Both measurements are volume-dependent and effort-dependent, so maximum patient effort is required for reliable recordings. The FEV1 is the preferred measurement because it is less variable and more likely to detect obstruction in the smaller airways (1,5). Comparison of both mea-surements in the acute care setting have shown that the PEFR underestimates the severity of airway obstruction (4).
SEVERE EXACERBATIONS: The measurement of FEV1 and PEFR requires a maximum inspiratory effort (to total lung capacity) followed by a maximum expiratory effort (to residual lung volume), and three consecutive measurements are recommended for optimal results. Patients with se-vere exacerbations of asthma and COPD are often unable to perform these maneuvers because of respiratory distress, and thus the FEV1 and PEFR have a limited role in the management of severe exacerbations of asthma and COPD (which includes most cases admitted to the ICU).
Intrinsic PEEP
For patients with asthma and COPD who require mechanical ventilation, the severity of airflow obstruction can be evaluated by monitoring a pressure known as intrinsic PEEP. This pressure is described in the final section of the chapter.
Aerosol Drug Therapy
Drug aerosols play a major role in the management of obstructive airways diseases. There are two basic designs for aerosol generators in clinical medicine: the jet nebulizer and the metered-dose inhaler. The operation of these devices is illustrated in Figure 24.1
Jet Nebulizer
The pneumatic or jet nebulizer uses the same principle as the air-entrainment device in Figure 22.5 (see page 435). A high-pressure gas source (e.g., 50 psi from a wall outlet) is passed through a narrow opening in the nebulizer, creating a high-velocity (jet) stream of gas that is passed over the opening of a narrow tube submerged in a drug solution. The gas jet draws the drug solution up the tube (by creating viscous drag) and then pulverizes the solution to create an aerosol spray that is inhaled by the patient. Standard jet nebulizers have a reservoir volume of 3–6 mL, and can completely aerosolize the reservoir volume in less than 10 minutes (6). A larger version (with a reservoir volume >200 mL) is available for continuous aerosol therapy (see later).
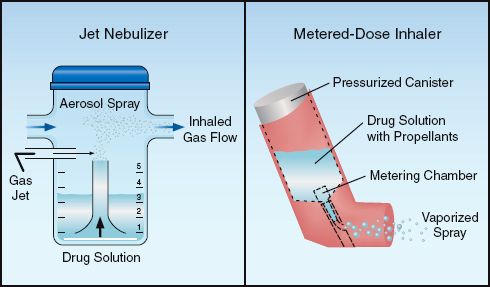
FIGURE 24.1 Devices used for aerosol drug therapy. See text for explanation.
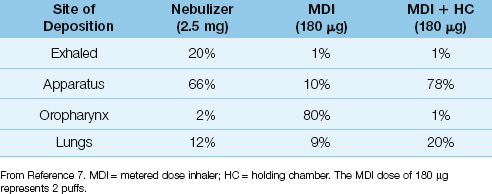
LUNG DEPOSITION: Although small-volume nebulizers can completely aerosolize a drug solution, only a fraction of the drug aerosol reaches the lungs. This is demonstrated in Table 24.1, which shows the distribution of aerosolized albuterol with different aerosol generator systems (7). When the nebulizer is used, most of the aerosol impacts on the delivery apparatus or is exhaled, and only 12% of the intended dose reaches the lungs. Inefficient drug delivery is a characteristic feature of aerosol drug therapy, and is not specific for the jet nebulizer.
Metered-Dose Inhaler
A metered-dose inhaler (MDI) operates like a canister of hair spray. The MDI has a pressurized canister that contains a drug solution with a boiling point below room temperature. When the canister is squeezed between the thumb and fingers, a valve opens that releases a fixed volume of the drug solution. The liquid immediately vaporizes when it emerges from the canister, and a liquid propellant in the solution creates a high-velocity spray.
LUNG DEPOSITION: The spray emerging from an MDI can have a velocity in excess of 30 meters per second (over 60 miles per hour) (8), and when this high-velocity spray is delivered directly into the mouth, most of the spray impacts on the posterior wall of the oropharynx and is not inhaled. This inertial impaction is reduced by using a spacer device or holding chamber to reduce the velocity of aerosol delivery. The influence of a holding chamber on drug delivery is shown in Table 24.1. When the MDI is used alone, 80% of the drug aerosol is deposited in the oropharynx, but when a holding chamber is used with the MDI, drug deposition in the mouth is almost completely eliminated, and the drug dose reaching the lungs is doubled. Results like this are the reason that holding chambers are recommended for all bronchodilator treatments with MDIs (6).
