Specially designed amino acid solutions are available for patients with severe metabolic stress (e.g., from multisystem trauma or burns), and for patients with renal or liver failure.
1. Solutions designed for metabolic stress (e.g., Aminosyn-HBC in Table 49.2) are enriched with branched chain amino acids (iso-leucine, leucine, and valine), which are preferred fuels in skeletal muscle when metabolic demands are high.
2. Renal failure solutions (e.g., Aminosyn RF in Table 49.2) are rich in essential amino acids, because the nitrogen in essential amino acids is partially recycled to produce nonessential amino acids, which results in smaller increments in blood urea nitrogen (BUN) when compared with breakdown of nonessential amino acids.
3. Solutions designed for hepatic failure (e.g., HepaticAid) are en-riched with branched chain amino acids, which block the transport of aromatic amino acids across the blood-brain barrier (which is implicated in hepatic encephalopathy).
It is important to emphasize that none of these specialized formulas have improved the outcomes in the disorders for which they are designed (3).
Glutamine
Glutamine is the principal metabolic fuel for rapidly dividing cells like intestinal epithelial cells and vascular endothelial cells (4). Because of studies showing that glutamine is important for maintaining the integrity of the bowel mucosa (5), and studies showing a glutamine-associated decrease in infectious complications in ICU patients (6,7), glutamine has been recommended as a daily nutrional supplement in ICU patients (0.2–0.4 g/kg/day) (1). However, a recent multicenter study has shown a glutamine-associated increase in mortality rate in ICU patients with multiorgan failure (8), and until this issue is resolved, the recommendation for daily glutamine administration in ICU patients must be re-evaluated. (Note: Glutamine is not included in any of the commercially available amino acid solutions, so it must be added to the solutions by the pharmacy. This alone will limit the popularity of daily glutamine supplementation.)
Table 49.3 Intravenous Lipid Emulsions for Clinical Use
Lipid Emulsions
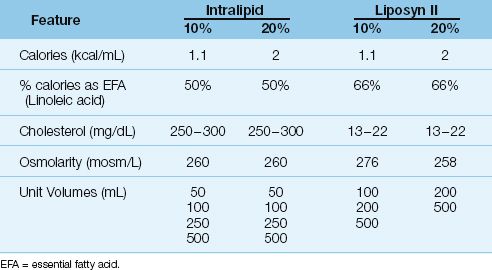
Lipids are provided as emulsions composed of submicron droplets of cholesterol, phospholipids, and triglycerides (9). The triglycerides are derived from vegetable oils (safflower or soybean oils) and are rich in linoleic acid, an essential fatty acid (10). Lipids are used to provide 30% of daily caloric requirements, and 4% of the daily calories should be provided as linoleic acid to prevent essential fatty acid deficiency (11).
As shown in Table 49.3, lipid emulsions are available in 10% and 20% strengths (the percentage refers to grams of triglyceride per 100 mL of solution). The 10% emulsions provide approximately 1 kcal/mL, and the 20% emulsions provide 2 kcal/mL. Unlike the hypertonic dextrose solutions, lipid emulsions are roughly isotonic to plasma and can be infused through peripheral veins. The lipid emulsions are available in unit volumes of 50 to 500 mL, and can be infused separately (at a maximum rate of 50 mL/hour) or added to the dextrose–amino acid mixtures. The triglycerides introduced into the bloodstream are not cleared for 8 to 10 hours, and lipid infusions often produce a transient, lipemic-appearing plasma.
ADDITIVES
Commercially available mixtures of electrolytes, vitamins, and trace elements are added directly to the dextrose–amino acid mixtures.
Electrolytes
There are more than 15 electrolyte mixtures available. Most have a volume of 20 mL, and contain sodium, chloride, potassium, and magnesium. You must check the mixture used at your hospital to determine if additional electrolytes must be added. Additional requirements for po-tassium or other electrolytes can be specified in the TPN orders.
Vitamins
Aqueous multivitamin preparations are added to the dextrose–amino acid mixtures. One unit vial of a standard multivitamin preparation will provide the normal daily requirements for most vitamins (see Table 47.3) (16). The daily vitamin requirements in ICU patients are not known (and probably varies with each patient). However, vitamin deficiencies are rcommon in ICU patients despite the provision of normal daily requirements, suggesting that critically ill patients have increased daily vitamin requirements.
