Henry VIII
The human body is a metabolic furnace that generates enough heat to raise the body temperature by 1°C every hour, even at rest (1). For-tunately, the external surface of the body acts like a radiator, and discharges excess heat into the surrounding environment. The behavior of this radiator is guided by a thermostat (the thermoregulatory system) that limits the daily variation in body temperature to ±0.6°C (2). This chapter describes what happens when this thermostat fails, and allows the body temperature to rise or fall to life-threatening levels.
HEAT-RELATED ILLNESS
Hyperthermia vs. Fever
The distinction between hyperthermia and fever deserves mention at the outset. Both conditions are characterized by an elevated body temperature, but hyperthermia is the result of a defect in temperature regulation, while fever is the result of a normal thermoregulatory system operating at a higher set point. The elevations in body temperature in this chapter represent hyperthermia, not fever. Because the underlying mechanisms involved in the production of hyperthermia and fever are different, the antipyretic agents used to treat fever (e.g., acetaminophen) are ineffective in hyperthermia.
Response to Thermal Stress
The maintenance of body temperature in conditions of thermal stress (e.g., hot weather, strenuous exercise) is primarily achieved by enhanced blood flow to the skin (convective heat loss) and the loss of sweat (evaporative heat loss).
Convective Heat Loss
When heat is lost from the skin, it warms the air just above the skin surface, and the increase in surface temperature limits the further loss of body heat by conduction. However, when an air current (e.g., from a fan or gust of wind) is passed across the skin, it displaces the warm layer of air above the skin and replaces it with cooler air, and this process facilitates the continued loss of body heat by conduction. The same effect is produced by increases in blood flow just underneath the skin. The action of currents (air and blood) that promotes heat loss is known as convection.
Evaporative Heat Loss
The transformation of water from a liquid to a gas requires heat (called the ‘latent heat of vaporization’), and the heat required for the evaporation of sweat from the skin is provided by body heat. The evaporation of one liter of sweat from the skin is accompanied by the loss of 580 kilocalories (kcal) of heat from the body (3). This is about one-quarter of the daily heat production by an average-sized adult at rest. Thermal sweating (as opposed to “nervous sweating”) can achieve rates of 1–2 liters per hour (3), which means that over 1,000 kcal of heat can be lost in one hour during profuse sweating. It is important to emphasize that sweat must evaporate to ensure loss of body heat. Wiping sweat off the skin will not result in heat loss, so this practice should be discouraged during strenuous exercise.
Syndromes
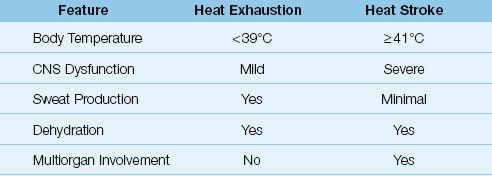
Heat-related illnesses are conditions where the thermoregulatory system is no longer able to maintain a constant body temperature in response to thermal stress. There are a number of minor heat-related illnesses, such as heat cramps and heat rash (prickly heat), but the following descriptions are limited to the major heat-related illnesses: heat exhaustion and heat stroke. The comparative features of these conditions are shown in Table 42.1
Table 42.1 Comparative Features of Heat Exhaustion and Heat Stroke
Heat Exhaustion
Heat exhaustion is the most common form of heat-related illness. Patients with heat exhaustion experience flu-like symptoms that include hyperthermia (usually <39°C or 102°F), muscle cramps, nausea, and malaise. The hallmark of this condition is volume depletion without signs of hemodynamic compromise. The volume loss can be accompanied by hypernatremia (from sweat loss) or hyponatremia (when sweat loss is partly replaced with water intake). There is no evidence of significant neurologic impairment.
The management of heat exhaustion includes volume repletion and other general supportive measures. Cooling measures to reduce body temperature are not necessary.
Heat Stroke
Heat stroke is a life-threatening condition characterized by extreme elevations in body temperature (≥41°C or 106°F), severe neurologic dysfunction (e.g., delirium, coma, and seizures), severe volume depletion with hypotension, and multiorgan involvement that includes rhabdomyolysis, acute kidney injury, disseminated intravascular coagulopathy (DIC), and marked elevation in serum transaminases, presumably from liver. The inability to produce sweat (anhidrosis) is a typical, but not universal, feature of heat stroke (4).