Table 24.1 Distribution of Inhaled Albuterol by Delivery System
Nebulizer vs. Metered-Dose Inhaler
One of the notable features of aerosol drug therapy is the equivalent bronchodilator responses produced by nebulizers and MDIs despite a large difference in drug dosage. This is demonstrated in Figure 24.2, which compares the bronchodilator response to albuterol delivered by nebulizer and MDI (with a holding chamber) in patients with acute exacerbation of asthma (9). There is no difference in the response in each of the 3 treatments. Using the distribution patterns in Table 24.1, the dose of albuterol deposited in the lungs in Figure 24.2 would be 12% of 2.5 mg or 250 µg for the nebulizer and 20% of 360 µg or 72 µg for the MDI with holding chamber. Thus, there is a 3.5-fold difference in drug dose in the airways, yet the bronchodilator responses are equivalent.
Mechanical Ventilation
Equivalent responses like those in Figure 24.2 have also been observed in ventilator-dependent patients (10,11). Drug deposition in the lungs is reduced further during mechanical ventilation (11) due to condensation on the endotracheal tube and ventilator tubing. However, the impact of this loss on bronchodilator responses is not clear (12). The response to MDIs is optimal when a holding chamber is used (11): the chamber is connected to the inspiratory limb of the ventilator tubing, and 5–8 puffs from the MDI are delivered into the chamber for inhalation during the ensuing lung inflations. Regardless of the aerosol device used, drug delivery into the airways can be enhanced by decreasing the inspiratory flow rate and increasing the duration of inspiration (13).
Summary
One thing is clear about aerosol therapy; i.e., it is an inefficient method of drug administration, regardless of the type of aerosol generator that is used. Yet, despite this inefficiency, drug aerosols are favored for administering bronchodilators in acute exacerbations of asthma and COPD, as described next.
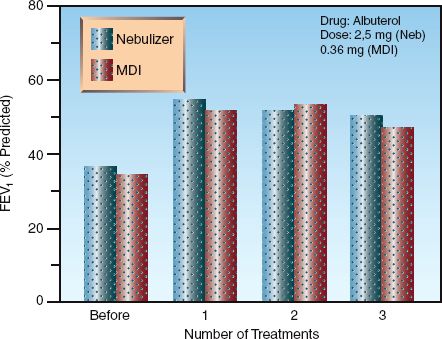
FIGURE 24.2 Equivalent responses to a bronchodilator (albuterol) given by nebulizer or metered-dose inhaler (MDI) with a holding chamber in patients with acute exacerbation of asthma. Treatments were given every 30 minutes using the doses indicated in the upper right portion of the graph (the MDI dose of 0.36 mg represents 4 puffs). FEV1 = forced expiratory volume in one second. Data from Reference 9.
ACUTE EXACERBATION OF ASTHMA
The flow diagram in Figure 24.3 summarizes the early management of adults with acute exacerbation of asthma (1). This protocol is based on objective measures of airway obstruction (i.e., FEV1 and peak expiratory flow rate), but clinical measures of disease severity (e.g., respiratory rate, use of accessory muscles) are also suitable (14,15). The recommended drugs and dosing regimens for acute asthma are summarized in Table 24.2.
β2-Receptor Agonists
The favored bronchodilators are drugs that stimulate α-adrenergic receptors in bronchial smooth muscle (β2 subtype). Aerosol delivery of these β2-agonists is preferred because it is more effective than oral (16) or intravenous (17) drug therapy, and has fewer side effects. Short-acting β2-agonists are preferred for the acute management of asthma because they can be given in rapid succession with less risk of drug accumulation (1).
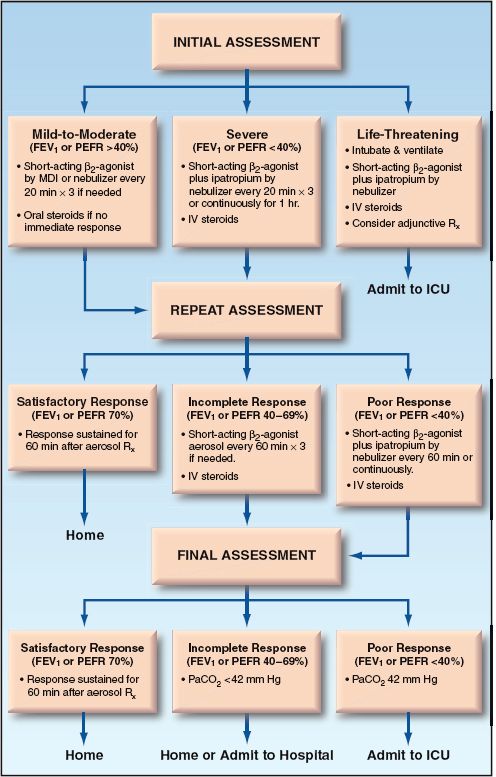
FIGURE 24.3 Flow diagram showing the early management of acute exacerbations of asthma, as recommended by the National Asthma Education Program (1). FEV1 = forced expiratory volume in one second, PEFR = peak expiratory flow rate.
