ca 500 B.C
The appearance of a new fever is always a matter of concern in a hospitalized patient. This chapter presents a practical approach to the ICU patient with a new-onset fever, and includes: (a) the definition of fever in ICU patients, (b) the appropriate sites for measuring body temperature, (c) the optimal method for obtaining blood cultures, and (d) the potential sources of fever in the ICU (1,2). The final section focuses on the practice of suppressing fever, and why Parmenides might disapprove of this practice.
BODY TEMPERATURE
Two scales (Celsius and Fahrenheit) are used to record body temperature, and the conversion from one scale to the other is shown in Table 43.1. Although readings on the Celsius scale are often called “degrees centigrade,” this unit is intended for the degrees on a compass, not for temperatures (3). The appropriate term for temperatures on the Celsius scale is degrees Celsius.
Normal Body Temperature
The definition of a normal body temperature is not straightforward, as demonstrated by the following observations.
1. The traditional norm of 37°C (98.6°F) is a mean value derived from a study of axillary temperatures in 25,000 healthy adults, conducted in the late 19th century (4). However, axillary temperatures can vary by as much as 1.0°C (1.8°F) from core body temperatures (5), and axillary temperatures are not advised in ICU patients (1).
2. Core body temperature can be 0.5°C (0.9°F) higher than oral temperatures (6), and 0.2–0.3°C lower than rectal temperatures (1).
3. Elderly subjects have a mean body temperature about 0.5°C (0.9°F) lower than younger adults (4,7).
4. Body temperature has a diurnal variation, with the nadir in the early morning (between 4 and 8 a.m.) and the peak in the late afternoon (between 4 and 6 p.m.). The range of diurnal variation varies, but can be as high as 1.3°C (2.4°F) (8).
These observations indicate that the normal body temperature is influenced by age, measurement site, and time of day. As such, the best definition of a normal body temperature is the usual range of temperatures for an individual patient, measured at the same site, over a 24-hour period.
Table 43.1 Converting Celsius and Fahrenheit Temperatures
Thermometry
The most recent guidelines on fever in the ICU (1) contain the following recommendations for measuring body temperature.
1. The most accurate measurements are obtained with thermistor-equipped catheters placed in the pulmonary artery, esophagus, or urinary bladder.
2. Less accurate measurements are obtained with rectal, oral, and tympanic membrane temperatures, in that order. Rectal temperatures are not advised in neutropenic patients (1), and oral temperatures should be measured with electronic probes (not mercury thermometers) placed in the right or left sublingual pockets.
3. The axillary and temporal artery sites are not recommended for temperature measurements in ICU patients.
Thermistor-equipped urinary bladder catheters seem ideal for patients who require a bladder drainage catheter (which includes most patients in an ICU). These devices not only provide accurate measurements of body temperature, they also permit continuous temperature monitoring, which allows you to identify the normal temperature range for each patient.
Definition of Fever
Fever is best defined as a temperature that exceeds the normal daily variation in temperature for each patient. However, this is not a practical definition because the normal temperature range for each patient cannot be determined with periodic measurements. The current recommendations for the definition of a fever in ICU patients are as follows (1):
1. A body temperature of 38.3°C (101°F) or higher represents a fever, and deserves further evaluation.
2. A lower threshold of 38.0°C (100.4°F) can be used for immunocompromised patients, particularly those with neutropenia.
The Febrile Response
Fever is the result of inflammatory cytokines (called endogenous pyrogens) that act on the hypothalamus to elevate the body temperature. Any condition that triggers a systemic inflammatory response will, therefore, produce a fever. Some implications of the febrile response are stated below.
1. Fever is a sign of inflammation, not infection, and about 50% of ICU patients who develop a fever have no apparent infection (9,10).
2. The severity of the fever does not correlate with the presence or severity of infection. High fevers can be the result of a noninfectious process such as a drug fever (see later), while fever can be absent in life-threatening infections (1).
The distinction between inflammation and infection is an important one, not only for the evaluation of fever, but also for curtailing the indiscriminate use of antibiotics.
Fever as an Adaptive Response
Unlike hyperthermia, which is the result of abnormal temperature regulation (see Chapter 42), fever is a condition where the thermoregulatory system is intact, but is operating at a higher set point (11). Elevated body temperatures serve to enhance immune function and inhibit bacterial and viral replication, indicating that fever can be viewed as an adaptive response that aids the host in defending against infection (12). The beneficial effects of fever are described in more detail later in the chapter.
Sources of Fever
Any condition capable of triggering an inflammatory response is capable of causing a fever. The notable sources of nosocomial fever in the ICU are shown in Figure 43.1.
