1978
For patients who are unable to eat, the preferred method of nutrition support is the infusion of liquid feeding formulas into the stomach or small bowel (1,2). This mimics the normal process of nutrition support, and also serves as an infection control measure, as will be described.
This chapter presents the fundamentals of nutrition support with enteral tube feedings, and will show you how to create a tube feeding regimen for each patient in the ICU.
GENERAL CONSIDERATIONS
Infectious Risk
The preference for enteral over parenteral nutrition is based on numerous studies showing that enteral nutrition is associated with fewer infections (1–4), particularly pneumonias. This is attributed to the trophic effects of nutritional bulk that maintain the barrier and immunological functions of the bowel, as described next.
Mechanisms
The role of enteral tube feedings in protecting against infections is summarized in the following statements.
1. The presence of food or tube feedings in the lumen of the bowel has a trophic influence on the bowel mucosa that preserves the structural integrity of the mucosa (5,6). This maintains the barrier function of the bowel mucosa, which protects against invasion from enteric pathogens, a phenomenon known as translocation (7).
2. The trophic influence of luminal nutrition also extends to the im-mune defenses in the bowel, such as the production of immunoglobulin A (IgA) by monocytes in the bowel wall, which blocks the attachment of pathogens to the bowel mucosa (8).
3. These effects are triggered by the presence of nutritional bulk in the lumen of the bowel (9), and are mediated, in part, by the release of gastrin and cholecystokinin in response to gastric distension (1). Specific nutrients in the bowel lumen also participate in these ef-fects. One of these nutrients is glutamine, which is a principal fuel for enterocytes in the bowel mucosa (10).
4. The trophic effects of nutritional bulk are lost during periods of bowel rest, and this results in progressive atrophy of the bowel mucosa (6), and can lead to translocation and the systemic spread of enteric pathogens (11). Parenteral nutrition does not prevent the deleterious effects of prolonged bowel rest (1,11).
The sum of these observations indicates that the normal antimicrobial defenses in the bowel are sustained by the presence of nutritional bulk in the bowel lumen. This is how nutrition support with enteral tube feedings serves as an infection control measure, as stated in the introduction to the chapter.
Who and When
Patients who are unable to eat and do not have the absolute contraindications described next are candidates for enteral tube feeding. The presence of bowel sounds is not required to initiate enteral tube feedings (1). Tube feedings should be started within 24–48 hours of admission to the ICU (1) to take advantage of the protective effects of tube feedings. There is evidence that early institution of enteral nutrition is associated with fewer septic complications and a shorter hospital stay (12).
Contraindications
Absolute contraindications to enteral tube feedings include complete bowel obstruction, bowel ischemia, ileus, and circulatory shock with high-dose vasopressor requirements (1,2). Gastric feedings can be attempted in stable patients on low doses of vasopressors (1), but any signs of intolerance should prompt immediate cessation of feedings.
FEEDING FORMULAS
There are at least 200 enteral feeding formulas that are commercially available, and many of the formulas used at individual hospitals are from the same manufacturer (because of contractual obligations). The following is a brief description of some characteristic features of feeding formulas. Examples are provided in Tables 48.1 and 48.2
Caloric Density
Feeding formulas are available with caloric densities of 1 kcal/mL, 1.5 kcal/mL, and 2 kcal/mL. Most tube feeding regimens use formulas with 1 kcal/mL. The high-calorie formulas (2 kcal/mL) are intended for patients with severe physiological stress (e.g., multisystem trauma and burns), but they are frequently used when volume restriction is a priority.
Table 48.1 Standard Formulas for Enteral Nutrition
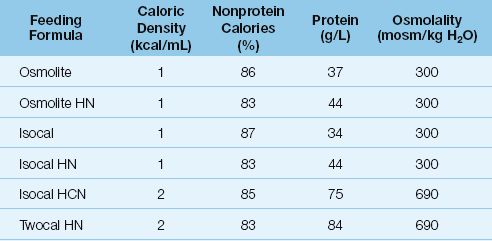
Nonprotein Calories
The caloric density of feeding formulas includes both protein and nonprotein calories, but daily caloric requirements should be provided by nonprotein calories (as mentioned in Chapter 47). In standard feeding formulas, nonprotein calories account for about 85% of the total calories (see Table 48.1).