Table 24.2 Drug Regimens for Acute Exacerbations of Asthma
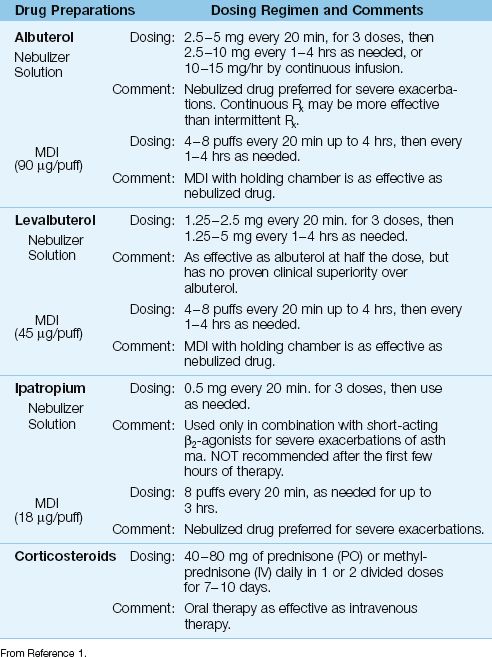
Albuterol is the most widely used short-acting β2-agonist for the acute management of asthma (1,14,15). Aerosolized albuterol has a rapid onset of action (less than 5 minutes), and a bronchodilator effect that lasts 2–5 hours (18). Levalbuterol is the R-enantiomer of albuterol (a more active form of the drug) that is equally effective at half the dose. However, clinical studies have not shown an advantage with levalbuterol over albuterol (1,14).
Aerosol Regimens
The following regimens of aerosolized albuterol are recommended for acute exacerbations of asthma (1,14,15):
1. The initial treatment involves up to three 20-minute treatments using 2.5 to 5 mg albuterol by nebulizer or 4–8 puffs (90 mg al-buterol per puff) by MDI with a holding chamber. Nebulizer delivery is preferred for patients with severe airflow obstruction (1), even though there is no evidence that nebulizers produce better bronchodilator responses than MDIs in acute asthma (19).
2. If further therapy is needed, albuterol can be given hourly for up to 3 hours, or can be given by continuous nebulization using doses of 5–15 mg/hr. Continuous aerosol therapy is popular, and may be more effective than intermittent aerosol therapy in patients with severe airflow obstruction (20).
3. For patients who are admitted to the hospital, albuterol (2.5–5 mg by nebulizer or 4–8 puffs by MDI) is given every 4–6 hours for the duration of the hospital stay.
Parenteral Therapy
For the rare asthmatic patient who does not tolerate bronchodilator aerosols (usually because of excessive coughing) parenteral therapy can be given using subcutaneous epinephrine (0.3 to 0.5 mg every 20 minutes for 3 doses) or subcutaneous terbutaline (0.25 mg every 20 minutes for 3 doses) (1). However, it is important to emphasize that parenteral β-agonist therapy is not more effective than aerosol therapy, and is more likely to produce unwanted side effects (21).
Side Effects
High-dose aerosol therapy with β2-agonists can produce a number of side effects, including tachycardia, tremors, hyperglycemia, and a decrease in serum potassium, magnesium, and phosphate levels (22,23). Cardiac ischemia has been reported, but is rare (22). The decrease in serum potassium is the result of a β-Receptor-mediated shift of potassium into cells. This effect is notable because large doses of inhaled β2-agonists (e.g., 20 mg albuterol) have been used for the acute management of hyperkalemia (24). (Note: This is not a favored treatment for hyperkalemia because of the side effects of high-dose β2-agonists.)
Anticholinergic Agents
Anticholinergic agents offer only marginal benefits in acute asthma, and their use is restricted to combination therapy with short-acting β2-agonists for severe exacerbations, and only for the first 3–4 hours of management (1,25). The only anticholinergic agent approved for use in the United States is ipatropium bromide, a derivative of atropine that blocks muscarinic receptors in the airways. The dose in acute asthma is 0.5 mg (which can be mixed with albuterol in the nebulizer) every 20 minutes for 3 doses, then as needed, or 8 puffs (18 ∝g per puff) by MDI every 20 min as needed for up to 3 hours (1). Systemic absorption is minimal, and there is little risk of anticholinergic side effects (e.g., tachycardia, dry mouth, blurred vision, urinary retention). Ipatropium has no proven benefit beyond the first few hours of management, and it should be discontinued in patients who are admitted for continued asthma management (1).