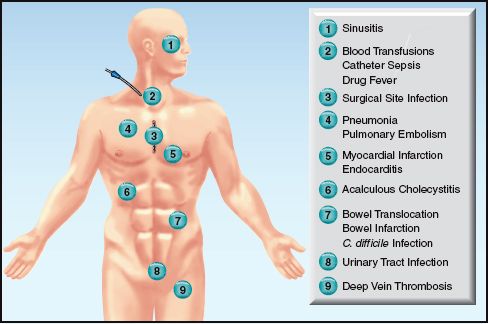
FIGURE 43.1 Potential sources of nosocomial fever in the ICU.
NONINFECTIOUS SOURCES
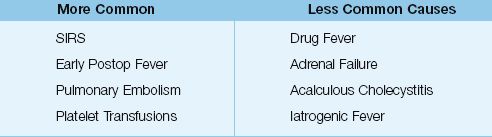
As mentioned earlier, infection is responsible for only half of ICU-acquired fevers (9,10). The conditions described in this section are responsible for most of the remaining 50% of ICU-acquired fevers. The ones that deserve mention are included in Table 43.2.
Table 43.2 Noninfectious Causes of ICU-Acquired Fever
SIRS
The clinical entity known as the systemic inflammatory response syndrome (SIRS) is characterized by signs of systemic inflammation (see Table 14.2), and may not be associated with infection. Noninfectious sources of SIRS include tissue injury (e.g., from ischemia or major surgery), and translocation of endotoxin and inflammatory cytokines from the GI tract. SIRS can be accompanied by inflammatory injury in one or more vital organs (e.g., acute respiratory distress syndrome), and can have a fatal outcome. This condition is described in more detail in Chapter 14.
Early Postoperative Fever
Major surgery is itself a source of tissue injury. (In the words of Dr. John Millili, a surgeon and close friend, major surgery is like being hit with a baseball bat!) Because inflammation and fever are the normal response to tissue injury, it is no surprise that fever in the first postoperative day is reported in 15–40% of cases of major surgery (13–15) and, in most cases, there is no apparent infection (13,14). These fevers are short-lived, and usually resolve within 24–48 hours.
Atelectasis Does Not Cause Fever
There is a long-standing misconception that atelectasis is a common cause of fever in the early postoperative period. One possible source of this misconception is the high incidence of atelectasis in patients who develop a postoperative fever. This is demonstrated in Figure 43.2 (see the graph on the left), which is from a study involving patients who underwent open heart surgery (15). Close to 90% of the patients with a fever on the first postoperative day had radiographic evidence of atelectasis. This, however, is not evidence that the atelectasis is the source of fever. In fact, the graph on the right in Figure 43.2 shows that most (75%) of the patients with atelectasis did not have a fever. The inability of atelectasis to produce fever was demonstrated over 50 years ago in an animal study where lobar atelectasis produced by ligation of a mainstem bronchus was not accompanied by fever (16).
To summarize, atelectasis is a common complication of major surgery, and occurs in over 90% of cases of general anesthesia (17). However, it is not a common cause of postoperative fever. Most fevers that appear in the first 24 hours after surgery are the result of the tissue injury sustained during the procedure.
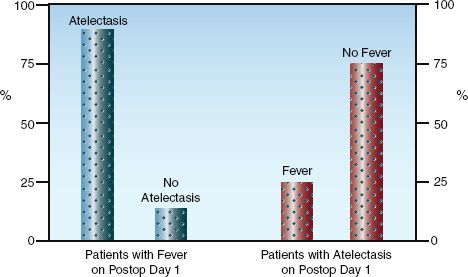
FIGURE 43.2 The relationships between fever and atelectasis in the first postoperative day in 100 consecutive patients who had open heart surgery. The graph on the left shows that most patients with fever had atelectasis, but the graph on the right shows that most patients with atelectasis did not have a fever. Data from Reference 15.
Malignant Hyperthermia
An uncommon but treatable cause of elevated body temperatures in the immediate postoperative period is malignant hyperthermia, an inherited disorder characterized by muscle rigidity, hyperpyrexia (temperature >40°C or 104°F), and rhabdomyolysis in response to halogenated inhalational anesthetics. This disorder is described in Chapter 42.
Venous Thromboembolism
Several groups of patients are at risk for venous thromboembolism (see Table 6.1 and Figure 6.1), but the risk is highest in trauma victims and postoperative patients. Most cases of hospital-acquired deep vein thrombosis are asymptomatic, but acute pulmonary embolism can produce a fever that lasts up to 1 week (18). The diagnostic approach to acute pulmonary embolism is outlined in Figure 6.2.