There are two types of heat stroke: (a) classic heat stroke, which is related to environmental temperatures, and (b) exertional heat stroke, which is related to strenuous exercise. Exertional heat stroke tends to be more severe, with a higher incidence of multiorgan dysfunction.
Management
The management of heat stroke includes volume resuscitation and body cooling to reduce the body temperature to 38°C (100.4°F).
EXTERNAL COOLING: External cooling is the easiest and quickest way to reduce the body temperature. This is accomplished by placing ice packs in the groin and axilla, and covering the upper thorax and neck with ice. Cooling blankets are then placed over the entire length of the body. The major drawback of external cooling is the risk of shivering, which is counterproductive because it raises the body temperature. Shivering occurs when the skin temperature falls below 30°C (86°F) (5).
The most effective external cooling method is evaporative cooling, which involves spraying the skin with cool water (at 15°C or 59°F) and then fanning the skin to promote evaporation of the water. This method can reduce the body temperature at a rate of 0.3°C (0.6°F) per minute (6). Evaporative cooling is used mostly in the field, and is particularly effective when the weather is hot and dry (which enhances evaporation from the skin).
INTERNAL COOLING: Internal cooling can be achieved with cold water lavage of the stomach, bladder, or rectum. These methods produce a more rapid reduction in body temperature than external cooling, but they are more labor-intensive. Internal cooling is usually reserved for cases where external cooling is ineffective or produces unwanted shivering.
Rhabdomyolysis
Skeletal muscle injury (rhabdomyolysis) is a common complication of hyperthermia syndromes, including heat stroke (particularly the exertional type) and drug-induced hyperthermia (described later in the chapter). Disruption of myocytes in skeletal muscle leads to the release of creatine kinase (CK) into the bloodstream, and the measurement of CK levels in plasma is used to determine the presence and severity of rhabdomyolysis. There is no standard CK level for the diagnosis of rhabdomyolysis, but CK levels that are five times higher than normal (or about 1,000 Units/liter) have been used to identify rhabdomyolysis in clinical studies (7). Plasma CK levels above 15,000 Units/L indicate severe rhabdomyolysis and an increased risk of acute renal failure from myoglobin released by disrupted myocytes (7).
Myoglobinuric Renal Failure
Renal tubular injury from myoglobin results in acute renal failure in about one-third of patients with rhabdomyolysis (8). This condition is described in Chapter 34.
DRUG-INDUCED HYPERTHERMIA
The heat-related illnesses just described are triggered by thermal stress in the environment. The source of the thermal stress in the following conditions is drug-induced metabolic heat production.
Malignant Hyperthermia
Malignant hyperthermia (MH) is an uncommon disorder that occurs once every 15,000 exposures to inhalational anesthesia, and affects approximately 1 in 50,000 adults (9). It is an inherited disorder with an autosomal dominant pattern, and is characterized by excessive release of calcium from the sarcoplasmic reticulum in skeletal muscle in response to halogenated inhalational anesthetic agents (e.g., halothane, isoflurane, servoflurane, and desflurane) and depolarizing neuromuscular blockers (e.g., succinylcholine) (9). The calcium release leads to uncoupling of oxidative phosphorylation and a marked rise in metabolic rate.
Clinical Manifestations
The clinical manifestations of MH include muscle rigidity, hyperthermia, depressed consciousness, and autonomic instability. The first sign of MH may be a sudden and unexpected rise in end-tidal PCO2 (reflecting the underlying hypermetabolism) in the operating room (9,10). This is followed (within minutes to a few hours) by generalized muscle rigidity, which can progress rapidly to widespread myonecrosis (rhabdomyolysis) and subsequent myoglobinuric renal failure. The heat generated by the muscle rigidity is responsible for the marked rise in body temperature (often above 40°C or 104°F) in MH. The altered mental status in MH can range from agitation to coma. Autonomic instability can lead to cardiac arrhythmias, fluctuating blood pressure, or persistent hypotension.
Management
The first suspicion of MH should prompt immediate discontinuation of the offending anesthetic agent.
DANTROLENE: Specific treatment for the muscle rigidity is available with dantrolene sodium, a muscle relaxant that blocks the release of calcium from the sarcoplasmic reticulum. When given early in the course of MH, dantrolene can reduce the mortality rate from 70% or higher (in untreated cases) to 10% or less (9,10). The dosing regimen for dantrolene in MH is as follows:
Regimen: 1–2 mg/kg as IV bolus, and repeat every 15 minutes if needed to a total dose of 10 mg/kg. Follow the initial dosing regimen with a dose of 1 mg/kg IV or 2 mg/kg orally four times daily for 3 days.