Corticosteroids
Corticosteroids are considered a staple in the management of both acute and chronic asthma. In acute asthma, the bulk of evidence shows that corticosteroids accelerate the rate of resolution and reduce the risk of relapses (26), although not all studies show a benefit from corticosteroids (27,28). The following features of steroid therapy deserve mention:
1. There is no difference in efficacy between oral and intravenous steroids (26,29).
2. The beneficial effects of steroids are often not apparent until 12 hours after therapy is started (29), so steroid therapy will not influence the clinical course of asthma in the emergency department.
3. There is no apparent dose-response curve for steroids (29), and no evidence that doses above 100 mg of prednisone daily (or equivalent doses of other steroids) provide added benefit in acute asthma (26).
4. A 10-day course of steroids can be stopped abruptly without a tapering dose (26,30).
Regimen in Acute Asthma
The National Asthma Education Program includes the following recommendations for corticosteroid therapy in acute exacerbations of asthma (1).
1. Steroids are recommended for all patients who do not show a satisfactory response after one or two bronchodilator treatments.
2. Oral steroids are recommended for patients who can tolerate oral medications.
3. The recommended dose is 40–80 mg daily of prednisone (for oral therapy) or methylprednisolone (for intravenous therapy) in one or two divided doses, which is continued until there is evidence of satisfactory resolution.
4. If the duration of therapy is less than 10 days, there is no need for a steroid taper.
5. Inhaled corticosteroids can be started at any time during treatment of an acute exacerbation of asthma, and are continued after systemic steroids are discontinued to reduce the risk of relapses.
Mechanism of Action?
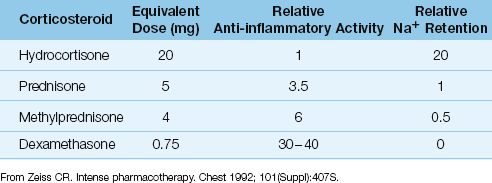
Acute asthma is considered more of an inflammatory condition than a bronchospastic condition, and the beneficial effects of steroids are attributed to their anti-inflammatory actions. However, as shown in Table 24.3, dexamethasone is the most potent anti-inflammatory corticosteroid, yet it is not recommended for the treatment of asthma. This is either an oversight, or it raises questions about the mechanism of action of steroids in asthma.
Table 24.3 Comparison of Therapeutic Corticosteroids
Steroid Myopathy
An acute myopathy has been reported in ventilator-dependent asthmatic patients treated with high-dose steroids and neuromuscular blocking agents (31). Unlike the traditional steroid myopathy, which is characterized by proximal muscle weakness, this condition involves both proximal and distal muscles, and is often associated with rhabdomyolysis. The muscle weakness can be prolonged (although it usually resolves) and can hamper weaning from mechanical ventilation. Because of the risk of this myopathy, it seems wise to avoid neuromuscular paralysis whenever possible in steroid-treated ventilator-dependent asthmatic patients.
Additional Considerations
The following are some additional considerations for the management of acute asthma:
1. Asthma exacerbations are often triggered by viral infections, and empiric antibiotic therapy is not advised the absence of a treatable infection (1).
2. Intravenous magnesium (2 grams over 20 min) has mild bronchodilator effects (possibly as a result of calcium channel blockade), and can be used as an adjunctive measure for severe exacerbations of asthma (1). However, magnesium administration has no impact on the clinical course of acute asthma (32).
3. An arterial blood gas is advised for patients who do not show a satisfactory response to bronchodilators in the emergency department. A normal PCO2 in acute asthma indicates severe airflow obstruction, and warrants admission to the ICU.
4. Intubation and mechanical ventilation can be problematic in acute exacerbations of asthma; this aspect of management is described in the final section of the chapter.
ACUTE EXACERBATION OF COPD
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States (33) and, despite a marked reduction in the prevalence of cigarette smoking in adults, hospital admissions for acute exacerbations of COPD increased by 60% over a recent 10-year period (34). About half of these admissions require care in an ICU (35).
An acute exacerbation of COPD is defined as “a change in the patient’s baseline dyspnea, cough, or sputum production that is beyond the normal day-to-day variation” (2). Most of these exacerbations are the result of infection (usually confined to the airways), but as many one of every four or five cases may be the result of an acute pulmonary embolism (36). In about one-third of patients, a precipitating event is never identified (2).