Blood Transfusions
Erythrocyte Transfusions
Febrile non-hemolytic transfusion reactions occur in 0.5% of erythrocyte transfusions. These reactions are the result of antileukocyte antibodies in the recipient that react with donor leukocytes, and they are more likely to occur in patients who have received multiple transfusions. The fever usually appears during, or up to 6 hours after, the transfusion. For more information on these reactions, see pages 360–361.
Platelet Transfusions
Fever is much more common with platelet transfusions; i.e., the reported incidence is as high as 30% (see page 380). These reactions are also caused by antibodies to donor leukocytes, and the frequent appearance of these reactions with platelet transfusions is probably due to the multiple donors used for routine platelet transfusions.
Drug Fever
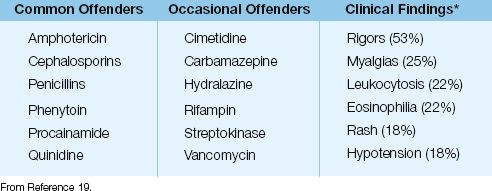
Drug-induced fever can be the result of a hypersensitivity reaction or an idiosyncratic reaction. Any drug can trigger a hypersensitivity reaction, but the drugs most often implicated in drug fever are listed in Table 43.3.
Drug fever is poorly understood. The onset of the fever varies from a few hours to more than three weeks after the onset of drug therapy (1). The fever can appear as an isolated finding, or can be accompanied by the other manifestations listed in Table 43.3 (19). Note that about half of patients have rigors, and about 20% develop hypotension, indicating that patients with a drug fever can appear seriously ill. Evidence of a hypersensitivity reaction (i.e., eosinophilia and rash) is absent in over 75% of cases of drug fever (19).
Suspicion of drug fever usually occurs when there are no other likely sources of fever. When suspected, possible offending drugs should be discontinued, if possible. The fever should disappear in 2–3 days, but it can persist for up to 7 days (20).
Table 43.3 Drug-Associated Fever in the ICU
Drug-Induced Hyperthermia Syndromes
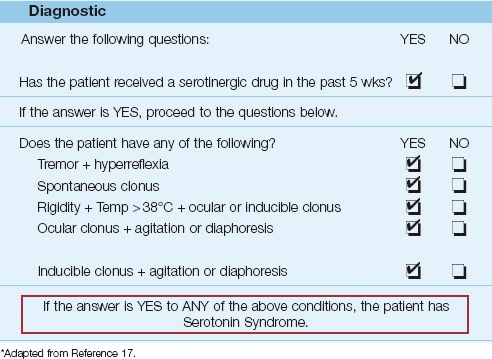
The drug-induced hyperthermia syndromes include malignant hyperthermia (mentioned earlier), neuroleptic malignant syndrome, and serotonin syndrome. These disorders are characterized by muscle rigidity, hyperpyrexia (temperature >40°C or 104°F), and rhabdomyolysis, and are described in detail in Chapter 42. The neuroleptic malignant syndrome is a particular concern in patients receiving haloperidol for sedation.
Other Sources
There are several other potential causes of noninfectious fever, and the most notable of these are included in the following text.
Acalculous Cholecystitis
Acalculous cholecystitis is an uncommon but serious disorder reported in 1.5% of critically ill patients (21). It is believed to be the result of ischemia and stasis within the gallbladder, resulting in edema of the cystic duct that blocks drainage of the gallbladder. The diagnosis and management of this condition is described in Chapter 40.
Endocrine Disorders
The endocrine disorders known to produce fever are thyrotoxicosis and adrenal crisis. Thyrotoxicosis is unlikely to appear de novo in the ICU, but adrenal crisis due to spontaneous adrenal hemorrhage is a recognized complication of anticoagulant therapy and disseminated intravascular coagulation (DIC). These endocrine disorders are described in Chapter 50.
Iatrogenic Fever
Faulty thermal regulators in water mattresses and aerosol humidifiers can cause fever by transference (22). It takes only a minute to check the temperature settings on heated mattresses and ventilators, but it can take far longer to explain why such a simple cause of fever was overlooked.
NOSOCOMIAL INFECTIONS
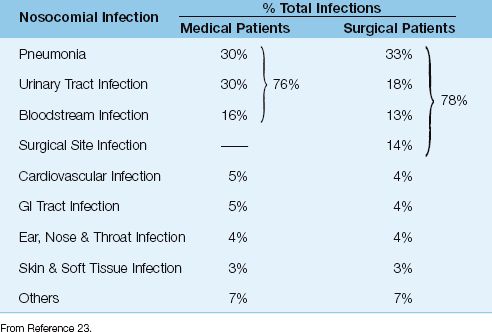
The incidence of ICU-acquired infections in medical and surgical ICU patients is shown in Table 43.4 (23). Four infections account for over three-quarters of ICU-acquired infections (pneumonia, urinary tract infections, bloodstream infections, and surgical site infections), and three of these infections involve indwelling plastic devices: i.e., 83% of the pneumonias occur in intubated patients, 97% of the urinary tract infections occur in catheterized patients, and 87% of bloodstream infections originate from intravascular catheters (23).