Treatment is extended to 3 days to prevent recurrences. The most common side effect of dantrolene is muscle weakness, particularly grip strength, which usually resolves in 2–4 days after the drug is discontinued (11). The most troublesome side effect of dantrolene is hepatocellular injury, which is more common when the daily dose exceeds 10 mg/kg (9). Active hepatitis and cirrhosis are contraindications to dantrolene therapy (11) but in light of the high mortality in MH if left untreated, these contraindications should not be absolute.
Prevention
All patients who survive an episode of MH should be given a medical bracelet that identifies their susceptibility to MH. In addition, because MH is a genetic disorder with an known inheritance pattern (autosomal dominant), immediate family members should be informed of their possible susceptibility to MH. A test is available to identify the responsible gene for MH in family members (10).
Neuroleptic Malignant Syndrome
The neuroleptic malignant syndrome (NMS) is strikingly similar to malignant hyperthermia in that it is a drug-induced disorder characterized by hyperthermia, muscle rigidity, altered mental status, and autonomic instability (12).
Pathogenesis
NMS is associated with drugs that influence dopamine-mediated synaptic transmission in the brain. A decrease in dopaminergic transmission in the basal ganglia and hypothalamic-pituitary axis may be responsible for many of the clinical manifestations of NMS (12). As indicated in Table 42.2, NMS can be the result of therapy with drugs that inhibit dopaminergic transmission (most cases), or can be triggered by discontinuing drugs that facilitate dopaminergic transmission. (Note that not all drugs associated with NMS are neuroleptic drugs.) The drugs most frequently implicated in NMS are haloperidol and fluphenazine (12). The incidence of NMS during therapy with neuroleptic agents is reported at 0.2–1.9% (13).
Table 42.2 Drugs Implicated in Neuroleptic Malignant Syndrome
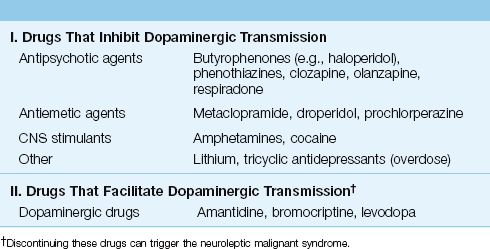
There is no relationship between the intensity or duration of drug therapy and the risk of NMS (12), so NMS is an idiosyncratic drug reaction and not a manifestation of drug toxicity. There is some evidence of a familial tendency, but a genetic pattern of transmission has not been identified (14).
Clinical Features
Most cases of NMS begin to appear 24–72 hours after the onset of drug therapy, and almost all cases are apparent in the first 2 weeks of drug therapy. The onset is usually gradual, and can take days to fully develop. In 80% of cases, the initial manifestation is muscle rigidity or altered mental status (12). The muscle rigidity has been described as lead-pipe rigidity to distinguish it from the rigidity associated with tremulousness (cogwheel rigidity). The change in mental status can range from agitation to coma. Hyperthermia (body temperature can exceed 41°C) is required for the diagnosis of NMS (12), but the increase in body temperature can be delayed for 8–10 hours after the appearance of muscle rigidity (15). Autonomic instability can produce cardiac arrhythmias, labile blood pressure, or persistent hypotension.
Laboratory Studies
Dystonic reactions to neuroleptic agents may be difficult to distinguish from the muscle rigidity in NMS. This is particularly relevant in the early stage of NMS, when muscle rigidity may be the only manifestation. The serum CK level can help in this regard because serum CK levels are only mildly elevated in dystonic reactions, but are higher than 1,000 Units/L in NMS (13).
The leukocyte count in blood can increase to 40,000/∝L with a leftward shift in NMS (12), so the clinical presentation of NMS (fever, leukocytosis, altered mental status) can be mistaken as sepsis. The serum CK level can help to distinguish NMS from sepsis.
Management
The single most important measure in the management of NMS is immediate removal of the offending drug. If NMS is caused by discontinuation of dopaminergic drugs, the drug should be restarted immediately, with gradual reduction of the drug dosage at a later time. General measures for NMS include volume resuscitation (for rhabdomyolysis or hypotension).