Osmolality
The osmolality of feeding formulas is determined primarily by the caloric density. Feeding formulas with 1 kcal/mL have an osmolality similar to plasma (280–300 mosm/kg H2O), and feeding formulas with 2 kcal/mL have an osmolality about twice that of plasma. Hypertonic feedings create little risk of diarrhea when they are delivered into the stomach, where the large volume of gastric secretions attenuates the osmolality.
Protein Content
Standard feeding formulas provide 35–40 grams of protein per liter. High-protein formulas, often designated by the suffix HN (for “high nitrogen”), provide about 20% more protein than the standard formulas (compare Isocal and Isocal HN in Table 48.1).
Most enteral formulas contain intact proteins that are broken down into amino acids in the upper GI tract. These are called polymeric formulas. Feeding formulas are also available that contain small peptides (called semi-elemental formulas) and individual amino acids (called elemental formulas) that are absorbed more readily than intact protein. Semi-elemental and elemental formulas promote water reabsorption from the bowel, and could benefit patients with troublesome diarrhea. However, the clinical benefit of these formulas is unproven (14). Examples of semi-elemental and elemental formulas include Optimental, Peptamen, Perative, Vital HN, and Vivonex T.E.N.
Carbohydrate Content
Carbohydrates (usually polysaccharides) are the major source of calories in feeding formulas, and provide 40–70% of the total calories. Low-carbohydrate formulas, in which carbohydrates provide 30–40% of the calories, are available for diabetics. One example of a low-carbohydrate formula is Glucerna.
Fiber
The term “fiber” refers to polysaccharides from plants that are not digested by humans. Fiber is fermented by bacteria in the colon, and is broken down into short-chain fatty acids, which are an important energy source for the mucosal cells in the large bowel (13). The uptake of these fatty acids into the bowel mucosa also promotes sodium and water absorption. This “fermentable” fiber promotes the growth and viability of the surface mucosa in the large bowel, and can also reduce the water content of stool. There is also “nonfermentable” fiber that is not broken down by gut bacteria. This type of fiber draws water into the bowel and increases the water content of stool.
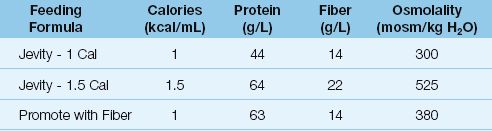
Fiber is added to some feeding formulas to promote the viability of the mucosa in the large bowel, and some examples of fiber-containing formulas are shown in Table 48.2. The fiber in most feeding formulas is a mixture of fermentable and nonfermentable varieties.
Table 48.2 Feeding Formulas Enriched With Fiber
Lipid Content
Standard feeding formulas contain polyunsaturated fatty acids from vegetable oils. The lipid content is adjusted to provide about 30% of the caloric density of the formula.
Omega-3 Fatty Acids
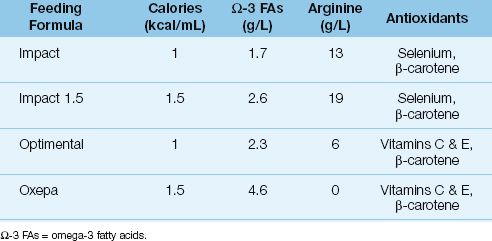
Polyunsaturated fatty acids from vegetable oils (which make up the lipid content of standard feeding formulas) can serve as precursors for inflammatory mediators (eicosanoids) that are capable of promoting inflammatory cell injury. This concern has prompted the introduction of feeding solutions that contain polyunsaturated fatty acids from fish oils (omega-3 fatty acids), which do not promote the production of inflammatory mediators. Some of these feeding formulas are shown in Table 48.3. The use of feeding formulas that influence the inflammatory response is known as immunonutrition (14).
Table 48.3 Immune-Modulating Feeding Formulas
Clinical studies have shown that patients with acute respiratory distress syndrome (ARDS) derive some benefit (fewer days on the ventilator) from feeding formulas enriched with omega-3 fatty acids and antioxidants (15). However, the benefits are marginal, and there is a general reluctance to adopt these feeding formulas for patients with ARDS.
Conditionally Essential Nutrients
Non-essential nutrients can become essential (i.e., require exogenous support) in conditions of increased utilization. Two of these conditionally essential nutrients deserve mention.