Table 43.4 Nosocomial Infections in Medical and Surgical ICU Patients
Common Nosocomial Infections
The three most common ICU-acquired infections in Table 43.4 are described elsewhere in this book. The diagnosis and management of these conditions can be found in Chapter 3 (for vascular catheter-related infections), Chapter 29 (for ventilator-associated pneumonia), and Chapter 41 (for urinary tract infections). The following are some other nosocomial infections that should be considered in a patient with ICU-acquired fever.
Surgical Site Infections
Surgical site infections (SSIs) continue to be a considerable source of postoperative morbidity despite attention to preventive measures (24). These infections typically appear 5–7 days after surgery. Superficial infections are less likely to produce fever than infections with deep tissue involvement. Sternal wound infections following open heart surgery show a particular propensity for deep tissue involvement (i.e., mediastinitis) (25). For patients with fever following open heart surgery, sternal instability can be an early sign of sternal wound infection.
The pathogens involved in SSIs are determined by the surgical procedure. SSIs from clean surgical procedures (where the chest or abdomen has not been opened) usually involve Staphylococcus aureus, while SSIs from contaminated procedures (where the chest or abdomen has been opened) often involve organisms that are part of the indigenous flora of the organ that was surgically repaired (e.g., infections following bowel surgery typically involve Gram-negative aerobic bacilli and anaerobes) (1).
Management
Superficial infections can usually be managed with debridement only. The management of deep-seated infections is dependent on the character of the infection. Localized collections (abscesses) can often be managed with drainage only, while more diffuse involvement of deep tissues should prompt antimicrobial therapy.
Necrotizing Wound Infections
Necrotizing wound infections are produced by Clostridium species or α-hemolytic streptococci (1). Unlike other wound infections (which typically appear 5–7 days after surgery), necrotizing infections are evident in the first few postoperative days. There is often marked edema, and fluid-filled bullae, around the incision, and crepitance may be present. Spread to deeper structures is rapid, and progressive disease is often accompanied by rhabdomyolysis and myoglobinuric renal failure. Treatment involves extensive debridement and intravenous penicillin. The mortality rate is high (>60%) when treatment is delayed.
Paranasal Sinusitis
Indwelling nasogastric and nasotracheal tubes can block the ostia that drain the paranasal sinuses, leading to accumulation of infected secretions in the sinuses. The maxillary sinuses are almost always involved, and the resulting acute sinusitis can be an occult source of fever. Paranasal sinusitis is reported in 15–20% of patients with indwelling nasal tubes (26,27), and can be a source of fever and bacteremia. However, the clinical significance of this condition, in many cases, is unclear (see later).
Microbiology
The pathogens involved in ICU-acquired sinusitis are the same ones that colonize the oropharynx in critically ill patients. The most frequent isolates are Gram-negative aerobic bacilli (in 60% of cases), followed by Gram-positive aerobic cocci (particularly Staph. aureus and coagulase-negative staphylococci) in 30% of cases, and yeasts (mostly Candida albicans) in 5–10% of cases (1).
Diagnosis
Purulent drainage from the nares is absent in about 75% of cases (1), and the diagnosis is suggested by radiographic features of sinusitis (i.e., opacification or air–fluid levels in the involved sinuses). Although CT scans are recommended for the diagnosis of nosocomial sinusitis (26,27), portable sinus films obtained at the bedside can also be revealing, as shown in Figure 43.3. The maxillary sinuses can be viewed with a single occipitomental view, also called a “Waters view,” which can be obtained at the bedside (28). (Avoiding a CT scan also avoids the risks and manpower involved in transporting a patient out of the ICU.)
Radiographic or CT evidence of sinusitis is not sufficient for the diagnosis of sinusitis because 30–40% of patients with evidence of sinusitis on imaging studies do not have an infection documented by aspiration of the involved sinus (26,27). The diagnosis requires aspiration of the involved sinus with a quantitative culture that grows ≥103 colony forming units per mL (26,27).
Management
A trial of empiric antibiotic therapy is warranted when there is radiographic evidence of sinusitis in a febrile patient with no other apparent source of fever. The antibiotic regimen should provide coverage for Gram-negative aerobic bacilli and staphylococci. Single-agent therapy with imipenem or meropenem should be adequate if MRSA has not been isolated on routine nasal swabs. If MRSA has been isolated on a nasal swab, or MRSA is a frequent isolate in your ICU, then vancomycin should be added to the Gram-negative coverage. In addition, nasal tubes should be removed and replaced with oral tubes. If there is no improvement with empiric antibiotics, sinus puncture for Gram stain and quantitative culture is warranted (1).