Bronchodilator Therapy
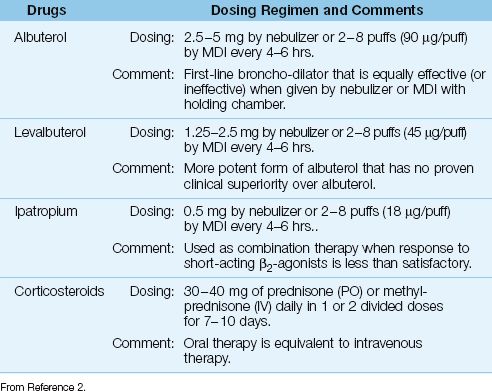
Although a distinguishing feature of COPD is lack of responsiveness to bronchodilators (in contrast to asthma), bronchodilator therapy is used routinely in patients with COPD (2). The same bronchodilator drugs used in acute ex-acerbations of asthma are also recommended for acute exacerbations of COPD, but the dosing regimens differ, as shown in Table 24.4. Ipatropium is used as combination therapy when the response to short-acting β2-agonists is less than satisfactory, although at least three clinical studies do not support this practice (37).
Corticosteroids
A short course (7–10 days) of corticosteroid therapy is associated with fewer treatment failures and a shorter duration of mechanical ventilation in acute exacerbations of COPD (38,39). However, at least 10 patients must be treated with corticosteroids to produce one favorable response (38), so the impact of steroid therapy is limited. The recommended steroid regimen for acute exacerbations of COPD is shown in Table 24.4 (2). Note that the dose range is slightly lower than the dose range for treating acute asthma. Intravenous steroids offer no advantage over oral steroids in acute exacerbations of COPD (40), similar to the observations in acute asthma (26,29).
Antibiotics
Airways infections (viral and bacterial) are responsible for at least 50% of acute exacerbations of COPD (2), but many are nontreatable viral infections, and this limits the benefit of antimicrobial therapy.
Indications
Antibiotic therapy is not determined by sputum cultures in acute exacerbations of COPD because the same organisms are often isolated during clinically stable periods and during acute exacerbations (41). Instead, the clinical severity of disease is used to determine the need for antibiotic therapy. Clinical studies have shown that antibiotic therapy is most likely to improve clinical outcomes when exacerbations of COPD are severe enough to require hospitalization (34,42), and particularly when mechanical ventilation is required (2,43). This means that all ICU patients with acute exacerbations of COPD are candidates for antibiotic therapy.
Table 24.4 Drug Regimens for Acute Exacerbations of COPD
Antibiotic Regimens
The pathogens most often isolated from the lower respiratory tract in acute exacerbations of COPD are Hemophilus influenzae and Streptococcus pneumoniae (2,41). Pseudomonas aeruginosa is also a prominent isolate in advanced cases of COPD and in ventilator-dependent patients (44). Antibiotics with activity against all these pathogens are advised for ICU patients with acute exacerbations of COPD. These antibiotics include levofloxacin, pipericillin-tazobactam, and imipenem or meropenem. Duration of antibiotic therapy is typically 5-7 days.
Oxygen Therapy
In cases of severe COPD with chronic hypercapnia, high concentrations of inhaled O2 can promote further increases in arterial PCO2. This was originally attributed to loss of hypoxic ventilatory drive (45 ), but more recent studies have shown that the oxygen-induced rise in arterial PCO2 is not accompanied by a decrease in ventilatory drive (46). Oxygen unloading of CO2 from hemoglobin may play a role in this phenomenon. Regardless of the mechanism involved, it is important to avoid high concentrations of inhaled O2 in patients with chronic CO2 retention.
The best practice for oxygen therapy in patients with chronic CO2 retention is to keep the FIO2 (fractional concentration of inhaled O2) as low as possible, and use air-entrainment devices to maintain a constant FIO2. (These devices are described on pages 434–435). If a high FIO2 is needed to maintain adequate arterial oxygenation, then you should monitor the patient’s mental status closely (for signs of CO2 narcosis), and check the arterial PCO2 and pH periodically. Undesirable increases in arterial PCO2 in this situation is an indication for ventilatory assistance (noninvasive ventilation or conventional mechanical ventilation).
MECHANICAL VENTILATION
Over 50% of patients admitted to the ICU because of asthma or COPD are placed on mechanical ventilation (47,48), and the following are some of the major considerations related to positive pressure ventilation in these patients.