Arginine
Arginine is a preferred metabolic substrate for injured muscle, and can become depleted in conditions like multisystem trauma. Arginine also promotes wound healing, and is a precursor of nitric oxide (16). At least 8 enteral feeding formulas contain arginine in concentrations of 8–19 g/L, but the optimal intake of arginine is not known because there is no daily requirement for this amino acid.
POTENTIAL HARM: Arginine is a common additive in immune-modulating feeding formulas, and postoperative patients seem to benefit most from arginine-enriched feeding formulas (14). However, there are reports of increased mortality associated with arginine-enriched feeding formulas in patients with severe sepsis (1,17). The presumed mechanism is arginine-induced formation of nitric oxide, with subsequent vasodilation and hypotension. At the present time, arginine-containing feeding formulas are not advised for patients with severe sepsis (1).
Carnitine
Carnitine is necessary for the transport of fatty acids into mitochondria for fatty acid oxidation. Hypercatabolic conditions can promote carnitine deficiency (18), which is characterized by a myopathy involving the heart and skeletal muscle. A deficiency state is suggested by plasma carnitine concentration <20 mmol/L.
The recommended daily intake of carnitine is 20–30 mg/kg in adults (19). Feeding formulas that provide supplemental carnitine include Glucerna, Isocal HN, Jevity, and Peptamen.
One Feeding Formula for All?
Despite the confusing array of liquid feeding formulas, including the “designer formulas,” there is very little consistent or convincing evidence that one feeding formula, or one type of formula, is superior to the others. In other words a single feeding formula could be used for all ICU patients (with an occasional exception), as long as it is used appropriately.
CREATING A FEEDING REGIMEN
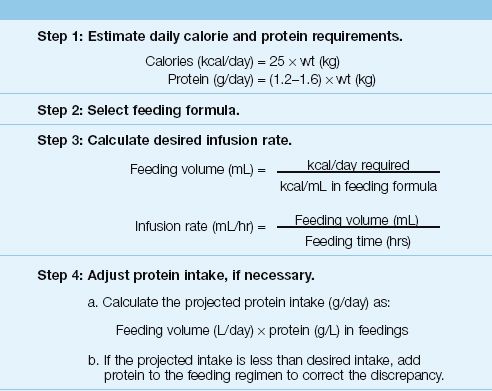
This section describes a simple four-step method for creating an enteral feeding regimen. This method is summarized in Table 48.4.
Step 1. Estimate daily energy and protein requirements.
The very first consideration is the patient’s daily requirement for calories and protein, and both requirements can be estimated with the simple predictive formulas in Table 48.2. (See Chapter 47 for more information on these formulas.) You can use actual body weight in the formulas as long as actual body weight is not above 125% of the ideal weight. If actual body weight exceeds 125% of ideal body weight, you should use an adjusted body weight, which is derived in Equation 47.3. If available, indirect calorimetry should be used to measure the resting energy expenditure (see Chapter 47).
Step 2. Select a feeding formula.
A standard formula, with 1–1.5 kcal/mL, should be sufficient for most patients. Use formulas with higher caloric densities if volume restriction is a priority.
Step 3. Calculate the desired infusion rate.
To determine the desired infusion rate for feeding, first calculate the volume of the feeding formula that must be infused to meet the daily re-quirement for calories (i.e., the daily caloric requirement in kcal/day divided by the caloric density of the feeding formula in kcal/mL). Next, divide the feeding volume (L/hr) by the number of hours each day that the feeding formula will be infused.
There are two considerations at this stage:
a. If propofol is being infused, subtract the calories provided by propofol (1 kcal/mL) from the daily caloric requirement. Propofol is infused in a 10% lipid emulsion, which has a caloric density of 1 kcal/mL. There-fore, the hourly infusion rate of propofol (mL/hr) is equivalent to the hourly yield of calories from propofol (kcal/hr).
b. Use nonprotein calories to provide the daily caloric requirement (so protein can be used for muscle strength, etc). This requires an ad-justment of the calories provided by the feeding formula. (In standard feeding formulas, nonprotein calories account for about 85% of the total calories.)
Step 4. Adjust the protein intake, if necessary.