DANTROLENE: Dantrolene sodium (the same muscle relaxant used in the treatment of MH) can be given intravenously for severe cases of muscle rigidity. The optimal dose is not clearly defined, but one suggestion is shown below (12,16):
Regimen: 2–3 mg/kg as IV bolus, and repeat every few hours if needed to a total dose of 10 mg/kg. Follow with oral dantrolene in doses of 50–200 mg daily (given in divided doses every 6–8 hrs).
BROMOCRIPTINE: Bromocriptine mesylate is a dopamine agonist that has been successful in treating NMS when given orally in a dose of 2.5–10 mg three times daily (16). Some improvement in muscle rigidity can be seen within hours after the start of therapy, but the full response often takes days to develop. Hypotension is a troublesome side effect. There is no advantage with bromocriptine over dantrolene, except in patients with advanced liver disease (where dantrolene is not advised).
Treatment of NMS should continue for about 10 days after clinical resolution because of delayed clearance of many neuroleptics (when depot preparations are implicated, therapy should continue for 2–3 weeks after clinical resolution) (12). There is a heightened risk of venous thromboembolism during NMS (12), so heparin prophylaxis is recommended. The mortality rate in NMS is about 20% (13), and it is unclear if dantrolene or bromocriptine has a favorable effect on mortality (12,13).
Serotonin Syndrome
Overstimulation of serotonin receptors in the central nervous system produces a combination of mental status changes, autonomic hyperactivity, and neuromuscular abnormalities that is known as the serotonin syndrome (SS) (17). The recent growth in popularity of seritonergic drugs such as selective serotonin reuptake inhibitors (SSRIs) has led to a marked increase in the prevalence of SS in recent years. The severity of illness can vary widely, and the most severe cases can be confused with the other drug-induced hyperthermia syndromes.
Pathogenesis
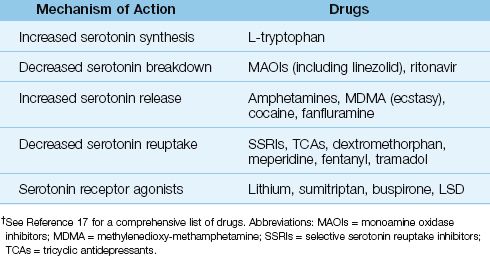
Serotonin is a neurotransmitter that participates in sleep–wakefulness cycles, mood, and thermoregulation. A variety of drugs can enhance serotonin neurotransmission and produce SS, and a list of these drugs is shown in Table 42.3. Many of these drugs work in combination to produce SS, although single-drug therapy can also result in SS. Many of the drugs involved in SS are mood enhancers, including illegal substances like “ecstasy,” an amphetamine derivative implicated in life-threatening cases of SS (18).
Table 42.3 Drugs That Can Produce Serotonin Syndrome†
Clinical Manifestations
The onset of SS is usually abrupt (in contrast to NMS, where the full syndrome can take days to develop), and over half of the cases are evident within 6 hours after ingestion of the responsible drug(s) (17). The clinical findings include mental status changes (e.g., confusion, delirium, coma), hyperthermia, autonomic hyperactivity (e.g., mydriasis, tachycardia, hypertension), and neuromuscular abnormalities (e.g., hyperkinesis, hyperactive deep tendon reflexes, clonus, and muscle rigidity). The clinical presentation can vary markedly (17). Mild cases may include only hyperkinesis, hyperreflexia, tachycardia, diaphoresis, and mydriasis. Moderate cases often have additional findings of hyperthermia (temperature >38°C) and clonus. The clonus is most obvious in the patellar deep-tendon reflexes, and horizontal ocular clonus may also be present. Severe cases of SS often present with delirium, hyperpyrexia (temperature >40°C), widespread muscle rigidity, and spontaneous clonus. Life-threatening cases are marked by rhabdomyolysis, renal failure, metabolic acidosis, and hypotension.
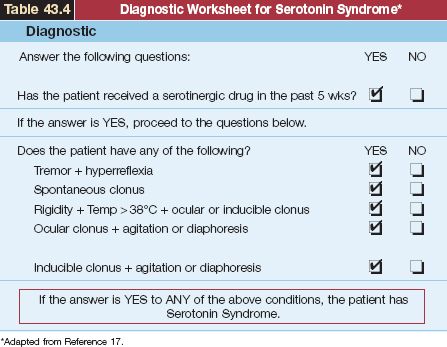
A useful worksheet for the diagnosis of SS is shown in Table 42.4. The first step in the diagnostic evaluation is to establish recent ingestion of seritonergic drugs. Although the worksheet in Table 42.2 indicates drug ingestion in the past five weeks, most cases of SS follow within hours of drug ingestion (17). Hyperthermia and muscle rigidity can be absent in mild cases of the illness. The features that most distinguish SS from other drug-induced hyperthermia syndromes are hyperkinesis, hyperreflexia, and clonus. However, muscle rigidity can mask these clinical findings in severe cases of SS.