Clinical Significance
Despite the fact that nosocomial sinusitis is documented in 15–20% of patients with indwelling nasal tubes (26,27), sinusitis is often overlooked in the evaluation of ICU-acquired fever, without apparent harm. This creates uncertainty about the clinical significance of ICU-acquired sinusitis.
Clostridium difficile Infection
ICU-acquired fever that is associated with new-onset diarrhea should always prompt suspicion of Clostridium difficile enterocolitis. The diagnosis and management of this condition are described in Chapter 40 (see pages 738–742).
Patient-Specific Infections
Infections that should be considered in specific patient populations include: (a) abdominal abscesses in patients who have had a laparotomy or laparoscopy (see pages 744–745), (b) endocarditis in patients with prosthetic or damaged valves, (c) meningitis in neurosurgery patients, and (d) spontaneous bacterial peritonitis in patients with cirrhosis and ascites (see pages 725–726).
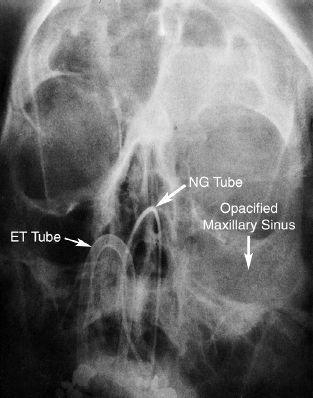
FIGURE 43.3 Portable sinus film (Waters view) showing opacification of the left maxillary and frontal sinuses in a patient with indwelling nasotracheal and nasogastric tubes. The diagnosis of paranasal sinusitis was subsequently confirmed by a maxillary sinus puncture at the bedside, with the aspirate growing Staph. epidermidis at 103 cfu/mL.
INITIAL APPROACH
The appearance of an ICU-acquired fever is not a license to perform an extensive evaluation and start empiric antimicrobial therapy. Instead, an evaluation should be made to determine the likelihood of a noninfectious or infectious source of fever. If a noninfectious source of fever is unlikely, the following measures are relevant.
Blood Cultures
Blood cultures are recommended for all cases of ICU-related fever where a noninfectious source is unlikely (1). The yield from blood cultures is dependent on the volume of blood withdrawn during a venipuncture, and the number of venipuncture sites.
Influence of Volume
The yield from blood cultures is optimal when 20–30 mL of blood is withdrawn from each venipuncture site (1). The standard practice is to withdraw 20 mL of blood from a venipuncture site: one-half (10 mL) is then injected into each of the two bottles of broth (one aerobic and one anaerobic) provided in a blood culture set. Increasing from 20 mL to 30 mL of blood increases the yield from blood cultures by about 10% (29). When using 30 mL per venipuncture, the extra 10 mL aliquot of blood should be injected into an aerobic broth bottle (29).
Number of Blood Cultures
In the terminology of blood cultures, one blood culture refers to a single venipuncture site. (For example, culture of blood specimens from each lumen of a multilumen catheter still represents one blood culture.) The relationship between the number of blood cultures and the detection of bacteremia is shown in Figure 43.4 (30). This is from a study of patients with bacteremia documented by four or more blood cultures drawn over a 24 hour period. The two curves in the graph represent patients with endocarditis and patients with other infections. The majority of the bacteremias (94%) were detected with two blood cultures in the patients with endocarditis, while three blood cultures were required to detect over 90% of the bacteremias in patients with other infections. The enhanced detection rate in endocarditis is due to the continuous bacteremia associated with endocarditis.
Based on the data in Figure 43.4, three blood cultures drawn over a 24-hour period will detect a majority (>90%) of bacteremias (1). However, two blood cultures will detect a majority of bacteremias in patients with endocarditis.
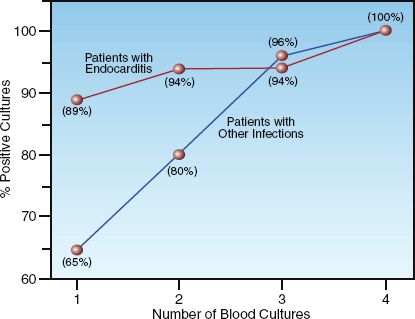
FIGURE 43.4 Relationship between the number of blood cultures drawn over a 24-hour period (20 mL per blood culture) and the detection rate for bacteremia. See text for explanation. Data from Reference 30.
Empiric Antimicrobial Therapy
Empiric antibiotic therapy is recommended when the likelihood of infection is high. Prompt initiation of antimicrobial therapy is considered essential, particularly in patients with neutropenia (absolute neutrophil count <500), where delays of only a few hours can have a negative impact on outcomes (31).