The final step in the process is to determine if the feeding regimen will provide enough protein to satisfy the daily protein requirement (from step 1). The projected protein intake is simply the daily feeding volume multiplied by the protein concentration in the feeding formula. If the projected protein intake is less than the desired protein intake, powdered protein is added to the tube feedings to correct the discrepancy.
Table 48.4 Creating an Enteral Feeding Regimen
INITIATING TUBE FEEDINGS
Placement of Feeding Tubes
Feeding tubes are inserted through the nares and advanced blindly into the stomach or duodenum. The distance required to reach the stomach can be estimated by measuring the distance from the tip of the nose to the earlobe and then to the xiphoid process (typically 50–60 cm) (20). Once the tube is advanced the desired length, a portable chest x-ray is required to verify proper tube position before the feeding formula is infused. The common practice of evaluating tube placement by pushing air through the tube and listening for bowel sounds is not reliable, because sounds emanating from a misplaced tube in the distal airways or pleural space can be transmitted into the upper abdomen (21,22).
Tube Misplacement
Feeding tubes end up in the trachea during 1% of insertions (23). In-tubated patients often do not cough when feeding tubes enter the trachea (unlike healthy subjects); as a result, feeding tubes can be advanced deep into the lungs without any warning signs, and can puncture the visceral pleura and create a pneumothorax (21,22). The portable chest x-ray in Figure 48.1 shows a feeding tube that has been advanced almost to the edge of the right lung in a patient with a tracheostomy. This was a routine post-insertion chest x-ray, and there was no evidence that the tube was in the airways other than the chest x-ray. This illustrates the value of a portable chest x-ray immediately after a feeding tube is inserted, and before the feeding formula is infused. (The pleural effusion on the right in Figure 48.1 was present prior to insertion of the feeding tube.)
Gastric vs. Duodenal Placement
Advancing the tip of the feeding tube into the duodenum, to reduce the risk of aspiration, is not necessary (1) because most studies show that there is no difference in the risk of aspiration with gastric versus duodenal feedings (24,25). However, duodenal placement may be necessary in patients who regurgitate intragastric feedings.
Starter Regimens
The traditional practice is to begin tube feedings at a low infusion rate (10–20 ml/hr), and then gradually advance to the target infusion rate over the next 6–8 hours. However, gastric feedings can begin at the desired (target) rate in most patients without the risk of vomiting or aspiration (26,27). Starter regimens are more appropriate for small bowel feedings (particularly in the jejunum) because of the limited reservoir capacity of the small bowel.
COMPLICATIONS
The complications associated with enteral tube feedings include occlusion of the feeding tube, regurgitation of the feeding formula into the mouth and airways, and diarrhea.
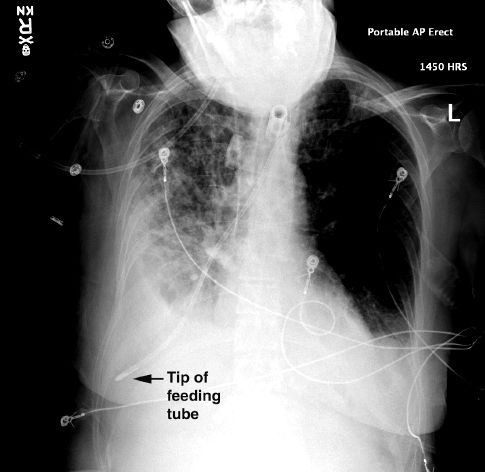
FIGURE 48.1 Routine chest x-ray following the insertion of a feeding tube. See text for explanation. Image digitally enhanced.
Tube Occlusion
Narrow-bore feeding tubes can become occluded by protein precipitates that form when acidic gastric secretions reflux into the feeding tubes (28). Standard preventive measures include flushing the feeding tubes with 30 mL of water every 4 hours, and using a 10-mL water flush after medications are instilled.
Restoring Patency
If flow through the feeding tube is sluggish, flushing the tube with warm water can restore flow in 30% of cases (29). If this is ineffective, pancreatic enzyme (Viokase) can be used as follows (17):
Regimen: Dissolve 1 tablet of Viokase and 1 tablet of sodium carbonate (324 mg) in 5 mL of water. Inject this mixture into the feeding tube and clamp for 5 minutes.
Follow with a warm water flush. This should relieve the obstruction in about 75% of cases (17).