Dynamic Hyperinflation
During spontaneous breathing in normal subjects, inhaled gas is completely exhaled before the end of expiration. In this situation, there is no expiratory airflow at the end of expiration, so the pressure in the distal airspaces is equivalent to atmospheric (zero reference) pressure. This is illustrated in the lower pressure-volume loop in Figure 24.4. In patients with airways obstruction from asthma or COPD, exhalation is prolonged, and when the airways obstruction is severe, exhalation is not completed before the next inhalation. This results in hyperinflation, called dynamic hyperinflation, and the trapped gas in the distal airspaces creates a positive end-expiratory pressure (PEEP), which is called intrinsic PEEP or auto-PEEP (49). This is illustrated by the upper hysteresis loop in Figure 24.4. Note that, in the presence of hyperinflation and intrinsic PEEP, breathing occurs on a flatter portion of the pressure-volume curve, which means that the respiratory muscles must generate a higher transpulmonary pressure to draw the normal volume of air into the lungs. This creates an increased work of breathing in patients with severe airflow obstruction.
Positive Pressure Ventilation
The shift in the pressure volume curves for breathing caused by dynamic hyperinflation means that positive-pressure mechanical ventilation will create positive intrathoracic pressures that are higher than normal in patients with dynamic hyperinflation. Furthermore, mechanical ventilation can add to the hyperinflation (e.g., by delivering high inflation volumes) (50). Dynamc hyperinflation can have two adverse consequences: (a) overdistention of alveoli, which can lead to ventilator-induced lunginjury, and (b) an increase in intrathoracic pressure, which can impede venous return to the heart. The following measures can help to reduce the risk of these adverse consequences.
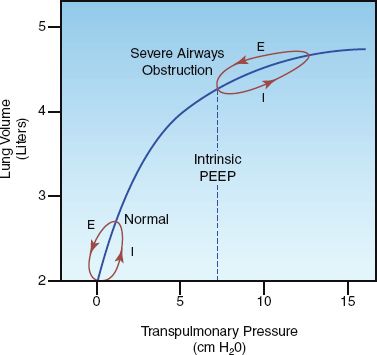
FIGURE 24.4 Pressure-volume curves showing the effects of severe airways obstruction on lung volumes and transpulmonary pressures. The hysteresis loops show the pressure and volume changes during inspiration (I) and expiration (E) for a single breath. See text for further explanation.
Monitoring
Dynamic hyperinflation can be detected by monitoring the flow waveforms displayed by mechanical ventilators. This is illustrated in Figure 24.5. The normal flow waveforms in the upper panel show that the expiratory flow ceases before the next lung inflation, while the flow waveforms in the lower panel show that expiratory flow is continuing when the next lung inflation is delivered. The presence of expiratory flow at the end of expiration is evidence of dynamic hyperinflation.
INTRINSIC PEEP: When there is evidence of dynamic hyperinflation on the flow waveforms, the severity of the problem can be evaluated by monitoring the level of intrinsic PEEP. (The measurement of intrinsic PEEP is described in Chapters 25 and 27). The intrinsic PEEP level provides a measure of the severity of airways obstruction in patients with asthma and COPD who require mechanical ventilation.
Ventilator Strategies
The following measures are designed to limit dynamic hyperinflation:
1. Ventilate with low tidal volumes (6 mL/kg) using the lung protective ventilation protocol in Table 23.4 (see page 455).
2. Maximize the time for expiration by: (a) preventing rapid respiratory rates (with sedation, if possible, or neuromuscular paralysis, if necessary) and (b) maintaining inspiratory:expiratory ratio of 1:2 or higher.
Noninvasive Ventilation
Positive pressure ventilation can be delivered with tight-fitting face masks instead of endotracheal tubes. This type of noninvasive mechanical ventilation avoids the adverse effects of endotracheal intubation (e.g., patient discomfort, increased risk of nosocomial pneumonia), but is not appropriate for all patients. Patients are not candidates for noninvasive ventilation if they are unresponsive, have facial trauma, have severe circulatory compromise, have impending cardiac or respiratory arrest, or have copious respiratory secretions and are unable to clear secretions effectively.
Noninvasive ventilation has been used most successfully in acute exacerbations of COPD associated with progressive hypercapnia (51). There is far less experience in acute asthma, but the few available studies show that noninvasive ventilation can hasten the resolution of the acute episode and reduce the number of patients who require intubation (52).