1. Empiric coverage should always include an antibiotic that is active against Gram-negative aerobic bacilli, which are the most prevalent pathogens in ICU-acquired infections. Popular choices include the carbapenems (imipenem or meropenem), piperacillin/tazobactam, or cefepime.
2. Coverage for staphylococci (S. aureus and coagulase-negative staphylococci) should be included if vascular catheter-related septicemia is a possibility. Vancomycin is the antibiotic-of-choice for this purpose.
3. An antifungal agent should be considered when unexplained fever persists for longer than 3 days after the start of empiric antibiotics. This is most appropriate for patients who are at-risk for disseminated candidiasis (e.g., long hospital stay, recent antimicrobial therapy, immunosuppressed, Candida colonizing multiple sites). Fluconazole is adequate for most patients, while an alternative agent (e.g., caspofungin) is recommended for neutropenic patients.
See Chapter 52 for more information on the antimicrobial agents just mentioned, including dosing recommendations.
ANTIPYRETIC THERAPY
The popular perception of fever as a malady that must be corrected is rooted in hearsay. In fact, fever is a normal adaptive response that enhances the ability to eradicate infection (12). This section contains some observations about fever that are intended to make you think twice about starting antipyretic therapy in critically ill patients.
Fever as a Host Defense Mechanism
An increase in body temperature can enhance immune function by increasing the production of antibodies and cytokines, activating T-lymphocytes, facilitating neutrophil chemotaxis, and enhancing phagocytosis by neutrophils and macrophages (32,33). In addition, high temperatures inhibit bacterial and viral replication. The effect of body temperature on the growth of bacteria in blood is demonstrated in Figure 43.5 (34). Note that an increase in temperature of 4°C completely suppresses growth. Similar results have been demonstrated in an animal model of pneumococcal meningitis (35).
Clinical Studies
The benefits of fever as a host defense against infection is supported in clinical studies showing that septic patients who develop hypothermia have at least twice the mortality rate of septic patients who develop a fever (36,37). The results of these studies are shown in Figure 43.6. Although these studies cannot establish a causal relationship between body temperature and outcomes, they do show that higher body temperatures are associated with improved outcomes. A more recent observational study showed that antipyretic therapy was associated with higher mortality rates in septic patients (38).
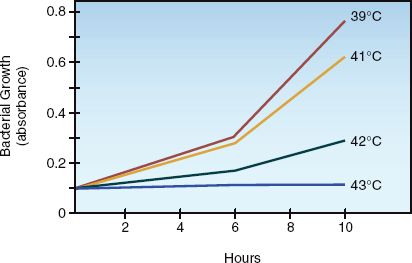
FIGURE 43.5 The influence of body temperature on the growth of Pasteurella multocida in the blood of infected laboratory animals. The range of temperatures in the figure is the usual range of febrile temperatures for the study animal (rabbits). Data from Reference 34.
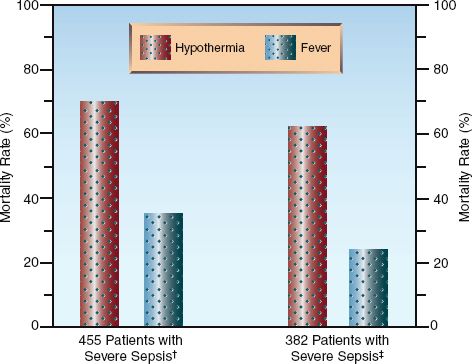
FIGURE 43.6 The relationship between body temperature and survival in two clinical studies of patients with severe sepsis. Data from References †36 and ‡37.
Is Fever Harmful?
Tachycardia
One of the claims in support of suppressing fever is the assumed effect of fever in promoting tachycardia, which can be harmful in patients with heart disease. However, the association between fever and tachycardia was established in animal models of sepsis, and it is likely that the inflammatory response to sepsis is the source of tachycardia, and not an elevation in body temperature.
Neurologic Injury
There is convincing evidence that increased body temperatures aggravate ischemic brain injury following cardiac arrest (see Chapter 17) and ischemic stroke (see Chapter 46). However, the effects of increased body temperatures in the non-ischemic brain have not been adequately studied. The popular claim that hyperpyrexia (temperature ≥40°C or ≥104°F) promotes injury in the non-ischemic brain can neither be supported nor refuted because hyperpyrexia is rarely left untreated in clinical practice.
Summary Statements
The available evidence at the present time indicates the following:
1. Fever is not a pathological condition, but is a normal adaptive response that serves an antimicrobial defense mechanism.
2. Except for the early period following cardiac arrest or ischemic stroke, fever provides a documented benefit in patients with infection.