If the tube is completely occluded, advance a flexible wire or a drum cartridge catheter through the feeding tube in an attempt to clear the obstruction. If this is unsuccessful, replace the feeding tube without delay.
Regurgitation/Aspiration
Retrograde regurgitation of feeding formula is reported in as many as 80% of patients receiving gastric or duodenal feedings (18). The following measures are available for reducing the risk of regurgitation and aspiration pneumonia.
Gastric Residual Volume
A standard practice during enteral tube feeding is to measure the gastric residual volume periodically, and discontinue the feeding temporarily if the residual volume exceeds a preselected threshold. This results in frequent interruptions in feeding, and is a common cause of inadequate nutrition support. However, this practice is flawed, because there is no agreement on the residual volume that causes regurgitation.
WHAT VOLUME? Residual volumes of 150–250 mL are typically used to stop enteral feedings, but clinical studies have shown that residual volumes up to 500 mL do not increase the risk of aspiration pneumonia (31). In fact, a recent study shows that managing ventilator-dependent patients without monitoring gastric residual volumes has no adverse consequences on the risk of ventilator-associated pneumonia, or on clinical outcomes (32). This observation creates doubt about the benefits of monitoring gastric residual volumes routinely in the ICU.
RECOMMENDATIONS The most recent guidelines on nutrition support in the ICU recommends that gastric residual volumes of 200–500 mL should raise concern about the risk of aspiration, but tube feedings should not be stopped when the residual volumes are <500 mL unless there are other signs of intolerance to feedings (e.g., vomiting) (1).
When regurgitation of enteral feedings is evident, the head of the bed should be elevated to 45º above horizontal, and the feeding tube should be advanced into the small bowel (if it’s not already there). Prokinetic therapy is an additional option, but the benefits are questionable.
Prokinetic Therapy
The prokinetic agents and recommended dosing regimens are shown in Table 48.5. Prokinetic therapy can produce short-term improvement in indices of gastric motility, but the clinical significance of these effects have been difficult to demonstrate (33).
ERYTHROMYCIN: The macrolide antibiotic, erythromycin, promotes gastric emptying by stimulating motilin receptors in the GI tract (34). At a dose of 200 mg IV every 12 hours, erythromycin can decrease gastric residual volumes by 60% after 24 hours, but this effect diminishes rapidly over a few days (35). Erythromycin may be more effective than metoclopramide, but it is usually not favored because of concerns about antimicrobial resistance. Erythromycin is more effective when used in combination with metoclopramide (36).
Table 48.5 Prokinetic Therapy
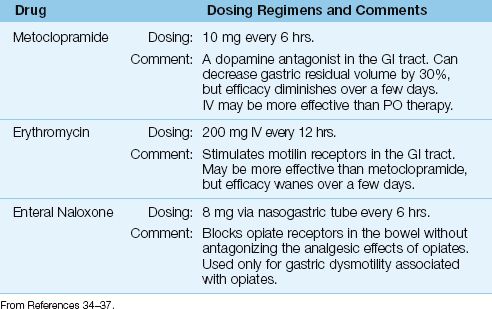
METOCLOPRAMIDE: Metoclopramide promotes gastric emptying by antagonizing the actions of dopamine in the GI tract. At a dose of 10 mg IV every 6 hours, metoclopramide can decrease gastric residual volumes by 30% after 24 hours, but the effect wanes rapidly (35). Metoclopramide is more effective when given in combination with erythromycin (36).
ENTERAL NALOXONE: In critically patients who have gastric dysmotility associated with opiates, direct intragastric administration of the opioid antagonist naloxone (8 mg via nasogastric tube every 6 hours) can selectively block opioid receptors in the bowel and promote gastric emptying without antagonizing the analgesic effects of the opiate (37). The opiate antagonist methylnaltrexone, has also been shown to promote postoperative recovery of bowel function in opiate users (38).
RECOMMENDATION: For a trial of prokinetic therapy, either start with erythromycin, and add metoclopramide after 24 hours if needed, or start with both drugs. And don’t expect much.
The Intolerant Patient
For patients who continue to be intolerant of tube feedings (e.g., repeatedly regurgitate feedings or develop abdominal distention), a switch to parenteral nutrition may be necessary. However, the infusion of tube feedings should be continued at a lower, tolerable rate, whenever possible, to provide some support for the antimicrobial defenses in the bowel.