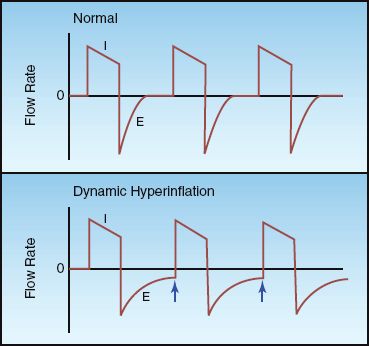
FIGURE 24.5 Flow waveforms during positive pressure mechanical ventilation. The waveforms in the lower panel show continuing expiratory flow at the end of expiration (indicated by the arrows), which indicates the presence of dynamic hyperinflation. I = inspiration, E = expiration.
Start Early
Noninvasive ventilation is a consideration in patients with acute exacerbation of asthma or COPD who do not respond adequately to initial medical management, and it is most likely to be successful when instituted early, before progression to life-threatening CO2 retention and impending respiratory arrest. This is particularly important in patients with severe asthma, who do not develop hypercapnia until late in the progression to life-threatening ventilatory failure (52).
(Note: Noninvasive ventilation is presented in detail in Chapter 27.)
A FINAL WORD
Keeping It Simple
The management of patients admitted to the ICU because of severe exacerbations of asthma or COPD can be summarized in the following two statements:
1. Give bronchodilators and corticosteroids to all patients, plus antibiotics for patients with severe COPD.
2. If the condition progresses despite the above measures, use noninvasive ventilation if possible, or conventional mechanical ventilation if necessary.
That’s about it.
REFERENCES
Clinical Practice Guidelines
1. National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Full Report 2007. NIH Publication No. 07–4051; August, 2007. (Available at www.nhlbi.nih.gov/ guidelines/asthma)
2. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. The GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555.
Measures of Airway Obstruction
3. Shim CS, Williams MH. Evaluation of the severity of asthma: patients versus physicians. Am J Med 1980; 68:11–13.
4. Langhan ML, Spiro DM. Portable spirometry during acute exacerbations of asthma in children. J Asthma 2009; 46:122–125.
5. Pellegrino R, Viegl G, Brusasco V, et al. Interpretive strategies for lung function tests. Eur Respir J 2005; 26:948–968.
Aerosol Drug Therapy
6. Fink J. Aerosol drug therapy. In Wilkins RL, Stoller JK, Kacmarek RM, eds. Egan’s Fundamentals of Respiratory Care. St. Louis, MO: Mosby, Inc. 2009; 801–839.
7. Fink JB. Metered-dose inhalers, dry powder inhalers, and transitions. Respir Care 2000; 45:623–635.
8. Clarke SW, Newman SP. Differences between pressurized aerosol and stable dust particles. Chest 1981; 80(Suppl):907–908.
9. Idris AH, McDermott MF, Raucci JC, et al. Emergency department treatment of severe asthma. Metered-dose inhaler plus holding chamber is equivalent in effectiveness to nebulizer. Chest 1993; 103:665–672.
10. Dhand R, Tobin MJ. Pulmonary perspective: Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med 1997; 156:3–10.
11. AARC Clinical Practice Guideline. Selection of device, administration of bronchodilator, and evaluation of response to therapy in mechanically ventilated patients. Respir Care 1999; 44:105–113.
12. Smalldone GC. Aerosolized bronchodilators in the intensive care unit. Much ado about nothing? Am Rev Respir Crit Care Med 1999; 159:1029–1030.
13. Mantous CA, Hall JB. Update on using therapeutic aerosols in mechanically ventilated patients. J Crit Illness 1996; 11:457–468.
Acute Exacerbations of Asthma
14. Lazarus SC. Emergency treatment of asthma. N Engl J Med 2010; 363:755–764.
15. Mannam P, Seigel MD. Management of life-threatening asthma in adults. J Intensive Care Med 2010; 25:3–15.
16. Shim C, Williams MH. Bronchial response to oral versus aerosol metaproterenol in asthma. Ann Intern Med 1980; 93:428–431.
17. Salmeron S, Brochard L. Mal H, et al. Nebulized versus intravenous albuterol in hypercapnic acute asthma. Am J Respir Crit Care Med 1994; 149:1466–1470.
18. Dutta EJ, Li JTC. β-agonists. Med Clin N Am 2002; 86:991–1008.
19. Dhuper S, Chandra A, Ahmed A, et al. Efficacy and cost comparisons of bronchodilator administration between metered dose inhalers with disposable spacers and nebulizers for acute asthma treatment. J Emerg Med 2011; 40:247–255.
20. Peters SG. Continuous bronchodilator therapy. Chest 2007; 131:286–289.
21. Travers AH, Rowe BH, Barker S, et al. The effectiveness of IV beta-agonists in treating patients with acute asthma in the emergency department: A meta-analysis. Chest 2002; 122:1200–1207.