3. The professed harm of hyperpyrexia (≥40°C or ≥104°F) in a nonischemic brain is more assumption than documented fact.
Antipyretic Drugs
Prostaglandin E mediates the febrile response to endogenous pyrogens, and drugs that interfere with prostaglandin E synthesis are effective in reducing fever (39). These drugs include aspirin, acetaminophen, and nonsteroidal anti-inflammatory agents (NSAIDS). Only the latter two are used for fever suppression in the ICU.
Acetaminophen
Acetaminophen is the favored drug for antipyresis, despite the fact that it is the leading cause of acute liver failure in the United States (see Chapter 54). Acetaminophen is contraindicated in patients with hepatic insufficiency.
DOSING REGIMENS: Acetaminophen is usually given orally or by rectal suppository in a dose of 650 mg every 4–6 hrs, with a maximum daily dose of 4 grams. An intravenous preparation is now available in the United States (OFIRMEV™), and the recommended dose for adults ≥50 kg is 650 mg every 4 hrs, or 1,000 mg every 6 hrs, with a maximum dose of 4 grams daily (40). This dosing regimen is equivalent to oral acetaminophen for suppressing fever in adults (41). Intravenous acetaminophen is costly, and is recommended only for patients who cannot tolerate oral or rectal drug administration.
NSAIDs
Ibuprofen is apopular over-the-counter NSAID that provides safe and effective antipyresis at an intravenous dose of 400–800 mg every six hours (42). Ketorolac is another intravenous NSAID that has been effective in suppressing fever (in a single dose of 0.5 mg/kg) (43). See Chapter 51 for more information on these drugs.
Cooling Blankets
Cooling blankets are inappropriate for the treatment of fever. The febrile response raises the body temperature by promoting cutaneous vasoconstriction and increasing skeletal muscle activity (via rigors and shivering). This is what the body normally does in response to a cold environment, so the febrile response mimics the physiological response to cold. Stated another way, the febrile response makes the body behave like it is wrapped in a cooling blanket. Adding a cooling blanket will only aggravate the cutaneous vasoconstriction and increased muscle activity involved in the febrile response. This explains why cooling blankets are notoriously ineffective in reducing fever.
Cooling blankets are more appropriate for hyperthermia syndromes, when normal thermoregulation is faulty (see Chapter 42).
A FINAL WORD
Right vs. Wrong
There’s a wrong way and a right way to approach a new-onset fever in the ICU. The wrong way is to culture everything available, order a barrage of laboratory tests and imaging studies, and start antibiotics without hesitation. The right way is to make sure the fever is real (and not the result of an iatrogenic problem), and then evaluate the patient for the likelihood of an infectious or noninfectious source of the fever. Remem-ber that there is a 50-50 chance of finding an underlying infection, so don’t start antibiotics unless an infection is apparent or highly suspected, or the patient is immunocompromised. And finally, please think twice about suppressing fever, and stay away from cooling blankets.
REFERENCES
Reviews
1. O’Grady NP, Barie PS, Bartlett J, et al. Guidelines for the evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Disease Society of America. Crit Care Med 2008; 36:1330–1349.
2. Laupland KB. Fever in the critically ill medical patient. Crit Care Med 2009; 37(Suppl):S273–S278.
Body Temperature
3. Stimson HF. Celsius versus centigrade: the nomenclature of the temperature scale of science. Science 1962; 136:254–255.
4. Wunderlich CA, Sequine E. Medical thermometry and human temperature. New York: William Wood, 1871.
5. Mellors JW, Horwitz RI, Harvey MR, et al. A simple index to identify occult bacterial infection in adults with acute unexplained fever. Arch Intern Med 1987; 147:666–671.
6. Tandberg D, Sklar D. Effect of tachypnea on the estimation of body temperature by an oral thermometer. N Engl J Med 1983; 308:945–946.
7. Marion GS, McGann KP, Camp DL. Core body temperature in the elderly and factors which influence its measurement. Gerontology 1991; 37:225–232.
8. Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6°F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA 1992; 268:1578–1580.
9. Commichau C, Scarmeas N, Mayer SA. Risk factors for fever in the intensive care unit. Neurology 2003; 60:837–841.
10. Peres Bota D, Lopes Ferriera F, Melot C, et al. Body temperature alterations in the critically ill. Intensive Care Med 2004; 30:811–816.
11. Saper CB, Breder CB. The neurologic basis of fever. N Engl J Med 1994; 330:1880–1886.
12. Kluger MJ, Kozak W, Conn CA, et al. The adaptive value of fever. Infect Dis Clin North Am 1996; 10:1–20.
Noninfectious Sources of Fever
13. Fry DE. Postoperative fever. In: Mackowiak PA, ed. Fever: basic mechanisms and management. New York: Raven Press, 1991; 243–254.