Diarrhea
Diarrhea occurs in approximately 30% of patients receiving enteral tube feedings (26). The feeding formulas were originally implicated, but the consensus opinion now is that other factors are involved (39). The principal offender in tube-feeding diarrhea may be liquid drug preparations.
Liquid Drug Preparations
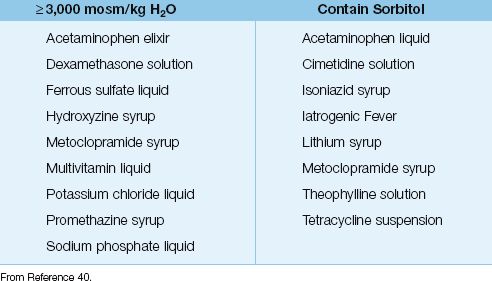
Liquid preparations are favored for drug delivery through narrow-bore feeding tubes because there is less risk of obstruction. However, liquid preparations have two features that create a risk for diarrhea: (a) they can be extremely hyperosmolar (≥3,000 mosm/kg H2O), and (b) they can contain sorbitol (to improve palatability), a well-known laxative that draws water into the bowel lumen (40). Table 48.6 includes a list of diarrhea-prone liquid preparations that might be used in ICU patients. These preparations should be discontinued, if possible, in any patient who develops diarrhea of uncertain etiology during enteral nutrition.
Table 48.6 Liquid Drug Preparations That Promote Diarrhea
A FINAL WORD
Eating as a Physical Defense Mechanism
The mucosal surface of the bowel is constantly changing, with new cells to replace the old ones every few days, and the force behind this dynamic process is the presence of food in the lumen of the bowel. Removing the nutritional bulk from the bowel lumen disrupts the normal process of renewal in the bowel mucosa, and makes us vulnerable to invasion by the hordes of enteric pathogens that inhabit the bowel. This is one of the major advantages of enteral tube feedings over total parenteral nutrition, and it also means that eating is a defense against infection.
REFERENCES
Clinical Practice Guidelines
1. McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. J Parent Ent Nutr 2009; 33:277–316.
2. Kreymann KG, Berger MM, Deutz NEP, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr 2006; 25:210–223.
General Considerations
3. Simpson F, Doig GS. Parenteral vs enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med 2005; 31:12–23.
4. Moore FA, Feliciano DV, Andrassay RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications: the results of a meta-analysis. Ann Surg 1992; 216:172–183.
5. Levine GM, Derin JJ, Steiger E, et al. Role of oral intake in maintenance of gut mass and disaccharide activity. Gastroenterology 1974; 67:975–982.
6. Alpers DH. Enteral feeding and gut atrophy. Curr Opin Clin Nutr Metab Care 2002; 5:679–683.
7. Wiest R, Rath HC. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol 2003; 17:397–425.
8. Ohta K, Omura K, Hirano K, et al. The effect of an additive small amount of a low residue diet against total parenteral nutrition-induced gut mucosal barrier. Am J Surg 2003; 185:79–85.
9. Spaeth G, Specian RD, Berg R, Deitch EA. Bulk prevents bacterial translocation induced by the oral administration of total parenteral nutrition solution. J Parenter Ent Nutrit 1990; 14:442–447.
10. Herskowitz K, Souba WW. Intestinal glutamine metabolism during critical illness: a surgical perspective. Nutrition 1990; 6:199–206.
11. Alverdy JC, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery 1988; 104:185–190.
12. Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med 2001; 29:2264–2270.
Enteral Feeding Formulas
13. Lefton J, Esper DH, Kochevar M. Enteral formulations. In: The A.S.P.E.N Nutrition Support Core Curriculum. Silver Spring, MD: American Society for Parenteral and Enteral Nutrition, 2007: 209–232.
14. Heyland DK, Novak F, Drover JW. et al. Should immunonutrition become routine in critically ill patients? JAMA 2007; 286:944–953.
15. SingerP, Theilla M, Fisher H, et al. Benefit of an enteral diet enriched with eicosapentanoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med 2006; 34:1033–1038.
16. Kirk SJ, Barbul A. Role of arginine in trauma, sepsis, and immunity. J Parenter Ent Nutr 1990; 14(Suppl):226S–228S.
17. Bertolini G, Iapichino G, Radrizzani D, et al. Early enteral immunonutrition in patients with severe sepsis: results of an interim analysis of a randomized multicentre clinical trial. Intensive Care Med 2003; 29:834–840.