Trace Elements
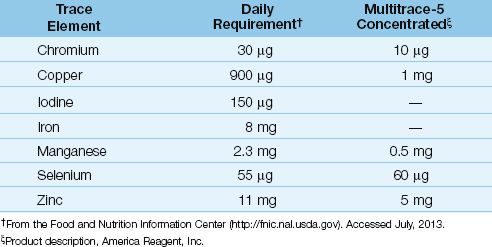
A variety of trace element additives are available, and one of the commercial preparations is shown in Table 49.4, along with the recommended daily requirement for trace elements. Note the poor correlation between the daily requirements and the trace element content of the commercial mixture. Trace element mixtures don’t contain iron and iodine, and some don’t contain selenium. Iron is not advised in critically ill patients because of its pro-oxidant effects (see Chapter 47 for more information on iron and oxidant injury). However, selenium should be given daily to ICU patients, particularly those with severe sepsis.
Selenium
The most important consideration regarding trace elements is selenium, which is a co-factor for glutathione peroxidase, an enzyme that participates in endogenous antioxidant protection (see Figure 22.7). Plasma levels of selenium are reduced in severe sepsis, and selenium replacement is associated with improved survival (12). The proposed daily requirement for selenium is 55 µg, but this is probably inadequate for critically ill patients. A daily dose of 200 µg is used in many studies, and doses of 400 µg daily are considered safe (see Table 47.3).
Table 49.4 Dietary Allowances for Essential Trace Elements
CREATING A TPN REGIMEN
The following is a stepwise approach to creating a standard TPN regimen for an individual patient. The patient in this example will be a 70-kg adult who is not malnourished and has no volume restrictions.
Step 1
The first step is to determine the daily requirement for calories and protein. There are two very simple approximations that can be used: i.e., the daily requirement for calories is 25 kcal/kg, and the daily protein requirement is 1.2–1.6 g/kg. (See Chapter 47 for more information on these estimates.) You can use actual body weight in these estimates as long as actual body weight is within 125% of the ideal weight. If actual body weight exceeds 125% of ideal body weight, you can use an adjusted body weight (see equation 47.3). If available, indirect calorimetry should be used to measure the resting energy expenditure (see Chapter 47).
For the 70 kg patient, we’ll use actual body weight, and a daily protein requirement of 1.4 g/kg. Therefore, the daily requirement for calories and protein will be:
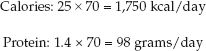 (49.1)
(49.1)
Note: If propofol is being infused, you should determine the calories provided by propofol and subtract those calories from the daily caloric requirement. Propofol is infused in a 10% lipid emulsion, which has a caloric density equivalent to 10% Intralipid (1 kcal/mL). Therefore, the hourly infusion rate of propofol (mL/hr) is equivalent to the hourly yield of calories (kcal/hr).
Step 2
The next step is to take a standard mixture of 10% amino acids (500 mL) and 50% dextrose (500 mL) and determine the volume of this mixture that is needed to deliver the estimated daily protein requirement. Although the dextrose–amino acid mixture is referred to as A10-D50, the final mixture actually represents 5% amino acids (50 grams of protein per liter) and 25% dextrose (250 grams of dextrose per liter). The volume of the A10-D50 mixture that will provide the daily protein requirement is equivalent to the daily protein requirement (98 g/day), divided by the protein concentration in the amino acid mixture (50 g/L); i.e.,
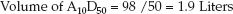 (49.2)
(49.2)
If this mixture is infused over 24 hours, the infusion rate will be:
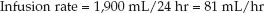 (49.3)
(49.3)
Step 3
Now, determine how many nonprotein calories will be provided by 1.9 liters of A10-D50. (Only nonprotein calories are used to provide the daily energy needs.) First, determine how much dextrose is in 1.9 liters of A10-D50:
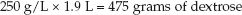 (49.4)
(49.4)
Now, using an energy yield of 3.4 kcal/g for dextrose, calculate the calories provided by 475 grams of dextrose:
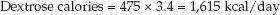 (49.5)
(49.5)
Step 4
The next step is to use lipid calories to make up the difference between the calories supplied by dextrose and the daily requirement for calories, as shown below:
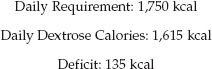 (49.6)
(49.6)
The remaining 135 calories will be provided by lipids. If a 10% lipid emulsion (1 kcal/mL) is used, the volume will be 135 mL/day. (Lipid emulsions are available in unit volumes of 50 mL, so the volume can be adjusted to 150 mL to avoid wastage). The maximum infusion rate is 50 mL/hr.
Step 5
The TPN orders for this example can be written as follows:
1. A10-D50 to run at 80 mL/hour.
2. 10% Intralipid, 150 mL, to infuse over 3 hours.
3. Add standard electrolytes, multivitamins, and trace elements.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