Table 43.4 Diagnostic Worksheet for Serotonin Syndrome*
Management
As in other drug-induced hyperthermia syndromes, removal of the precipitating drug(s) is the single most important task in the management of SS. The remainder of the management includes measures to control agitation and hyperthermia, and the use of serotonin antagonists. Many cases of SS will resolve within 24 hours after initiation of therapy, but seritonergic drugs with long elimination half-lives can produce more prolonged symptomatology.
Sedation with benzodiazepines is important for controlling agitation in SS. Physical restraints should be avoided because they encourage isometric muscle contraction, which can aggravate skeletal muscle injury and promote lactic acidosis (19).
CYPROHEPTADINE: Cyproheptadine is a serotonin antagonist that can be given in severe cases of SS (20). This drug is available for oral administration only, but tablets can be broken up and administered through a nasogastric tube.
Regimen: The initial dose is 12 mg, followed by 2 mg every 2 hrs for persistent symptoms. The maintenance dose is 8 mg every 6 hours.
Cyproheptadine can be sedating, but this should aid in the control of agitation in SS.
Neuromuscular paralysis may be required in severe cases of SS to control muscle rigidity and extreme elevations of body temperature (>41°C). Nondepolarizing agents (e.g., vecuronium) should be used for muscle paralysis because succinylcholine can aggravate the hyperkalemia that accompanies rhabdomyolysis. Dantrolene does not reduce muscle rigidity or hyperthermia in SS (17).
HYPOTHERMIA
Hypothermia is defined as a decrease in body temperature below 35°C (95°F), and can be the result of environmental forces (accidental hypo-thermia), a metabolic disorder (secondary hypothermia), or a therapeutic intervention (induced hypothermia). This section will focus primarily on environmental (accidental) hypothermia.
Adaptation to Cold
Physiologically, the human body is better equipped to survive in hot rather than cold environments. The physiological response to cold includes cutaneous vasoconstriction (to reduce convective heat loss) and shivering (which can double metabolic heat production). These physiological adaptations are protective only in mild hypothermia (see later in the text); otherwise, protection from the cold is dependent on behavioral responses (e.g., wearing warm clothing and seeking shelter from the cold). Because of the importance of behavioral responses, hypothermia is particularly pronounced when these responses are impaired (e.g., in the intoxicated or confused patient).
Accidental Hypothermia
Environmental hypothermia is most likely to occur in the following situations: (a) prolonged submersion in cold water (the transfer of heat to cold water occurs much more readily than the transfer of heat to cold air), (b) exposure to cold wind (wind promotes heat loss by convection, as described earlier in the chapter), (c) when the physiological response to cold is impaired (e.g., the vasoconstrictive response to cold is impaired by alcohol consumption), and (d) when the behavioral responses to cold are impaired (as mentioned in the previous paragraph).
Temperature Recordings
Most standard thermometers measure temperatures down to 34°C (94°F). For more accurate recordings in hypothermia, electronic temperature probes are available that can record temperatures down to 25°C (77°F), and can be placed in the bladder, rectum, or esophagus.
Clinical Features
The consequences of progressive hypothermia are summarized in Table 42.5.
Table 42.5 Manifestations of Progressive Hypothermia
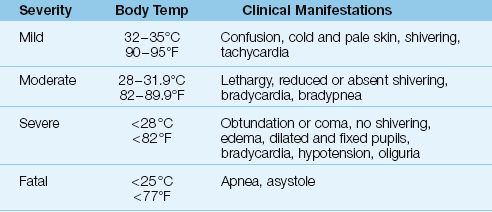
MILD HYPOTHERMIA: In mild hypothermia (32–35°C or 90–95°F), pa-tients are usually confused, and show signs of adaptation to cold; i.e., cold, pale skin from cutaneous vasoconstriction. There is brisk shivering, and a rapid heart rate.
MODERATE HYPOTHERMIA: In moderate hypothermia (28–31.8°C or 82–89°F), shivering may be absent, and patients are lethargic. Bradycardia and a decreased respiratory rate (bradypnea) become evident, and pupillary light reflexes can be absent.