18. Rebouche CJ. Carnitine. In: Shils ME, et al., eds. Modern nutrition in health and disease. 10th ed. Philadelphia, PA: Lippincott, Williams & Wilkins, 2006; 537–544.
19. Karlic H, Lohninger A. Supplementation of L-carnitine in athletes: does it make sense? Nutrition (Burbank, CA) 2004; 20:709–715.
Initiating Tube Feedings
20. Stroud M, Duncan H, Nightingale J. Guidelines for enteral feeding in adult hospital patients. Gut 2003; 52 Suppl 7:vii1–vii12.
21. Kolbitsch C, Pomaroli A, Lorenz I, et al. Pneumothorax following nasogastric feeding tube insertion in a tracheostomized patient after bilateral lung transplantation. Intensive Care Med 1997; 23:440–442.
22. Fisman DN, Ward ME. Intrapleural placement of a nasogastric tube: an unusual complication of nasotracheal intubation. Can J Anaesth 1996; 43:1252–1256.
23. Baskin WN. Acute complications associated with bedside placement of feeding tubes. Nutr Clin Pract 2006; 21:40–55.
24. Neumann DA, DeLegge MH. Gastric vsersus small bowel tube feeding in the intensive care unit: a prospective comparison of efficacy. Crit Care Med 2002; 30:1436–1438.
25. Marik PE, Zaloga GP. Gastric versus post-pyloric feeding: a systematic review. Crit Care 2003; 7:R46–R51.
26. Rees RG, Keohane PP, Grimble GK, et al. Elemental diet administered nasogastrically without starter regimens to patients with inflammatory bowel disease. J Parenter Enteral Nutr 1986; 10:258–262.
27. Mizock BA. Avoiding common errors in nutritional management. J Crit Illness 1993; 10:1116–1127.
Complications
28. Marcuard SP, Perkins AM. Clogging of feeding tubes. J Parenter Enteral Nutr 1988; 12:403–405.
29. Marcuard SP, Stegall KS. Unclogging feeding tubes with pancreatic enzyme. J Parenter Enteral Nutr 1990; 14:198–200.
30. Metheny N. Minimizing respiratory complications of nasoenteric tube feedings: state of the science. Heart Lung 1993; 22:213–223.
31. Montejo JC, Minambres E, Bordejé L, et al. Gastric residual volume during enteral nutrition in ICU patients: the REGANE study. Intensive Care Med 2010; 36:1386–1393.
32. Reignier K, Mercier E, Le Gouge A, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding. JAMA 20113: 309:249–256.
33. Booth CM, Heyland DK, Paterson WG. Gastrointestinal promotility drugs in the critical care setting: a systematic review of the evidence. Crit Care Med 2002; 30:1429–1435.
34. Hawkyard CV, Koerner RJ. The use of erythromycin as a gastrointestinal prokinetic agent in adult critical care: benefits and risks. J Antimicrob Chemother 2007; 59:347–358.
35. Nguyen NO, Chapman MJ, Fraser RJ, et al. Erythromycin is more effective than metoclopramide in the treatment of feed intolerance in critical illness. Crit Care Med 2007; 35:483–489.
36. Nguyen NO, Chapman M, Fraser RJ, et al. Prokinetic therapy for feed intolerance in critical illness: one drug or two? Crit Care Med 2007; 35:2561–2567.
37. Meissner W, Dohrn B, Reinhart K. Enteral naloxone reduces gastric tube reflux and frequency of pneumonia in critical care patients during opioid analgesia. Crit Care Med 2003; 31:776–780.
38. Ladanyi A, Temkin SM, Moss J. Subcutaneous methylnaltrexone to restore postoperative bowel function in a long-term opiate user. Int J Gynecol Cancer 2010; 20:308–310 (abstract).
39. Edes TE, Walk BE, Austin JL. Diarrhea in tube-fed patients: feeding formula not necessarily the cause. Am J Med 1990; 88:91–93.
40. Williams NT. Medication administration through enteral feeding tubes. Am J Heath-Sys Pharm 2008; 65:2347–2357.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