14. Freischlag J, Busuttil RW. The value of postoperative fever evaluation. Surgery 1983; 94:358–363.
15. Engoren M. Lack of association between atelectasis and fever. Chest 1995; 107:81–84.
16. Shelds RT. Pathogenesis of postoperative pulmonary atelectasis: an experimental study. Arch Surg 1949; 48:489–503.
17. Warlitier DC. Pulmonary atelectasis. Anesthesiology 2005; 102:838–854.
18. Murray HW, Ellis GC, Blumenthal DS, et al. Fever and pulmonary thrombo- embolism. Am J Med 1979; 67:232–235.
19. Mackowiak PA, LeMaistre CF. Drug fever: a critical appraisal of conventional concepts. Ann Intern Med 1987; 106:728–733.
20. Cunha B. Drug fever: The importance of recognition. Postgrad Med 1986; 80:123–129.
21. Walden DT, Urrutia F, Soloway RD. Acute acalculous cholecystitis. J Inten-sive Care Med 1994; 9:235–243.
22. Gonzalez EB, Suarez L, Magee S. Nosocomial (water bed) fever. Arch Intern Med 1990; 150:687 (letter).
Nosocomial Infections
23. Richards MJ, Edwards JR, Culver DH, Gaynes RP. The National Nosocomial Infections Surveillance System. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol 2000; 21:510–515.
24. Alexander JW, Solomkin JS, Edwards MJ. Updated recommendations for control of surgical site infections. Ann Surg 2011; 253:1082–1093.
25. Loopp FD, Lytle BW, Cosgrove DM, et al. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990; 49:179–187.
26. Holzapfel L, Chevret S, Madinier G, et al. Influence of long-term oro- or nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: results of a prospective, randomized, clinical trial. Crit Care Med 1993; 21:1132–1138.
27. Rouby J-J, Laurent P, Gosnach M, et al. Risk factors and clinical relevance of nosocomial maxillary sinusitis in the critically ill. Am Rev Respir Dis 1994; 150:776–783.
28. Diagnosing sinusitis by x-ray: is a single Waters view adequate? J Gen Intern Med 1992; 7:481–485.
Initial Approach
29. Patel R, Vetter EA, Harmsen WS, et al. Optimized pathogen detection with 30- compared to 20-milliliter blood culture draws. J Clin Microbiol 2011; 49:4047–4051.
30. Cockerill FR, Wilson JW, Vetter EA, et al. Optimal testing parameters for blood cultures. Clin Infect Dis 2004; 38:1724–1730.
31. Hughes WH, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 2002; 34(6):730–751.
Antipyretic Therapy
32. van Oss CJ, Absolom DR, Moore LL, et al. Effect of temperature on the chemotaxis, phagocytic engulfment, digestion, and O2 consumption of human polymorphonuclear leukocytes. J Reticuloendothel Soc 1980; 27:561–565.
33. Azocar J, Yunis EJ, Essex M. Sensitivity of human natural killer cells to hyperthermia. Lancet 1982; 1:16–17.
34. Kluger M, Rothenburg BA. Fever and reduced iron: their interaction as a host defense response to bacterial infection. Science 1979; 203:374–376.
35. Small PM, Tauber MG, Hackbarth CJ, Sande MA. Influence of body temperature on bacterial growth rates in experimental pneumococcal meningitis in rabbits. Infect Immun 1986; 52:484–487.
36. Arons MM, Wheeler AP, Bernard GR, et al. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Critical Care Med 1999; 27:699–707.
37. Clemmer TP, Fisher CJ, Bone RC, et al. Hypothermia in the sepsis syndrome and clinical outcome. Crit Care Med 1990; 18:801–806.
38. Lee BH, Inui D, Sun GY, et al, for Fever and Antipyretic in Critically Ill patients Evaluation (FACE) Study Group. Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: multi-centered prospective observational study. Crit Care 2012; 16:R33.
39. Plaisance KI, Mackowiak PA. Antipyretic therapy. Physiologic rationale, diagnostic implications, and clinical consequences. Arch Intern Med 2000; 160:449–456.
40. Drug prescribing information on IV acetaminophen. Cadence Pharmaceuti-cals. Available at www.ofirmev.com (accessed 6/22/2013).
41. Peacock WF, Breitmeyer JB, Pan C, et al. A randomized study of the efficacy and safety of intravenous acetaminophen compared to oral acetaminophen for the treatment of fever. Acad Emerg Med 2011; 18:360–366.
42. Scott LJ. Intravenous ibuprofen. Drugs 2012; 72:1099–1109.
43. Gerhardt RT, Gerharst DM. Intravenous ketorolac in the treatment of fever. Am J Emerg Med 2000; 18:500–501 (Letter).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