22. Truwit JD. Toxic effect of bronchodilators. Crit Care Clin 1991; 7:639–657.
23. Bodenhamer J, Bergstrom R, Brown D, et al. Frequently nebulized beta-agonists for asthma: effects on serum electrolytes. Ann Emerg Med 1992; 21:1337–1342.
24. Allon M, Dunlay R, Copkney C. Nebulized albuterol for acute hyperkalemia in patients on hemodialysis. Ann Intern Med 1989; 110:426–429.
25. Rodrigo G, Rodrigo C. The role of anticholinergics in acute asthma treatment. An evidence-based evaluation. Chest 2002; 121:1977–1987.
26. Krishnan JA, Davis SQ, Naureckas ET, et al. An umbrella review: corticosteroid therapy for adults with acute asthma. Am J Med 2009; 122:977–991.
27. Stein LM, Cole RP. Early administration of corticosteroids in emergency room treatment of asthma. Ann Intern Med 1990; 112:822–827.
28. Morrell F, Orriols R, de Gracia J, et al. Controlled trial of intravenous corticosteroids in severe acute asthma. Thorax 1992; 47:588–591.
29. Rodrigo G, Rodrigo C. Corticosteroids in the emergency department therapy of acute adult asthma. An evidence-based evaluation. Chest 1999; 116:285–295.
30. Cydulka RK, Emerman CL. A pilot study of steroid therapy after emergency department treatment of acute asthma: Is a taper needed? J Emerg Med 1998; 16:15–19.
31. Griffin D, Fairman N, Coursin D, et al. Acute myopathy during treatment of status asthmaticus with corticosteroids and steroidal muscle relaxants. Chest 1992; 102:510–514.
32. Rowe BH, Bretzlaff JA, Bourdon C, et al. Intravenous magnesium for treatment of acute asthma in the emergency department: a systematic review of the literature. Ann Emerg Med 2000; 36:181–190.
Acute Exacerbations of COPD
33. National Center for Health Statistics. Health, United States, 2011: with Special Feature on Socioeconomic Status and Health. Hyattville, MD, 2012.
34. Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD. Chest 2008; 133:756–766.
35. Stoller JK. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002; 346:988–994.
36. Rizkallah J, Man P, Sin DD. Prevalence of pulmonary embolism in acute exacerbations of COPD. Chest 2009; 135:786–793.
37. McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD. A summary and appraisal of published evidence. Chest 2001; 119:1190–1209.
38. Walters JAE, Gibson PG, Wood-Baker R, et al. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews, 2009; 1:CD001288.
39. Immaculada A, de la Cal MA, Esteban A, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Arch Intern Med 2011; 171:1939–1946.
40. Lindenauer PK, Pekow PS, Lahiti MC, et. al. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA 2010; 303:2359–2367.
41. Monso E, Ruiz J, Rosell J, et al. Bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152:1316–1320.
42. Rothberg MR, Pekow PS, Lahti M, et al. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA 2010; 303:2035–2042.
43. Nouira S, Marghli S, Belghith M, et al. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized, placebo-controlled trial. Lancet 2001; 358:2020–2025.
44. Murphy TF. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr Opin Pulm Med 2009; 15:138–142.
45. Campbell EJM. The J. Burns Amberson Lecture. The management of acute respiratory failure in chronic bronchitis and emphysema. Am Rev Respir Crit Care Med 1967; 96:626–639.
46. Aubier M, Murciano D, Fournier M, et al. Central respiratory drive in acute respiratory failure or patients with chronic obstructive pulmonary disease. Am Rev Respir Crit Care Med 1980; 122:191–199.
Mechanical Ventilation
47. Peters JI, Stupka JE, Singh H, et al. Status asthmaticus in the medical intensive care unit: a 30-year experience. Respir Med 2012; 106:344–348.
48. Soo Hoo GW, Hakimian N, Santiago SM. Hypercapnic respiratory failure in COPD patients” response to therapy. Chest 2000; 117:169–177.
49. Blanch L, Bernabe F, Lucangelo U. Measurement of air trapping, intrinsic positive end-expiratory pressure, and dynamic hyperinflation in mechanically ventilated patients. Respir Care 2005; 50:110–123.
50. Pepe P, Marini JJ. Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction. The auto-PEEP effect. Am Rev Respir Dis 1982; 126:166–170.
51. Boldrini R, Fasano L, Nava S. Noninvasive mechanical ventilation. Curr Opin Crit Care 2012; 18:48–53.
52. Scala R. Noninvasive ventilation in severe acute asthma? Still far from the truth. Respir Care 2010; 55:630–637.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