SEVERE HYPOTHERMIA: In severe hypothermia (<28°C or <82°F), patients are usually obtunded or comatose with dilated, fixed pupils (which are not a sign of brain death in this situation). Additional findings include hypo-tension, severe bradycardia, oliguria, and generalized edema. Apnea and asystole are expected at body temperatures below 25°C (77°F).
Laboratory Evaluation
The laboratory tests of interest in hypothermia include arterial blood gases, serum electrolytes (particularly potassium), tests of coagulation, and tests of renal function. A generalized coagulopathy (with elevation of the INR and prolonged partial thromboplastin times) is common in hypothermia (21), but may not be evident if the coagulation profile is run at normal body temperatures. Arterial blood gases (which should be run at normal body temperatures) can reveal a respiratory acidosis or a metabolic acidosis (21). Serum electrolytes can reveal hyperkalemia, which is presumably due to potassium release by skeletal muscle from shivering or rhabdomyolysis. Serum creatinine levels can be elevated as a result of rhabdomyolysis, acute renal failure, or cold diuresis (caused by diminished renal tubular responsiveness to antidiuretic hormone).
Electrocardiogram
About 80% of patients with hypothermia will have prominent J waves at the QRS–ST junction on the electrocardiogram (see Figure 42.1). These waves, which are called Osborn waves, are not specific for hypothermia, and can occur with hypercalcemia, subarachnoid hemorrhage, cerebral injuries, and myocardial ischemia (22). Despite the attention these waves have received, they are merely a curiosity, and have little or no diagnostic or prognostic value in hypothermia (21–13).
ARRHYTHMIAS: A multitude of rhythm disturbances can occur in hypo-thermia, including first, second, and third-degree heart block, sinus and junctional bradycardia, idioventricular rhythm, premature atrial and ventricular beats, and atrial and ventricular fibrillation (22).
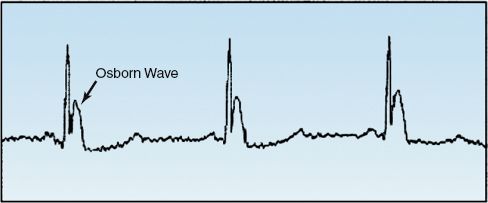
FIGURE 42.1 The (overhyped) Osborn wave.
Rewarming
EXTERNAL REWARMING: External rewarming (removing wet clothes, covering the patient in blankets, etc.) can increase body temperature at a rate of 1–2°C per hour (21), and is adequate for most cases of hypothermia (23). There is a risk of a further decrease in body temperature during external rewarming (called afterdrop), which can trigger ventricular fibrillation (24). This phenomenon is attributed to central displacement of cold blood in cutaneous blood vessels. Fortunately, serious cardiac arrhythmias are not common, and do not contribute to mortality during external rewarming for severe hypothermia (23,24).
INTERNAL REWARMING: There are several methods of internal rewarming, but they are invasive, time-consuming, and are needed only in the most severe cases of hypothermia. The easiest internal warming technique is to increase the temperature of inhaled gases to 40–45°C (104–113°F), which can raise the core temperature at a rate of 2.5°C per hour in intubated patients (21). Other internal warming techniques include peritoneal lavage with heated fluids (21), extracorporeal blood rewarming (25), and heated intravenous fluids (26). Warmed gastric lavage is ineffective (21).
REWARMING SHOCK: Rewarming from moderate or severe hypothermia is often accompanied by hypotension (rewarming shock). This is attributed to a combination of factors, including hypovolemia (from cold diuresis), myocardial depression, and vasodilation (23,24). Volume infusion will help to alleviate this problem, but the infusion of fluids at room temperature (21°C or 70°F) can aggravate the hypothermia, so the infused fluids should be heated. Vasoactive drugs are required in about half of patients with severe hypothermia, and this requirement indicates a poor prognosis (24).
Induced Hypothermia
Intentional cooling to a body temperature of 32–34°C (89.6–93.2°F) is now a popular treatment modality for patients who remain comatose after resuscitation from cardiac arrest. This topic is presented in Chapter 17 (see pages 336–339).
A FINAL WORD
The Adaptable Human
The number of deaths from heat exposure is estimated at only 400 per year in the United States (27), and in a 20-year survey of a large urban hospital in France, severe hypothermia accounted for only 0.4% of admissions to the ICU (24). These small numbers are a testament to the human ability to adapt (both physiologically and behaviorally) to environmental extremes.
REFERENCES
1. Keel CAm Neil E, Joels N. Regulation of body temperature in man. In: Samson Wright’s Applied Physiology, 13th ed. New York: Oxford University Press, 1982:346.
2. Guyton AC, Hall JE. Body temperature, temperature regulation, and fever. In: Medical Physiology, 10th ed. Philadelphia, WB Saunders, 2000:822–833.
Heat-Related Illness
3. Khosla R, Guntupalli KK. Heat-related illnesses. Crit Care Clin 1999; 15:251–263.
4. Lugo-Amador NM, Rothenhaus T, Moyer P. Heat-related illness. Emerg Med Clin N Am 2004; 22:315–327.
5. Glazer JL. Management of heat stroke and heat exhaustion. Am Fam Physician 2005; 71:2133–2142.
6. Hadad E, Rav-Acha M, Heled Y, et al. Heat stroke: a review of cooling methods. Sports Med 2004; 34:501–511.
7. Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med 1988; 148:1553–1557.
8. Sharp LS, Rozycki GS, Feliciano DV. Rhabdomyolysis and secondary renal failure in critically ill surgical patients. Am J Surg 2004; 188:801–806.
Malignant Hyperthermia
9. Rusyniakn DE, Sprague JE. Toxin-induced hyperthermic syndromes. Med Clin N Am 2005;89:1277–1296.
10. Litman RS, Rosenberg H. Malignant hyperthermia. J Am Med Assoc 2005; 293:2918–2924.
11. McEvoy GK, ed. AHFS Drug Information, 2001. Bethesda, MD: American Society of Health-System Pharmacists, 2001, pp. 1328–1331.
Neuroleptic Malignant Syndrome
12. Bhanushali NJ, Tuite PJ. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin N Am 2004; 22:389–411.
13. Khaldarov V. Benzodiazepines for treatment of neuroleptic malignant syndrome. Hosp Physician, 2003 (Sept):51–55.
14. Otani K, Horiuchi M, Kondo T, et al. Is the predisposition to neuroleptic malignant syndrome genetically transmitted? Br J Psychiatry 1991; 158:850–853.
15. Lev R, Clark RF. Neuroleptic malignant syndrome presenting without fever: case report and review of the literature. J Emerg Med 1996; 12:49–55.
16. Guze BH, Baxter LR. Neuroleptic malignant syndrome. N Engl J Med 1985; 313:163–166.
Serotonin Syndrome
17. Boyer EH, Shannon M. The serotonin syndrome. N Engl J Med 2005; 352:1112–1120.
18. Demirkiran M, Jankivic J, Dean JM. Ecstacy intoxication: an overlap between serotonin syndrome and neuroleptic malignant syndrome. Clin Neurophar-macol 1996; 19:157–164.
19. Hick JL, Smith SW, Lynch MT. Metabolic acidosis in restraint-associated cardiac arrest. Acad Emerg Med 1999; 6:239–245.
20. Graudins A, Stearman A, Chan B. Treatment of serotonin syndrome with cyproheptadine. J Emerg Med 1998; 16:615–619.
Hypothermia
21. Hanania NA, Zimmerman NA. Accidental hypothermia. Crit Care Clin 1999; 15:235–249.
22. Aslam AF, Aslam AK, Vasavada BC, Khan IA. Hypothermia: evaluation, electrocardiographic manifestations, and management. Am J Med 2006; 119:297–301.
23. Cornell HM. Hot topics in cold medicine: controversies in accidental hypothermia. Clin Ped Emerg Med 2001; 2:179–191.
24. Vassal T, Bernoit-Gonin B, Carrat F, et al. Severe accidental hypothermia treated in an ICU. Chest 2001; 120:1998–2003.
25. Ireland AJ, Pathi VL, Crawford R, et al. Back from the dead: Extracorporeal rewarming of severe accidental hypothermia victims in accidental emergency. J Accid Emerg Med 1997; 14:255–303.
26. Handrigen MT, Wright RO, Becker BM, et al. Factors and methodology in achieving ideal delivery temperatures for intravenous and lavage fluid in hypothermia. Am J Emerg Med 1997; 15:350–359.
A Final Word
27. Morbidity and Mortality Weekly Report, 2002; 51:567–570.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








